ABSTRACT
Recent studies indicate an important role for vitamin D3 in autism spectrum disorder (ASD), although its mechanism is not completely understood. The most puzzling aspect of ASD is that identical twins, who share identical DNA, do not have 100% concordance rates (∼88% for identical and ∼31% for fraternal twins). These findings provide major clues into the etiology: ASD must involve an environmental factor present in the prenatal milieu that both identical twins are not always exposed to because they do not always share it (i.e., placentas). Combined with the exponential increasing rates of ASD around the world, these observations suggest a contagious disease is probably transferred to the fetus via the placenta becoming infected by a cervical virus. Vitamin D3 boosts immune responses clearing viral infections and increases serotonin and estrogen brain levels. Here we review the different roles and untangle the most probable one vitamin D3 plays in ASD.
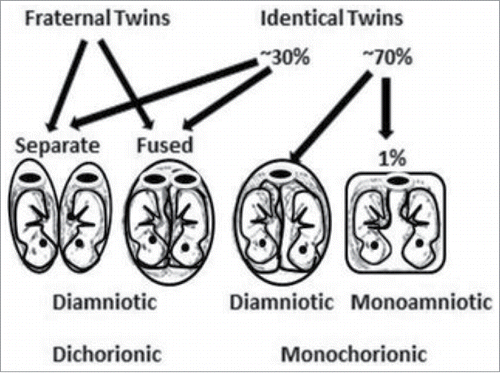
The most puzzling aspect of autism spectrum disorder (ASD) is that identical twins, who share identical DNA, do not have 100% concordance rates. Identical, monozygotic twins only have concordance rates of ∼88%, while fraternal, dizygotic twins have concordance rates of ∼31%.Citation1,2 These twin observations alone provide clues into the etiology: ASD must involve something present in the prenatal environment that both identical twins are not always exposed to because they do not always share it. Identical twins can share the same placenta and amniotic sac (occurrence ∼1%) or they can share the same placenta but not their amniotic sac (occurrence ∼69%) or they can have their own placentas and amniotic sacs (occurrence ∼30%), while fraternal twins always have their own placentas and amniotic sacsCitation3 (see ). The subtle differences between the prenatal environments of identical twins, especially their placentas, might explain why a 100% concordance rate of ASD does not exist for them. Thus, we need to closely examine the prenatal environment of twins.
Figure 1. Identical and fraternal twin placenta and amniotic sac possibilities and percent occurrences: shared placentas (monochorionic), separate placentas (dichorionic), shared amniotic sacs (monoamniotic) and separate amniotic sacs (diamniotic). The placenta is represented by an oval with a large dark disc in the middle.
Prenatal soluble factors
Many soluble factors exist in the prenatal environment that can possibly affect the developing fetus: vitamins, hormones, cytokines, chemicals, alcohol, drugs, medications, etc. A prenatal environment with low levels of vitamin D3, measured as 25-hydroxyvitamin D3, was hypothesized to cause ASD.Citation4 Recent investigations confirm low maternal 25-hydroxyvitamin D3 levels during gestation are associated with ASD-related traits in a large population-based sampleCitation5 and supplementing children with vitamin D3 improve the signs and symptoms of ASD.Citation6 However, although vitamin D3 treatment is beneficial for improving and possibly preventing some of the symptoms of ASD or even lowering its occurrence, those observations do not prove a causal relationship exists because low 25-hydroxyvitamin D3 levels are shared between twins in their prenatal environment whether they are identical or not. In fact, any circulating soluble factor like cytokines, medications, alcohol, drugs, or exposure to any chemical in our environment would give a 100% concordance rate for either identical or fraternal twins, but this is not observed. Thus, something else must be awry that vitamin D3 somehow differentially affects.
Vitamin D3 – estrogen, serotonin and immune function
The hormonal form of vitamin D3, 1, 25-dihydroxyvitamin D3, affects over 200 genes through the vitamin D3 receptor,Citation7 but more importantly it increases estrogen levels in the placentaCitation8 and the brainCitation9 and is probably necessary for regulating serotonin production.Citation10 Estrogen brain levels might help to somewhat explain why males are 4 to 5 times more likely to become autistic than femalesCitation11 because estrogen is extremely important in brain development.Citation12 Of note, ASD subjects displayed dysregulation of the estrogen receptor beta, aromatase, and estrogen receptor co-activators in the middle frontal gyrus region of their brains.Citation13 Racial disparity supports a causal role for estrogen rather than vitamin D3 in ASD because African-American blacks have lower vitamin D3 status than whitesCitation14 but instead of having higher incidences of ASD their incidences are actually lowerCitation15–17 probably because their male's estrogen levels are significantly higher than white males.Citation18 Estrogen increases the synthesis of tryptophan hydroxylase-2, the rate-limiting enzyme in the production of serotonin,Citation19 and vitamin D3 also increases tryptophan hydroxylase-2.Citation10 Apparently some of the positive effects of vitamin D3, or 1, 25-dihydroxyvitamin D3, on ASD is its ability to raise both estrogen and serotonin levels during fetal brain development.Citation20
Another important biological function of vitamin D3 is its ability to activate the mother's immune system,Citation21 especially T cellsCitation22 that remove infected cells and infectious agents like bacteria and viruses in the mother, especially from her cervix. Thus, if ASD is caused by an infectious agent, boosting the immune system with vitamin D3 might also help to explain its positive effects.
Infectious placental and cervical diseases
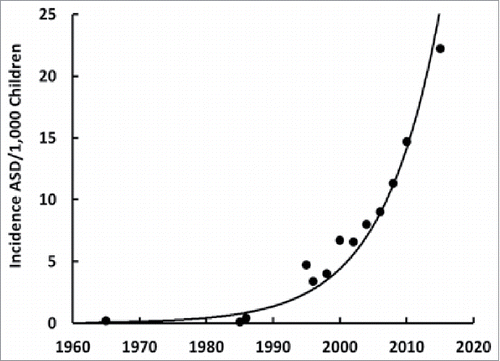
The increase in the incidence of ASD is much faster than that predicted from genetic inheritance or from exposure to environmental pollutantsCitation23 but rather displays characteristics of an infectious disease because it is increasing at an exponential rate in the United States (see , plotted from the data in reference 24) and around the world.Citation24 This infectious disease is probably either a bacterium or a virus.Citation25 We can rule out bacterial infections because they would have been noticed on hematoxylin and eosin stained slides of different tissues and would have produced other health problems. So, if it is an infection, it is most likely a viral infection.Citation26 Human immunodeficiency virus was a candidate over two decades ago that did not pan outCitation27 but co-infections are common so that other viruses should be considered. Evidently, the virus that may cause ASD does not cause any obvious symptoms or it would have been discovered years ago.
Which viral infection?
