Abstract
The accumulation of lactate in muscle and blood during high-intensity exercise is negatively correlated with the duration exercise can be sustained. Removal of lactate is a key component of acute recovery between consecutive bouts of such exercise. Low-intensity exercise enhances recovery by accelerating lactate turnover in metabolically active tissues, largely mediated by blood flow to these tissues. Therefore, the purpose of this research was to clarify if L-citrulline, a nutritional supplement purported to promote vasodilation via enhanced nitric oxide availability, would augment the removal of blood lactate during active recovery (AR). L-citrulline ingestion will augment the rate of blood lactate concentration decrease during AR, reduce the oxygen-cost of submaximal exercise, and increase time-to-exhaustion and peak oxygen uptake (V̇O2peak) during a test of maximal aerobic power. Healthy university students (five males & five females) participated in this double-blind, randomized, placebo-controlled study. Participants exercised on a cycle ergometer at submaximal steady-state intensities followed by progressively increasing intensity to exhaustion, 10 min of AR, and then supramaximal intensity exercise to exhaustion. Oxygen uptake was measured throughout the trial and blood lactate was sampled repeatedly during AR. The protocol elicited very high peak blood lactate concentrations after exercise (11.3 + 1.3 mmol/L). L-citrulline supplementation did not significantly alter blood lactate kinetics during AR, the oxygen cost of exercise, V̇O2peak, or time-to-exhaustion. Despite a strong theoretical basis by which L-citrulline could augment lactate removal from the blood, L-citrulline supplementation showed no effect as an exercise-recovery supplement.
Keywords:
- active recovery
- aerobic power
- amino acid
- bicycle ergometry
- blood lactate
- blood-flow
- cycling
- dietary L-citrulline supplementation
- ergogenic aids
- exercise capacity
- exercise metabolism
- exercise physiology
- exercise
- exhaustive exercise
- high-intensity exercise
- human
- lactate kinetics
- lactate
- L-citrulline supplementation
- L-citrulline
- metabolism
- mitochondrial function
- nitric oxide
- nutrition
- oral L-citrulline
- oxygen consumption
- oxygen cost of exercise
- oxygen cost
- oxygen uptake kinetics
- performance
- physiology
- recovery
- sport science
- sports nutrition
- steady-state exercise
- submaximal exercise
- supplementation
- supramaximal exercise
- total nitrate
- vascular function
- vasodilation
- VO2peak
- young adults
Background
Supramaximal-intensity exercise is defined for the purposes of this report as an exercise intensity with a metabolic demand that exceeds an individual’s ability to meet the energy requirement via aerobic metabolism, i.e. the demand exceeds an individual’s maximal aerobic power. The dependency on anaerobic energy metabolism for such exercise is associated with a well-established accumulation of high concentrations of lactate in muscle and blood. However, commencing high intensity exercise with preexisting high levels of lactate in blood has been reported to negatively affect performance (Klausen et al. Citation1972; Greenwood et al. Citation2008). Therefore, an acceleration of lactate removal from blood or muscle is considered a key component of acute recovery, particularly between bouts of supramaximal intensity exercise. Active recovery after high-intensity exercise, involving low-intensity exercise, enhances recovery by accelerating lactate turnover via consumption in metabolically active tissues (Menzies et al. Citation2010). This process is mediated largely by an increased metabolism and removal of lactate facilitated by blood flow to and from lactate-containing tissues (Gladden Citation2004).
L-citrulline is a non-essential amino acid that is commercially available as a nutritional supplement and is frequently promoted as a vasodilatory ergogenic aid. It is found in nature (Cutrufello et al. Citation2015; Figueroa et al. Citation2017) and synthesized within the body through the urea cycle and the citrulline-nitric-oxide cycle (Barbul Citation1986; Alderton et al. Citation2001; Schwedhelm et al. Citation2008). Following a two-step enzymatic reaction, L-citrulline is converted into L-arginine (Barbul Citation1986; Alderton et al. Citation2001; Flam et al. Citation2007), which, in turn, is converted back into L-citrulline while also producing nitric oxide (NO), a small, short-lived molecule involved in vasodilation (Moncada and Higgs Citation1993; Jobgen et al. Citation2006; Flam et al. Citation2007; Schwedhelm et al. Citation2008). Resynthesis of L-citrulline enables cyclic generation of nitric oxide from L-citrulline to occur and is an essential process for maintaining nitric oxide bioavailability within the body (Geller and Billiar Citation1998; Alderton et al. Citation2001; Solomonson et al. Citation2003; Flam et al. Citation2007). Thus, the citrulline-nitric-oxide cycle is a critical process for the maintenance of NO bioavailability, and thus, vasodilatory function in the body.