We know viral infections can affect the developing fetal brain from the Zika virus that causes microencephaly. Evidence that ASD might be caused by a virus is obtained from affected children's increased levels of the virally-induced enzyme, alpha-N- acetylgalactosaminidase,Citation28 similar to women with cervical cancer whose enzyme levels are elevated by the Human Papillomavirus (HPV).Citation29
HPV is vertically transmitted from the mother's infected cervix to her placenta and then to her fetus rather than through her blood.Citation30,31 Further proof of placental transmission of HPV rather than blood is obtained from the prevalence of HPV DNA, which was found to be considerably lower in newborns (∼1.5% or ∼1/68) of infected mother's (∼30% population of pregnant women).Citation32 Coincidentally, this infection rate matches the incidence of 1 in 68 children with ASD eight years later. HPV is known to infect the trophoblasts of the placentaCitation33 where inclusions in it have been found to be predictive of ASD.Citation34 Moreover, the discordant rates of identical twins might be explained by the initial location of one of their placentas being juxtapose to the cervix, where HPV infection occurs; a low-lying placenta, which is a fairly common occurrence, moves back up 90% of the time during the second trimesterCitation35 and can sometimes take HPV with it. Fraternal twins with only a 31% concordant rate of ASD might be explained by one twin having its placenta in closer proximity to the cervix than the other twin whether or not it moves back up (placenta previa if it remains low). And besides the differences in estrogen levels, the higher male to female ratio of ASD might be explained by the male fetuses' tendency to implant their placentas lower in the uterus during the first trimester, as demonstrated by male fetuses having a higher incidence of placenta previa (the reason is unknown).Citation36,37 Twins also have a higher rate of placenta previa than singletonsCitation38 and they have a higher rate of ASD as well.Citation39,40 Furthermore, cesarean births increase the risk for placenta previaCitation41 and they also increase the risk for ASD.Citation42 Finally, the increase in ASD with increasing age of the mother might be explained by the increasing incidence of placenta previa as women age.Citation43 Thus, timing of HPV infection during pregnancy might be important because HPV probably has to be replicating in the cervix during the first trimester while the placenta is low-lying in the uterus prior to moving back up;Citation44,45 however, if placenta previa occurs or if the infection travels higher into the uterus, then transfer of the infection can occur at any time during pregnancy.
Supporting evidence HPV is the primary infecting agent
Some argue that HPV cannot exist in the brain because it only infects mucosal (alpha genus) or epithelial (beta and gamma genera) cells and those cell types are not present inside the brain. It is true that no mucosal or epithelial cells exist inside the brain, but there is an epithelial lining in the center of the brain known as the choroid plexus that forms the blood-cerebrospinal fluid barrier and is responsible for producing cerebrospinal fluids.Citation46 Although the choroid plexus appears to be a small structure, it affects every region of the brain during early embryogenesis and throughout brain development through the production of cerebral fluids;Citation47 we now know that every region of the ASD brain is affected to some degree and that there is serious disruption in the central nervous system.Citation48 The epithelial cells of the choroid plexus secrete cerebrospinal fluid by unidirectionally transporting sodium, chlorine and bicarbonate from the blood to the ventricles of the brain. The sodium-driven chloride bicarbonate exchanger, or SCL4A10 gene product,Citation49 is associated with ASDCitation50 and HPV integration into the host genome might lead to duplication of this gene. Disruption in this one gene augments neuronal excitability and synaptic short-term plasticityCitation51 and might cause complex partial epilepsy and mental retardation.Citation52 Although controversial, some studies found HPV in the post-mortem brains of children with focal cortical dysplasia (epilepsy and seizures),Citation53 a condition also associated with ASD.Citation54
Curiously, papillomas have been found in the choroid plexus of adults and childrenCitation55,56 and tumors have been found in the choroid plexus in children under the age of 2.Citation57 Another curious observation is the fact that about 75% of ASD children have gastrointestinal problems including inflammatory bowel diseaseCitation58 and a single layer of epithelium cells very similar to the choroid plexus with similar functions and immune responses exist in the gastrointestinal tract;Citation59 vitamin D3 reduces the symptoms. Furthermore, inflammation of the brain is a hallmark of ASD and inflammatory cytokines are in the cerebrospinal fluids of affected children.Citation60 Microglia cells are the first line of defense to rid the brain of infections resulting in an inflammatory responseCitation61 and differential M2 activation of microglia cells in ASD brains is driven by type 1 interferon responses to damage caused by viral infections.Citation62 This inflammatory response continues throughout the lifetime of an ASD individual, probably caused by HPV continually producing virions, and this inflammation might be reduced by vitamin D3.Citation63 Recently, the MRIs of children's brains at 6 or 12 months of age that showed abnormal amounts of cerebrospinal fluid displayed ASD traits at 2 yrs. of age; the more cerebrospinal fluid present, the worse the ASD symptoms were later in life.Citation64
Similarities between Cancer and ASD?
If HPV is involved in ASD, we would expect to see many biochemical similarities between cancer and ASD – and we do!Citation65 Most importantly, both cancer and ASD share disruptions in the PI3K-Akt-mTOR signaling pathway involving PTEN, FMR1, NF1, TSC1 and TSC2, and both share signal transduction pathways, chromatin remodeling and transcription factors. What is most amazing and specific for HPV in cancer is its E6 activation of mTORC1 signalingCitation66 and, coincidently, mTORC1 and PTEN are also associated with ASD.Citation67 The mTOR protein is a serine/threonine kinase involved in brain development mediating signaling pathways crucial for neuronal and glial differentiation and maintenance of the stemness of neural stem cells; abnormalities in its function are associated with ASD, seizures, pediatric brain tumors, learning disabilities, and mental retardation.Citation68 Another shared gene between ASD and HPV-induced cancers is UBE3A, which encodes the ubiquitin E3 ligase, E6-AP that degrades p53 and other proteins.Citation69,70 If HPV is involved in ASD, we would expect to see a higher incidence of cancers in this population – and we do!Citation71,72
Note here that HPV-16 or −18 might not be the strains that cause ASD; it could be HPV-11 or HPV-58, or one or more, or possibly all of the other 200+ available strains.Citation73 Alternatively, it might be another sexually transmitted infection of the cervix.Citation74 Other viruses and infections such as influenza, rubella, cytomegalovirus, parvovirus, Lyme's disease or toxoplasma gondii infection have sometimes been implicated in ASD. However, no correlation between any viral, bacterial, or parasitic infections could consistently be reliably made with ASD and appeared to be coincidental occurrences; whereas, HPV infection has never been explored probably because it is thought to only cause cancers. And the fact that HPV has been found in adult brain tumors such as glioblastoma multiformeCitation75 without any ASD symptoms can be explained by the mode of transmission; the adult becomes infected with HPV via sexual transmission after their brain has developed rather than the fetus that becomes infected with HPV via placentas while their brain is developing. Finally, HPV has also been found in neurons.Citation76 Thus, although other infections may sometimes be associated with ASD, HPV appears to be the primary infectious agent.
Do HPV's biochemical and epigenetic fingerprints match ASD's fingerprints?
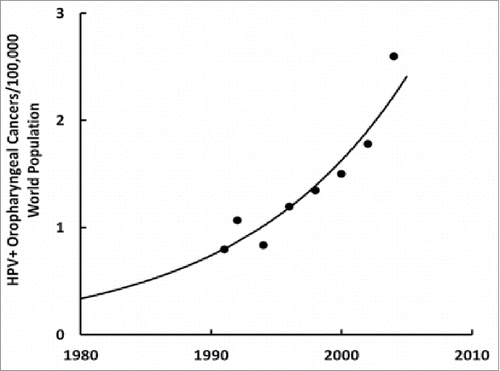
We can obtain evidence that HPV is a risk factor for ASD by looking at its incidence rate, and characteristic biochemical and epigenetic fingerprints. Unlike other sexually contagious diseases, the incidence of HPV infection has been exponentially increasing in the United States ()Citation77 like ASD ().Citation24 Meanwhile, vitamin D3 status has been dramatically decreasing over the last five decades, as shown by the inversely-related parathyroid hormone levels that have decreased nearly ten-fold during the same period.Citation78 Vitamin D3 is important for proper immune function,Citation79 especially enabling activated T cells to eliminate virally infected cellsCitation22 and to clear viral infections.Citation80 Vitamin D3 also decreases leptin levelsCitation81 that were found to be higher in ASD childrenCitation82 and older women who have a persistent HPV infection.Citation83 HPV causes preeclampsia, or high blood pressure, in pregnant womenCitation84 and so does ASD.Citation85 Furthermore, ASD children's brains have downregulated estrogen receptor beta in their middle frontal gyrus regionCitation13 where sexual dimorphisms have been observedCitation86 and HPV can downregulate estrogen receptor beta,Citation87 which in turn can downregulate the vitamin D3 receptor.Citation88 And among the multitudes of HPV's genomic integration sites, one happens to be at 12q13-1589 where the vitamin D3 receptor gene is located;Citation90 integration might decrease or annihilate its function. The fact that African-American blacks have lower vitamin D3 statusCitation14 while they have a higher prevalence of HPVCitation91 but lower incidences of ASDCitation15–17 might be explained by their males having significantly higher estrogen levels than white males.Citation18
Genetic or epigenetic component?
For the most part, ASD cannot be inherited or caused by sporadic genetic mutations because it is increasing at an exponential rate indicting it has a contagious epidemic aspect (); the belief that ASD's exponential increase is due to over diagnosis was laid to rest more than a decade ago by the CHARGE study.Citation92 Arguments that epigenetics plays a major role in ASD have been escalating over recent yearsCitation93 – with little evidence that ASD might begin with a sexually transmitted disease – except noted in the very religious and somewhat isolated Amish community by having a low ASD rate of 1 in 271 children compared with the overall United States having a high ASD rate of 1 in 91 children in 2009.Citation94 Because identical twins were 88% and fraternal twins were only 31% concordant for ASD along with an increased rate (10–20 times higher) over the general population's recurrence in siblings,Citation1 investigators thought ASD was a ‘highly’ inheritable, genetic disease. As a result, over 800 different predisposition genes have been identified so far with little consensus; these genes are involved in a multitude of different functions: chromatin remodeling, gene transcription regulation, cell growth and proliferation, ubiquitination, and neuronal-specific processes like synaptic activity and organization, dendritic morphology, and axonogenesis.Citation95 In addition, copy number variants have been implicated in ASDCitation50 but once again like the different infections noted in some cases of ASD, they were not found to be universal.Citation96 Logic dictates that for identical twins to be ∼12% discordant in ASD very few genes can be involved in its etiology due to the rare frequency of functional mutations. Some consensus has been reached on a few genetic loci that appear to be recurrently duplicated or deleted such as chromosomal regions 15q11-q13, 16p11 and 22q11; note that region 15q11-q13 encodes for UBE3A, the ubiquitin E3 ligase, E6-AP that degrades p53 and other proteins and is duplicated in ASDCitation97 possibly from viral intervention.
The fact that few genes are consistently changed but many genes appear to be involved might be explained by epigenetic events influencing multitudes of genetic pathways, which can make ASD appear to be genetic when it is not.Citation98 DNA methylation patterns, long noncoding RNAs and microRNAsCitation99,100 combined with the persistence of HPV infection might make ASD appear to be genetic because so many genes are affected. In addition, some mothers are not able to clear the virus before having another child, so that reoccurrence in siblings also makes ASD appear to be genetic. Over half the women were still infected with HPV after 2 years,Citation101 which might explain why the spacing between pregnancies of 1 year is 3 times riskier for having a second baby with ASD than 3 years.Citation2 Moreover, DNA methylation is significantly different between identical twins discordant for ASD,Citation102 and is aberrant in cervicalCitation103 and only in HPV+ not HPV- oropharyngeal cancers.Citation104 Another deceiving genetic connection is HPV integrating into the host genome at multiple sites (>190), mapped from cervical cancers,Citation105–107 but note that the integration sites in ASD might differ somewhat from cervical cancer or could be caused by another strain of HPV other than 16 or 18. Integration can result in deletion or duplication of a section of the DNA and considerable genomic instabilityCitation108 similar to what is observed in ASD.Citation109 Besides epigenetic events leading to aberrant gene expression in ASD, some of the point mutations observed might be due to methylation of cytosines leading to deamination that result in transition mutations.Citation110 Finally, because HPV is sometimes found in spermCitation111 but is usually transmitted vertically from the mother's placenta,Citation112 ASD can appear to be genetically transferred when it is not.
Epigenetic fingerprints of ASD and HPV might appear to be smudged sometimes because ASD and cancer researchers each concentrate on searching for endpoints related to their particular disease, so that they miss the relevant endpoints in each other's diseases; when they both might be looking at opposite sides of the same coin.
HPV strains found in cervical smears
The highest occurring strains of HPV in the uterine cervix associated with developing cervical carcinomas and precancerous lesions from the highest to lowest occurrence are HPV-16, −58, −18, −52, and −56 followed by the low risk strains.Citation113 This was determined by a PCR-based DNA chip microarray detection method of cervical smears that can assess 22 different strains of HPV including 15 from the high-risk group, HPV-16, −18, −31, −33, −35, −39, −45, −51, −52, −56, −58, −59, −66, −68, −69 and 7 from the low-risk group, HPV-6, −11, −34, −40, −42, −43, −44.Citation113 It is important to note once again that these strains of HPV are the high- and low-risk groups for cervical cancers and that they might not reflect the same risk for ASD. In addition, multiple strains of HPV might be required to produce ASD. Because Papanicolaou (Pap) smears only detect cancerous cells – not the presence of HPVCitation114 – this PCR method or another HPV detection method should be used in combination with a second, different HPV detection method for confirmationCitation115,116 during routine cervical exams. Be aware that other PCR-based methods sometimes detected HPV when this PCR-based microarray chip method did not, which is probably due to differences in their sensitivities.
This difference in sensitivities between detection methods points out how important it is to use multiple detection methods and approaches to find and confirm the existence of HPV, and to use broad spectrum probes before searching for specific strains. Furthermore, amplification of the signal is best because HPV might be missed due to a weak signal.Citation117 Alternatively, HPV might be missed due to the timing of infection or when some observations are performed, which might be critical because HPV can cause opposite biological effects during different phases of its life cycle. For example, in the beginning, HPV uses estrogen and its receptors to make more E6 and E7 proteins, but towards the end of its life cycle, in differentiated cells (and possibly third trimester fetuses), it downregulates estrogen and its receptorsCitation118 and increases p16 when it makes capsid proteins L1 and L2. Thus, it is best to cover both ends of HPV's life cycle.
Treatment and prevention options
If HPV is found guilty for being the contributing factor in ASD, the future incidence and current symptoms might be reduced by increasing both the mother's and child's 25-hydroxyvitamin D3 levels to at least 50 ng/mL using adequate amounts of vitamin D3119, 120, 121 to raise estrogen and serotonin levels, decrease leptin levels and inflammation, and improve brain functionCitation122 of the fetuses and children, while clearing existing HPV infections in the mother and children over 18 months of age. In addition, increasing estrogen and tryptophan (for serotonin synthesis) levels might help the brain to develop better. And recently new treatments for ASD target inhibiting the mTOR pathway;Citation123 Rapamycin shows promise for treating ASDCitation124 and coincidently it is also being used to improve clearance during treatment of HPV-positive head and neck cancers.Citation125 More importantly, vaccinating mothers prior to and even during pregnancy (found to be safe and provides passive immunity to the neonate and infant)Citation126 and giving at least two doses of the nine-valent HPV vaccine to ASD children (over 18 months old when they have a competent immune system) might boost their immune systems to clear HPV and help improve the symptoms in some ASD cases, because the quadrivalent vaccine was found to decrease or eliminate the occurrence of oral and genital warts.Citation127,128 However, these current HPV vaccines might not completely protect against the strain(s) of HPV that cause the entire spectrum of autism so that another or other vaccines might have to be developed. For example, along with HPV-16 and −18, HPV-38 has been found in melanomas and are implicated in its etiology.Citation129–131 Most importantly, note that the cervical Pap smears only detect cancerous cells and not the presence of HPV, so that it would be wise to include a broad-spectrum HPV test along with routine Pap smears in the future, especially for women who intend to get pregnant or who already are pregnant.