When comparing supplementation with L-citrulline and L-arginine, L-citrulline supplementation is reported to increase the concentration of L-arginine and nitric oxide metabolites in the blood more so than supplementation with L-arginine (Morita et al. Citation2014; Figueroa et al. Citation2017; Gonzales et al. Citation2017), and the difference has been attributed to L-arginine being subject to first-pass metabolism while L-citrulline is not (Schwedhelm et al. Citation2008; CitationFigueroa et al. 2017; Gonzales et al. Citation2017). Both acute (Raghavan and Dikshit Citation2001; Sureda et al. Citation2009; Mori et al. Citation2015; Le Roux-Mallouf et al. Citation2017) and prolonged supplementation (i.e. 7+ days) (Schwedhelm et al. Citation2008; Alsop and Hauton Citation2016; Gonzales et al. Citation2017; Safi et al. Citation2017) with L-citrulline have been previously reported to enhance vasodilation by facilitating production of nitric oxide.
Lactate concentration is associated with fatigue during acute high intensity exercise and its rate of removal from the muscle and blood influences the onset of fatigue during exercise and the rate of recovery afterwards (Klausen et al. Citation1972; Gladden Citation2000; Citation2004). This process is influenced by the rate at which lactate appears in blood and is transported to tissues capable of metabolizing it, a process which can be augmented by increasing blood flow (Madias Citation1986; Gladden Citation2000; Citation2004) both from the site of lactate production and to tissues where it is metabolized. It stands to reason that augmentation of vasodilation, and thus blood flow, should increase the rate at which lactate is transported to metabolically active tissues and its subsequent removal from the blood plasma.
In light of the reported vasodilatory effects of L-citrulline supplementation, the purpose of this study was to investigate the effects of L-citrulline on blood lactate kinetics during active recovery following exhaustive exercise and performance in a subsequent bout at a supramaximal intensity in healthy young adults. We hypothesized that five days of supplementation with exogenous L-citrulline would significantly increase the rate at which lactate is cleared from the blood during active recovery following supramaximal-intensity exercise. Additionally, we hypothesized that five days of supplementation with exogenous L-citrulline would significantly lower the oxygen-cost of steady-state exercise, and significantly increase time to exhaustion during a supramaximal bout of exercise following active recovery.
Methods
Study design
This study was a randomized, double-blind, placebo-controlled, crossover design that had participants engage in the same exercise protocol on three separate occasions to assess the effects of oral L-citrulline supplementation on: 1. blood lactate kinetics during active recovery following exhaustive exercise to fatigue (EXH), and 2. performance measures (i.e. time to exhaustion) on a subsequent supramaximal bout (SUP) of exercise immediately following a period of active recovery. Participants performed a control/familiarization (CON) trial first, in which the exercise protocol was completed with no intervention. The highest power output that was achieved during EXH of the CON trial determined the end point power outputs used for the remaining two trials. The second and third trials were intervention trials, in which participants were randomized to receive capsules containing either 100 mg/kg of L-citrulline (CIT) or 3 g of glucose (placebo; PLA) daily for five consecutive days, including the day of the trial. We based this protocol on the previously mentioned findings that both acute (Raghavan and Dikshit Citation2001; Sureda et al. Citation2009; Mori et al. Citation2015; Le Roux-Mallouf et al. Citation2017) and prolonged supplementation (i.e. 7+ days) (Schwedhelm et al. Citation2008; Alsop and Hauton Citation2016; Gonzales et al. Citation2017; Safi et al. Citation2017) with L-citrulline have been previously reported to enhance vasodilation by facilitating production of nitric oxide. Participants were contacted each day of their supplement dosing schedule to ensure protocol compliance. Following each trial, there was a minimum 2-day washout period, which allowed for 7 full days between testing days.