Conclusions
The fact that identical twins, who share identical DNA, do not have 100% concordance rates shows genetics plays a minor role (excluding epigenetic events) and some environmental factor that differs between twins plays a major role in ASD. Because identical twins only share their placentas about 70% of the time and their concordance rates are only ∼88%, an environmental factor probably plays its role through the placenta. Low levels of 25-hydroxyvitamin D3, or any other soluble factor, cannot be the cause of ASD because if it were, then all twins, identical or not, would be affected but they are not. Thus, the cause of ASD has to be something that vitamin D3 affects in the environment of the placenta. Based on observations of placenta inclusions, predictive of ASD and indicative of an infection, we proceeded to untangle the major role vitamin D3 plays in ASD and found it is probably to clear the viral infection that a low-lying placenta picked up from the cervix. The most probable viral infection is HPV because it is the only cervical infection that is increasing at an exponential rate around the world like ASD. HPV can exist in the epithelial lining in the center of the brain known as the choroid plexus that forms the blood-cerebrospinal fluid barrier. The choroid plexus secretes cerebrospinal fluid by unidirectionally transporting sodium, chlorine and bicarbonate from the blood to the ventricles of the brain via the sodium-driven chloride bicarbonate exchanger, or SCL4A10 gene product, which is associated with ASD;Citation50 HPV integration into the host genome might lead to duplication of this gene and elimination or disruption of the vitamin D3 receptor gene. More supporting evidence HPV is involved in ASD is obtained from the epigenetic fingerprints of HPV that match those of ASD, which also makes it appear to be of genetic origin when it is not. The roles of vitamin D3 in ASD include increasing the brain's estrogen and serotonin levels while decreasing inflammation via decreasing inflammatory cytokines produced by activated microglia cellsCitation132 and reducing leptin levels. The untangled major role for vitamin D3 in ASD and most cancersCitation133 is its ability to regulate 291 genes and boost the immune systemCitation134 in order to clear HPV and other viral infections.
Conflict of interest statement
The authors declare no conflict of interest.
Author contributions
DEG planned the study, analyzed the data in the literature, and was the primary author. SJM contributed to the scientific discussions and revising the final version.
Acknowledgments
We did not receive any financial support for this research.
References
- Rosenberg RE, Law JK, Yenokyan G, McGready J, Kaufmann WE, Law PA. Characteristics and concordance of autism spectrum disorders among 277 twin pairs. Arch Pediatr Adolesc Med. 2009;163(10):907–914. doi:10.1001/archpediatrics.2009.98.
- Newschaffer CJ, Croen LA, Fallin MD, Hertz-Picciotto I, Nguyen DV, Lee NL, Berry CA, Farzadegan H, Hess HN, Landa RJ, et al. Infant siblings and the investigation of autism risk factors. J Neurodev Disord. 2012;4(1):7. doi:10.1186/1866-1955-4-7. PMID:22958474
- Faye-Petersen OM, Heller DS, Joshi VV. Handbook of Placental Pathology, 2nd ed. London: Taylor & Francis; 2006. ISBN 9781842142325
- Cannell JJ. Autism and vitamin D. Med Hypotheses 2008;70(4):750–759. doi:10.1016/j.mehy.2007.08.016. PMID:17920208
- Vinkhuyzen AA, Eyles DW, Burne TH, Blanken LM, Kruithof CJ, Verhulst F, Jaddoe VW, Tiemeier H, McGrath JJ. Gestational vitamin D deficiency and autism-related traits: the Generation R Study. Mol Psychiatry. 2016. doi:10.1038/mp.2016.213. [Epub ahead of print]. PMID:27895322
- Saad K, Abdel-Rahman AA, Elserogy YM, Al-Atram AA, El-Houfey AA, Othman HA, Bjørklund G, Jia F, Urbina MA, Abo-Elela MG, Ahmad FA, Abd El-Baseer KA, Ahmed AE, Abdel-Salam AM Randomized controlled trial of vitamin D supplementation in children with autism spectrum disorder. J Child Psychol Psychiatry. 2016. doi:10.1111/jcpp.12652. [Epub ahead of print]. PMID:27868194
- Ramagopalan SV, Heger A, Berlanga AJ, Maugeri NJ, Lincoln MR, Burrell A, Handunnetthi L, Handel AE, Disanto G, Orton SM, Watson CT, Morahan JM, Giovannoni G, Ponting CP, Ebers GC, Knight JC. A ChIP-seq-defined genome-wide map of vitamin D receptor binding: Associations with disease and evolution. Genome Research. 2010;20(10):1352–60. doi:10.1101/gr.107920.110. [Epub 2010 Aug 24]. PMID:20736230
- Barrera D, Avila E, Hernández G, Méndez I, González L, Halhali A, et al. Estradiol and progesterone synthesis in human placenta is stimulated by calcitriol. J Steroid Biochem Mol Biol. 2007;103(3–5):529–522. doi:10.1016/j.jsbmb.2006.12.097.
- Yague JG, Garcia-Segura LM, Azcoitia I. Selective transcriptional regulation of aromatase gene by vitamin D, dexamethasone, and mifepristone in human glioma cells. Endocrine. 2009;35(2):252–261. doi:10.1007/s12020-008-9134-2.
- Kaneko I, Sabir MS, Dussik CM, Whitfield GK, Karrys A, Hsieh JC, Haussler MR, Meyer MB, Pike JW, Jurutka PW. 1,25-Dihydroxyvitamin D regulates expression of the tryptophan hydroxylase 2 and leptin genes: implication for behavioral influences of vitamin D. FASEB. 2015;29(9):4023–4035.doi:10.1096/fj.14-269811.
- https://www.cdc.gov/ncbddd/autism/data.html accessed on 01/02/2017
- McCarthy MM. Estradiol and the developing brain. Physiol Rev. 2008;88(1):91–124. doi:10.1152/physrev.00010.2007.
- Crider A, Thakkar R, Ahmed AO, Pillai A. Dysregulation of estrogen receptor beta (ERβ), aromatase (CYP19A1), and ER co-activators in the middle frontal gyrus of autism spectrum disorder subjects. Mol Autism. 2014;5:46. doi:10.1186/2040-2392-5-46. PMID:25221668
- Ginde AA, Liu MC, Camargo CA Jr. Demographic differences and trends of vitamin D insufficiency in the US population, 1988–2004. Arch Int Med. 2009;169:626–632. doi:10.1001/archinternmed.2008.604.
- Yeargin-Allsopp M, Rice C, Karapurkar T, Doernberg N, Boyle C, Murphy C. Prevalence of autism in a US metropolitan area. JAMA. 2003;289:49–55. doi:10.1001/jama.289.1.49.
- Bhasin TK, Schendel D. Sociodemographic risk factors for autism in a US metropolitan area. J Autism Dev Disord. 2007;37(4):667–77. doi:10.1007/s10803-006-0194-y.
- Christensen DL, Baio J, Braun KV, Bilder D, Charles J, Constantino JN, Daniels J, Durkin MS, Fitzgerald RT, Kurzius-Spencer M, et al. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years — Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2012. MMWR Surveill Summ. 2016;65(No. SS-3) (No. SS-3):1–23. doi:10.15585/mmwr.ss6503a1
- Rohrmann S, Nelson WG, Rifai N, Brown TR, Dobs A, Kanarek JD, et al. Serum estrogen, but not testosterone, levels differ between black and white men in a nationally representative sample of Americans. J Clin Endocrinol Metab. 2007;92:2519–2525. doi:10.1210/jc.2007-0028.
- Hiroi R, Handa RJ. Estrogen receptor-β regulates human tryptophan hydroxylase-2 through an estrogen response element in the 5′ untranslated region. J Neurochem. 2013;127(4):487–495. doi:10.1111/jnc.12401.
- Rubinow DR, Schmidt PJ, Roca CA. Estrogen-serotonin interactions: implications for affective regulation. Biol Psychiatry. 1998;44(9):839–850. doi:10.1016/S0006-3223(98)00162-0.
- Di Rosa M, Malaguarnera M, Nicoletti F, Malaguarnera L. Vitamin D3: a helpful immuno-modulator. Immunol. 2011;134(2):123–139. doi:10.1111/j.1365-2567.2011.03482.x.
- von Essen MR, Kongsbak M, Schjerling P, Olgaard K, Odum N, Geisler C. Vitamin D controls T cell antigen receptor signaling and activation of human T cells. Nat. Immunol. 2010;11:344–349. doi:10.1038/ni.1851.
- Nevison CD. A comparison of temporal trends in United States autism prevalence to trends in suspected environmental factors. Environ Health. 2014;13:73. doi:10.1186/1476-069X-13-73. PMID:25189402
- https://www.cdc.gov/ncbddd/autism/documents/asdprevalencedatatable2016.pdf Accessed on 01/07/2017
- Robbins JR, Bakardjiev AI. Pathogens and the placental fortress. Curr Opin Microbiol. 2012;15:36–43. doi:10.1016/j.mib.2011.11.006.
- Atladóttir HO, Thorsen P, Østergaard L, Schendel DE, Lemcke S, Addallah M, Parner ET. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J Autism Dev Disord. 2010;40(12):1423–1430. doi:10.1007/s10803-010-1006-y.
- Musetti L, Albizzati A, Grioni A, Rossetti M, Saccani M, Musetti C. Autistic disorder associated with congenital HIV infection. European Child & Adolescent Psychiatry. 1993 2(4):221–225. doi:10.1007/BF02098581.
- Bradstreet JJ, Vogelaar E, Thyer L. Initial observations of elevated alpha-n- acetylgalactosaminidase activity associated with autism and observed reductions from GC protein—macrophage activating factor injections. Autism Insights. 2012;4:31–38. doi:10.4137/AUI.S10485
- Reddi AL, Sankaranarayanan K, Arulraj HS, Devaraj N, Devaraj H. Serum alpha-N- acetylgalactosaminidase is associated with diagnosis/prognosis of patients with squamous cell carcinoma of the uterine cervix. Cancer Lett. 2000;158(1):61–64. doi:10.1016/S0304-3835(00)00502-4.
- Rombaldi RL, Serafini EP, Mandelli J, Zimmermann E, Losquiavo KP. Transplacental transmission of human papillomavirus. Virol J. 2008;5:106. doi:10.1186/1743-422X-5-106. PMID:18817577
- Weyn C, Thomas D, Jani J, Guizani M, Donner C, Van Rysselberge M, Hans C, Bossens M, Englert Y, Fontaine V. Evidence of human papillomavirus in the placenta. 2011;203(3):341–343. doi:10.1093/infdis/jiq056
- Smith EM, Parker MA, Rubenstein LM, Haugen TH, Hamsikova E, Turek LP. Evidence for vertical transmission of HPV from mothers to infants. Infect Dis Obstet Gynecol. 2010a;2010:326369. doi:10.1155/2010/326369.
- You H, Liu Y, Agrawal N, Prasad CK, Edwards JL, Osborne AF, Korourian S, Lowery CL, Hermonat PL. Multiple human papillomavirus types replicate in 3A trophoblasts. Placenta. 2008;29:30–38. doi:10.1016/j.placenta.2007.08.005.
- Walker CK, Anderson KW, Milano KM, Ye S, Tancredi DJ, Pessah IN, Hertz-Picciotto I, Kliman HJ. Trophoblast inclusions are significantly increased in the placentas of children in families at risk for autism. Biol Psychiatry. 2013;74(3):204–211. doi:10.1016/j.biopsych.2013.03.006.
- https://www.rcog.org.uk/globalassets/documents/guidelines/gtg_27.pdf Accessed on 01/11/2017
- Demissie K, Breckenridge MB, Joseph L, Rhoads GG. Placenta previa: preponderance of male sex at birth. Am J Epidemiol. 1999;149:824–830. doi:10.1093/oxfordjournals.aje.a009898.
- Wen SW, Demissie K, Liu S, Marcoux S, Kramer MS. Placenta praevia and male sex at birth: results from a population-based study. Paediatr Perinat Epidemiol. 2000;14(4):300–304. doi:10.1046/j.1365-3016.2000.00280.x
- Ananth CV, Demissie K, Smulian JC, Vintzileos AM. Placenta previa in singleton and twin births in the United States, 1989 through 1998: a comparison of risk factor profiles and associated conditions. Am J Obstet Gynecol. 2003;188:275–281. doi:10.1067/mob.2003.10.
- Greenberg DA, Hodge SE, Sowinski J, Nicoll D. Excess of twins among affected sibling pairs with autism: implications for the etiology of autism. Am J Hum Genet. 2001;69:1062–1067. doi:10.1086/324191. PMID:11590546
- Betancur C, Leboyer M, Gillberg C. Increased rate of twins among affected sibling pairs with autism. Am J Hum Genet. 2002;70:1381–1383. doi:10.1086/340364.
- Getahun D, Oyelese Y, Salihu HM, Ananth CV. Previous cesarean delivery and risks of placenta previa and placental abruption. Obstet Gynecol. 2006;107(4):771–778. doi:10.1097/01.AOG.0000206182.63788.80. PMID:16582111
- Curran EA, Dalman C, Kearney PM, Kenny LC, Cryan JF, Dinan TG, Khashan AS. Association between obstetric mode of delivery and autism spectrum disorder: A population-based sibling design study. JAMA Psychiatry. 2015;72(9):935–942. doi:10.1001/jamapsychiatry.2015.0846.
- Ananth CV, Wilcox AJ, Savitz DA, Bowes WA Jr., Luther ER. Effect of maternal age and parity on the risk of uteroplacental bleeding disorders in pregnancy. Obstet Gynecol. 1996;88(4 Pt 1):511–516.
- Gomez LM, Ma Y, Ho C, McGrath CM, Nelson DB, Parry S. Placental infection with human papillomavirus is associated with spontaneous preterm delivery. Human reproduction. 2008;23:709–715. doi:10.1093/humrep/dem404.
- Zuo Z, Goel S, Carter JE. Association of cervical cytology and HPV DNA status during pregnancy with placental abnormalities and preterm birth. Am J Clin Pathol. 2011;136:260–265. doi:10.1309/AJCP93JMIUEKRPIW.
- Wolburg H, Paulus W. Choroid plexus: biology and pathology. Acta Neuropathol. 2010;119(1):75–88. doi:10.1007/s00401-009-0627-8.
- Liddelow SA. Development of the choroid plexus and blood-CSF barrier. Front Neurosci. 2015;9: 32. doi:10.3389/fnins.2015.00032. PMID:25784848
- Minshew NJ, Williams DL. The new neurobiology of autism. Cortex, connectivity, and neuronal organization. Arch Neurol. 2007;64:945–950. doi:10.1001/archneur.64.7.945.
- Praetorius J, Nejsum LN, Nielsen S. A SCL4A10 gene product maps selectively to the basolateral plasma membrane of choroid plexus epithelial cells. Am J Physiol Cell Physiol. 2004;286(3):C601−610. doi:10.1152/ajpcell.00240.2003.
- Sebat J, Lakshmi B, Malhotra D, Troge J, Lese-Martin C, Walsh T, Yamrom B, Yoon S, Krasnitz A, Kendall J, et al. Strong association of de novo copy number mutations with autism. Science. 2007;316(5823):445–449. doi:10.1126/science.1138659.
- Sinning A, Liebmann L, Hübner CA. Disruption of Slc4a10 augments neuronal excitability and modulates synaptic short-term plasticity. Front Cell Neurosci. 2015;9:223. doi:10.3389/fncel.2015.00223. PMID:26136660
- Gurnett CA, Veile R, Zempel J, Blackburn L, Lovett M, Bowcock A. Disruption of sodium bicarbonate transporter SLC4A10 in a patient with complex partial epilepsy and mental retardation. Arch Neurol. 2008;65(4):550–552. doi:10.1001/archneur.65.4.550.
- Chen J, Tsai V, Parker WE, Aronica E, Baybis M, Crino PB. Detection of human papillomavirus in human focal cortical dysplasia type IIB. Ann Neurol. 2012;72:881–892. doi:10.1002/ana.23795.
- Casanova MF, El-Baz AS, Kamat SS, Dombroski BA, Khalifa F, Elnakib A, Soliman A, Allison-McNutt A, Switala AE. Focal cortical dysplasias in autism spectrum disorders. Acta Neuropathol Commun. 2013;1:67. doi:10.1186/2051-5960-1-67. PMID:24252498
- Abdulkader MM, Mansour NH, Van Gompel JJ, Bosh GA, Dropcho EJ, Bonnin JM, Cohen-Gadol AA. Disseminated choroid plexus papillomas in adults: A case series and review of the literature. J Clin Neurosci. 2016;32:148–154. doi:10.1016/j.jocn.2016.04.002.
- Prasad GL, Mahapatra AK. Case series of choroid plexus papilloma in children at uncommon locations and review of the literature. Surg Neurol Int. 2015;6:151. doi:10.4103/2152-7806.166167. PMID:26500797
- Ogiwara H, Dipatri AJ Jr., Alden TD, Bowman RM, Tomita T.Choroid plexus tumors in pediatric patients. Br J Neurosurg. 2012;26(1):32–37. doi:10.3109/02688697.2011.601820.
- Horvath K, Perman JA. Autistic disorder and gastrointestinal disease. Curr Opin Pediatr. 2002;14(5):583–587. doi:10.1097/00008480-200210000-00004.
- de Jonge WJ. The Gut's Little Brain in Control of Intestinal Immunity. ISRN Gastroenterol. 2013;2013:630159. doi:10.1155/2013/630159. PMID:23691339
- Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol. 2005;57(1):67–81. Erratum in: Ann Neurol. 2005;57(2):304. doi:10.1002/ana.20315.
- Shastri A, Bonifati DM, Kishore U. Innate immunity and neuroinflammation. Mediators Inflamm. 2013 (2013):342931. doi:10.1155/2013/342931. [Epub 2013 Jun 15]. Review. PMID:23843682
- Gupta S, Ellis SE, Ashar FN, Moes A, Bader JS, Zhan J, West AB, Arking DE. Transcriptome analysis reveals dysregulation of innate immune response genes and neuronal activity-dependent genes in autism. Nat Commun. 2014;5:5748–5755. doi:10.1038/ncomms6748.
- Cannell JJ, Grant WB, Holick MF. Vitamin D and inflammation. Dermato-Endocrinol. 2015;6(1):e983401. doi:10.4161/19381980.2014.983401.
- Shen MD, Kim SH, McKinstry RC, Gu H, Hazlett HC, Nordahl CW, Emerson RW, Shaw D, Elison JT, Swanson MR, Fonov VS, Gerig G, Dager SR, Botteron KN, Paterson S, Schultz RT, Evans AC, Estes AM, Zwaigenbaum L, Styner MA, Amaral DG, Piven J; Infant Brain Imaging Study Network; Infant Brain Imaging Study Network, The Infant Brain Imaging Study (IBIS) Network is a National Institutes of Health–funded Autism Center of Excellence project and consists of a consortium of eight universities in the United States and Canada, Piven J, Hazlett HC, Chappell C, Dager S, Estes A, Shaw D, Botteron K, McKinstry R, Constantino J, Pruett J, Schultz R, Zwaigenbaum L, Elison J, Evans AC, Collins DL, Pike GB, Fonov V, Kostopoulos P, Das S, Gerig G, Styner M, Gu H. Increased extra-axial cerebrospinal fluid in high-risk infants who later develop autism. Biological Psychiatry 2017;82(3):186–193. doi:10.1016/j.biopsych.2017.02.1095. [Epub 2017 Mar 6]. PMID: 28392081
- Crawley JN, Heyer WD, LaSalle JM. Autism and cancer share risk genes, pathways, and drug targets. Trends Genet. 2016;32(3):139–146. doi:10.1016/j.tig.2016.01.001.
- Spangle JM, Munger K. The human papillomavirus type 16 E6 oncoprotein activates mTORC1 signaling and increases protein synthesis. J Virol. 2010;84:9398–9407. doi:10.1128/JVI.00974-10.
- Tilot AK, Frazier TW, Eng C. Balancing proliferation and connectivity in PTEN-associated autism spectrum disorder. Neurotherapeutics. 2015;12(3):609–619. doi:10.1007/s13311-015-0356-8.
- Lee DY. Roles of mTOR signaling in brain development. Exp Neurobiol. 2015;24(3):177–185. doi:10.5607/en.2015.24.3.177.
- Yi JJ, Berrios J, Newbern JM, Snider WD, Philpot BD, Hahn KM, Zylka MJ. An autism-linked mutation disables phosphorylation control of UBE3A. Cell. 2015;162(4):795–807. doi:10.1016/j.cell.2015.06.045.
- Brimer N, Lyons C, Vande Pol SB. Association of E6AP (UBE3A) with human papillomavirus type 11 E6 protein. Virology. 2007;358(2):303–310. doi:10.1016/j.virol.2006.08.038.
- Crespi B. Autism and cancer risk. Autism Res. 2011;4(4):302–310. doi:10.1002/aur.208.
- Chiang HL, Liu CJ, Hu YW, Chen SC, Hu LY, Shen CC, Yeh CM, Chen TJ, Gau SS. Risk of cancer in children, adolescents, and young adults with autistic disorder. J Pediatr. 2015;166(2):418–423. doi:10.1016/j.jpeds.2014.10.029.
- Rosenblatt A, de Campos Guidi HG. Human Papillomavirus: A Practical Guide for Urologists. Berlin: Springer-Verlag; 2009.
- Satterwhite CL, Torrone E, Meites E, Dunne EF, Mahajan R, Ocfemia MCB, Su J, Xu F, Weinstock H. Sexually transmitted infections among US women and Men: Prevalence and incidence estimated, 2008. Sex. Trans. Dis. 2013;40:187–193. doi:10.1097/OLQ.0b013e318286bb53.
- Vidone M, Alessandrini F, Marucci G, Farnedi A, de Biase D, Ricceri F, Calabrese C, Kurelac I, Porcelli AM, Cricca M, et al. Evidence of association of human papillomavirus with prognosis worsening in glioblastoma multiforme. Neuro Oncol. 2014;16:298–302. doi:10.1093/neuonc/not140.
- Füle T, Máthé M, Suba Z, Csapó Z, Szarvas T, Tátrai P, Paku S, Kovalszky I. The presence of human papillomavirus 16 in neural structures and vascular endothelial cells. Virology. 2006;348(2):289–96. doi:10.1016/j.virol.2005.12.043.
- Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Jiang B, Goodman MT, Sibug-Saber M, Cozen W, Liu L, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294–4301. doi:10.1200/JCO.2011.36.4596.
- Griebeler ML, Kearns AE, Ryu E, Hathcock MA, Melton LJ 3, Wermers RA. Secular trends in the incidence of primary hyperparathyroidism over five decades (1965–2010). Bone. 2015;73:1–7. doi:10.1016/j.bone.2014.12.003.
- Prietl B, Treiber G, Pieber TR, Amrein K. Vitamin D and immune function. Nutrients. 2013;5(7):2502–2521. doi:10.3390/nu5072502.
- Beard JA, Bearden A, Striker R. Vitamin D and the anti-viral state. J Clin Virol. 2011;50(3):194–200. doi:10.1016/j.jcv.2010.12.006.
- Kasiappan R, Sun Y, Lungchukiet P, Quarni W, Zhang X, Bai W. Vitamin D suppresses leptin stimulation of cancer growth through microRNA. Cancer Res. 2014;74(21):6194–6204.doi:10.1158/0008-5472.CAN-14-1702.
- Ashwood P, Kwong C, Hansen R, Hertz-Picciotto I, Croen L, Krakowiak P, Walker W, Pessah IN, Van de Water J. Brief report: plasma leptin levels are elevated in autism: association with early onset phenotype? J Autism Dev Disord. 2008;38(1):169–175. doi:10.1007/s10803-006-0353-1.
- Baker R, Dauner JG, Rodriguez AC, Williams MC, Kemp TJ, Hildesheim A, Pinto LA. Increased plasma levels of adipokines and inflammatory markers in older women with persistent HPV infection. Cytokine. 2011;52(3):282–285. doi:10.1016/j.cyto.2010.11.014
- McDonnold M, Dunn H, Hester A, Pacheco LD, Hankins GD, Saade GR, Costantine MM. High risk human papillomavirus at entry to prenatal care and risk of preeclampsia. Am J Obstet Gynecol. 2014;210(2):138.e1−5. doi:10.1016/j.ajog.2013.09.040.
- Walker CK, Krakowiak P, Baker A, Hansen RL, Ozonoff S, Hertz-Picciotto I. Preeclampsia, placental insufficiency, and autism spectrum disorder or developmental delay. JAMA Pediatr. 2015;169(2):154–162. doi:10.1001/jamapediatrics.2014.2645.
- Goldstein JM, Seidman LJ, Horton NJ, Makris N, Kennedy DN, Caviness VS Jr., Faraone SV, Tsuang MT. Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cereb Cortex. 2001;11:490–497. doi:10.1093/cercor/11.6.490.
- Gingelmaier A, Gutsche S, Mylonas I, Shabani N, Kuhn C, Kunze S, Jeschke U, Friese K. Expression of HPV, steroid receptors (ERalpha, ERbeta, PR-A and PR-B) and inhibin/activin subunits (alpha, betaA and betaB) in adenosquamous endometrial carcinoma. Anticancer Res. 2007;27:2011–2017. http://ar.iiarjournals.org/content/27/4A/2011.long
- Gilad LA, Bresler T, Gnainsky J, Smirnoff P, Schwartz B. Regulation of vitamin D receptor expression via estrogen-induced activation of the ERK 1/2 signaling pathway in colon and breast cancer cells. J Endocrinol. 2005;185:577–592. doi:10.1677/joe.1.05770.
- Gallego MI, Lazo PA. Deletion in human chromosome region 12q13-15 by integration of human papillomavirus DNA in a cervical carcinoma cell line. JBC. 1995;270:24321–24326. doi:10.1074/jbc.270.41.24321.
- Taymans SE, Pack S, Pak E, Orban Z, Barsony J, Zhuang Z, Stratakis CA. The human vitamin D receptor gene (VDR) is localized to region 12cen-ql2 by fluorescent in situ hybridization and radiation hybrid mapping: genetic and physical VDR map. J Bone Miner Res. 1999;14: 1163–1166. doi:10.1359/jbmr.1999.14.7.1163.
- Schabath MB, Villa LL, Lin HY, Fulp WJ, Akoqbe GO, Abrahamsen ME, Papenfuss MR, Lazcano-Ponce E, Salmerón J, Quiterio M, et al. Racial differences in the incidence and clearance of human papilloma virus (HPV): the HPV in men (HIM) study. Cancer Epidemiol Biomarkers Prev. 2013;22(10):1762–1770. doi:10.1158/1055-9965.EPI-13-0303.
- Hertz-Picciotto I, Croen LA, Hansen R, Jones CR, van de Water J, Pessah IN. The CHARGE study: an epidemiologic investigation of genetic and environmental factors contributing to autism. Environ Health Perspect. 2006;114(7):1119–1125. doi:10.1289/ehp.8483.
- Poletaev AB, Shenderov BA. Autism: Genetics or Epigenetics? ARC J Immunol Vaccines. 2016;1(2):1–7. https://www.arcjournals.org/pdfs/ajiv/v1-i2/1.pdf
- Robinson JL, Nations L, Suslowitz N, Cuccaro ML, Haines J, Pericak-Vance M. Prevalence rates of autism spectrum disorders among the Old Order Amish, Abstract for International Meeting for Autism Research 2010 Paper 7336. https://imfar.confex.com/imfar/2010/webprogram/Paper7336.html
- Yin J, Schaaf CP. Autism genetics – an overview. Prenat Diagn. 2017;37(1):14–30. doi:10.1002/pd.4942.
- Laplana M, Royo JL, Aluja A, López R, Heine-Sunyer D, Fibla J. Absence of substantial copy number differences in a pair of monozygotic twins discordant for features of autism spectrum disorder. Case Rep Genet. 2014;2014:516529. doi:10.1155/2014/516529. PMID:24563798
- Bourgeron T. From the genetic architecture to synaptic plasticity in autism spectrum disorder. Nat Rev Neurosci. 2015;16(9):551–563. doi:10.1038/nrn3992.
- Loke YJ, Hannan AJ, Craig JM. The Role of Epigenetic Change in Autism Spectrum Disorders. Front Neurol. 2015;6:107. doi:10.3389/fneur.2015.00107. PMID:26074864
- Ziats MN, Rennert OM. The Evolving Diagnostic and Genetic Landscapes of Autism Spectrum Disorder. Front Genet. 2016;7:65. doi:10.3389/fgene.2016.00065. PMID:27200076
- Wu YE, Parikshak NN, Belgard TG, Geschwind DH. Genome-wide, integrative analysis implicates microRNA dysregulation in autism spectrum disorder. Nat Neurosci. 2016;19(11):1463–1476. doi:10.1038/nn.4373.
- Miranda PM, Silva NN, Pitol BC, et al. Persistence or clearance of human papillomavirus infections in women in Ouro Preto, Brazil. Biomed Res Int. 2013;2013:578276. doi:10.1155/2013/578276. PMID:24298551
- Wong CC, Meaburn EL, Ronald A, Price TS, Jeffries AR, Schalkwyk LC, Plomin R, Mill J. Methylomic analysis of identical twins discordant for autism spectrum disorder and related behavioural traits. Mol Psychiatry. 2014;19(4):495–503. doi:10.1038/mp.2013.41.
- Siegel EM, Riggs BM, Delmas AL, Koch A, Hakam A, Brown KD. Quantitative DNA methylation analysis of candidate genes in cervical cancer. PLoS One. 2015;10(3):e0122495. doi:10.1371/journal.pone.0122495. PMID:25826459
- Anayannis NV, Schlecht NF, Belbin TJ. Epigenetic mechanisms of human papillomavirus-associated head and neck cancer. Arch Pathol Lab Med. 2015;139(11):1373–1378. doi:10.5858/arpa.2014-0554-RA.
- Wentzensen N, Ridder R, Klaes R, Vinokurova S, Schaefer U, Doeberitz MK. Characterization of viral-cellular fusion transcripts in a large series of HPV16 and 18 positive anogenital lesions. Oncogene. 2002;21: 419–426. doi:10.1038/sj.onc.1205104.
- Wentzensen N, Vinokurova S, von Knebel Doeberitz M. Systematic review of genomic integration sites of human papillomavirus genomes in epithelial dysplasia and invasive cancer of the female lower genital tract. Cancer Res. 2004;64(11):3878–3884. doi:10.1158/0008-5472.CAN-04-0009.
- Schmitz M, Driesch C, Jansen L, Runnebaum IB, Dürst M. Non-random integration of the HPV genome in cervical cancer. PLoS One. 2012;7(6): e39632. doi:10.1371/journal.pone.0039632. PMID:22761851
- Akagi K, Li J, Broutian TR, Padilla-Nash H, Xiao W, Jiang B, Rocco JW, Teknos TN, Kumar B, Wangsa D, et al. Genome-wide analysis of HPV integration in human cancers reveals recurrent, focal genomic instability. Genome Res. 2014;24(2):185–199. doi:10.1101/gr.164806.113.
- Smith CL, Bolton A, Nguyen G. Genomic and epigenomic instability, fragile sites, schizophrenia and autism. Curr Genomics. 2010b;11(6):447–469. doi:10.2174/138920210793176001.
- Cooper DN, Mort M, Stenson PD, Ball EV, Chuzhanova NA. Methylation- mediated deamination of 5-methylcytosine appears to give rise to mutations causing human inherited disease in CpNpG trinucleotides, as well as in CpG dinucleotides. Hum Genomics. 2010;4(6):406–410. doi:10.1186/1479-7364-4-6-406.
- Kaspersen MD, Larsen PB, Ingerslev HJ, Fedder J, Petersen GB, Bonde J, Höllsberg P. Identification of multiple HPV types on spermatozoa from human sperm donors. PLoS One. 2011;6(3):e18095. doi:10.1371/journal.pone.0018095. PMID:21479232
- Lee SM, Park JS, Norwitz ER, Koo JN, Oh IH, Park JW, Kim SM, Kim YH, Park CW, Song YS. Risk of vertical transmission of human papillomavirus throughout pregnancy: a prospective study. PLoS One. 2013;8(6):e66368. doi:10.1371/journal.pone.0066368. PMID:23785495
- An HJ, Cho NH, Lee SY, Kim IH, Lee C, Kim SJ, Mun MS, Kim SH, Jeong JK. Correlation of cervical carcinoma and precancerous lesions with human papillomavirus (HPV) genotypes detected with the HPV DNA chip microarray method. Cancer. 2003;97(7):1672–1680. doi:10.1002/cncr.11235. PMID:12655524
- Dzuba IG, Díaz EY, Allen B, Leonard YF, Lazcano Ponce EC, Shah KV, Bishai D, Lorincz A, Ferris D, Turnbull B, et al. The acceptability of self-collected samples for HPV testing vs. the Pap test as alternatives in cervical cancer screening. Journal of Women's Health & Gender-Based Medicine. 2002;11:265–274. doi:10.1089/152460902753668466.
- Venuti A, Paolini F. HPV detection methods in head and neck cancer. Head Neck Pathol. 2012;6 Suppl 1:S63−74. doi:10.1007/s12105-012-0372-5
- Abreu AL, Souza RP, Gimenes F, Consolaro ME. A review of methods for detect human papillomavirus infection. Virol J. 2012;9:262. doi:10.1186/1743-422X-9-262. PMID:23131123
- Coras R, Korn K, Bien CG, Kalbhenn T, Rössler K, Kobow K, Giedl J, Fleckenstein B, Blumcke I. No evidence for human papillomavirus infection in focal cortical dysplasia IIb. Ann Neurol. 2015;77(2):312–319. doi:10.1002/ana.24328.
- den Boon JA, Pyeon D, Wang SS, Horswill M, Schiffman M, Sherman M, Sherman M, Zuna RE, Wang Z, Hewitt SM, et al. Molecular transitions from papillomavirus infection to cervical precancer and cancer: Role of stromal estrogen receptor signaling. Proc Natl Acad Sci USA. 2015;112(25):E3255–3264. doi:10.1073/pnas.1509322112.
- Heaney RP, Garland C, Baggerly C, French C, Gorham E. Letter to Veugelers, P.J. and Ekwaru, J.P., A statistical error in the estimation of the recommended dietary allowance for vitamin D. Nutrients. 2015;7:1688–1690. doi:10.3390/nu7031688
- Holick MF: The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev Endocr Metab Disord. 2017 18(2):153–165. Review. doi:10.1007/s11154-017-9424-1.
- Kimball SM, Mirhosseini N, Holick MF. Evaluation of vitamin D3 intakes up to 15,000 international units/day and serum 25-hydroxyvitamin D concentrations up to 300 nmol/L on calcium metabolism in a community setting. Dermatoendocrinol. 2017 9(1):e1300213. eCollection 2017. doi:10.1080/19381980.2017.1300213. PMID:28458767
- Holick MF. Vitamin D and brain health: the need for vitamin D supplementation and sensible sun exposure. J Intern Med. 2015 277(1):90–93. Epub 2014 Nov 13. doi:10.1111/joim.12308.
- Crino PB. The mTOR signalling cascade: paving new roads to cure neurological disease. Nat Rev Neurol. 2016 12(7):379–392. doi:10.1038/nrneurol.2016.81.
- Sato A. mTOR, a potential target to treat autism spectrum disorder. CNS Neurol Disord Drug Targets. 2016;15(5):523–543. doi:10.2174/1871527315666160413120638.
- Coppock JD, Wieking BG, Molinolo AA, Gutkind JS, Miskimins KW, Lee JH. Improved clearance during treatment of HPV-positive head and neck cancer through mTOR inhibition. Neoplasia. 2013;15(6):620–630. doi:10.1593/neo.13432.
- Scheller NM, Pasternark B, Molgaard-Nielsen D, Svanström H, Hviid A. Quadrivalent HPV Vaccination and the Risk of Adverse Pregnancy Outcomes. N Engl J Med. 2017;376:1223–1233. doi:10.1056/NEJMoa1612296.
- Cyrus N, Blechman AB, Leboeuf M, Belyaeva EA, de Koning MN, Quint KD, Stern JJ. Effect of quadrivalent human papillomavirus vaccination on oral squamous cell papillomas. JAMA Dermatol. 2015;151(12):1359–1363. doi:10.1001/jamadermatol.2015.2805.
- Ali H, Donovan B, Wand H, Read TR, Regan DG, Grulich AE, Fairley CK, Guy RJ. Genital warts in young Australians five years into national human papillomavirus vaccination programme: national surveillance data. BMJ. 2013;346:f2032. Erratum in: BMJ. 2013;346:F2942. doi:10.1136/bmj.f2032. PMID:23599298
- Godar DE, Subramanian M, Merrill SJ. Cutaneous malignant melanoma incidences analyzed worldwide by sex, age, and skin type over personal Ultraviolet-B dose shows no role for sunburn but implies one for vitamin D3. Dermatoendocrinol. 2016;9(1):e1267077. doi:10.1080/19381980.2016.1267077. eCollection 2017. PMID: 28924456
- Merrill SJ, Subramanian M, Godar DE. Worldwide cutaneous malignant melanoma incidences analyzed by sex, age, and skin type over time (1955–2007): Is HPV infection of androgenic hair follicular melanocytes a risk factor for developing melanoma exclusively in people of European-ancestry? Dermatoendocrinol. 2016;8(1):e1215391. doi:10.1080/19381980.2016.1215391. PMID:27588159
- Godar DE, Subramanian M, Merrill SJ. Cutaneous malignant melanoma incidences analyzed worldwide by skin type over advancing age of males and females: Evidence estrogen and androgenic hair are risk factors. J Epi Res. 2017;3(10): doi:10.5430/jer.v3n1p42
- Boontanrart M, Hall SD, Spanier JA, Hayes CE, Olson JK. Vitamin D3 alters microglia immune activation by an IL-10 dependent SOCS3 mechanism. J Neuroimmunol. 2016;292:126–36. Epub 2016 Jan 27. doi:10.1016/j.jneuroim.2016.01.015.
- Godar DE, Gurov R, Merrill SJ. All Sites but Skin Cancer Incidences Analyzed Worldwide by Sex, Age, and Skin Type over Time (1955–2007), Advancing Age, and UVB Dose Reveals Important Carcinogenic Drivers. J Epi Res. 2017;3(2):65–80.
- Hossein-nezhad A, Spira A, Holick MF. Influence of vitamin D status and vitamin D3 supplementation on genome wide expression of white blood cells: a randomized double-blind clinical trial. PLoS One. 2013;8(3):e58725. Epub 2013 Mar 20. doi:10.1371/journal.pone.0058725. PMID:23527013