Subjects
A sample size requirement of 10 participants was determined from previous research evaluating recovery conditions on blood lactate removal kinetics (Mota et al. Citation2017). This study found that active recovery hastened lactate clearance by a difference of 0.25 and within-subject SD of 0.17 mmol/L/min; we assumed that if supplementation with L-citrulline affected lactate removal kinetics, it would do so to a comparable magnitude. Therefore, we conducted a sample size calculation using online software (Schoenfeld Citation2010) with a two-sided alpha of .05 and power of 0.8 and with the delta and variance stated above. Healthy, moderately active men and women (n = 10; ♀️= 5) ages 18-25 were recruited to participate in the study ( – physical characteristics). Participant exclusion criteria included current use of any nutritional ergogenic aids (except protein and carbohydrate supplements). Participants were asked to maintain their regular diet throughout the duration of the study, and to abstain from caffeine consumption or exercise on trial days. All participants completed all trials without any complications. It was verbally confirmed that participants were unaware as to which supplement (CIT or PLA) they were ingesting for the trials.
Table 1. Mean values for participant characteristics.
Data collection
An Excalibur Sport (Lode B.V., Groninge, The Netherlands) electronically-braked cycle ergometer was used for all exercise. A portable metabolic cart, Metamax 3B (Cortex, Germany), was used to measure rate of oxygen uptake (VO2) throughout exercise. Blood lactate concentration was assayed in whole blood obtained from a finger prick using a Lactate Scout Plus (EKF, United Kingdom).
Participants followed an exercise protocol involving steady-state cycling at 75 W for 5 min and then 150 W for 5 min, followed by step increases in power output by 25 W every minute, until exhaustion. Each participant was instructed to maintain a cadence of 60-80 rpm throughout the entire exercise protocol. When the participant reached exhaustion (failure to maintain a cadence of 60 rpm) or reached the highest power output achieved during the control trial, the resistance was lowered and participants immediately began active recovery (AR), which consisted of cycling at 100 W at 75 rpm for 10 min. Following AR, participants immediately performed the supramaximal portion of the protocol (SUP), whereby the resistance was increased to the highest power output that was completed in a 60 s period during the exercise ramp protocol (EXH) and the participants were asked to maintain 60-80 rpm for as long as possible. Time to exhaustion in this study was defined as the time when participants could no longer maintain 60 rpm.
Blood was sampled from a finger-tip puncture and assayed for whole blood lactate concentration immediately following EXH and every 2 min during the 10 min of AR (t = 0, 2, 4, 6, 8, 10).
Respiratory gas exchange was monitored throughout the exercise protocol to determine oxygen consumption at submaximal − 75 W and 150 W (V75, V150; respectively) - and maximal (i.e. VO2peak) exercise intensities.
Results
Participants
Ten participants (♀️= 5) enrolled to participate in this study. All ten participants completed all three trials (CON, PLA, CIT). Participant characteristics are displayed in . There were no significant changes in weight, systolic, diastolic or mean arterial blood pressure (MAP = 1/3(SBP – DBP) + DBP) across all three trials ().
Oxygen cost
The average oxygen cost of each exercise intensity was determined for each participant by averaging the breath-by-breath oxygen consumption measured during the final 30 s of exercise at 75 W and at 150 W steady-state exercise phases, respectively. The mean values for each trial are displayed in and . There were no differences between trials for the oxygen cost of exercise at either 75 or 150 W .
Figure 1. Oxygen cost of exercise for all three trials (control, placebo, and L-citrulline). Data represents mean values and standard deviation for all participants (n = 10) during each trial. Statistical significance is indicated as *p < 0.05.
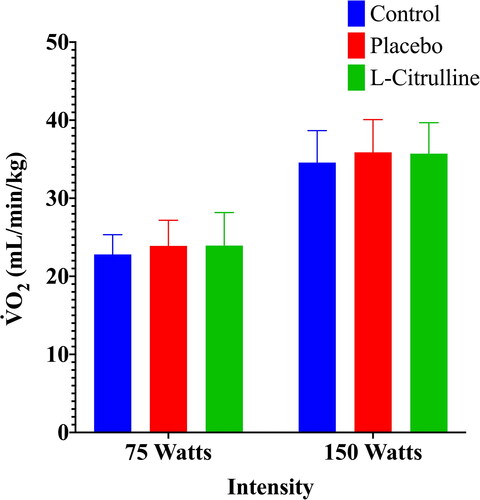
Table 2. Mean values for measured variables.
Aerobic power
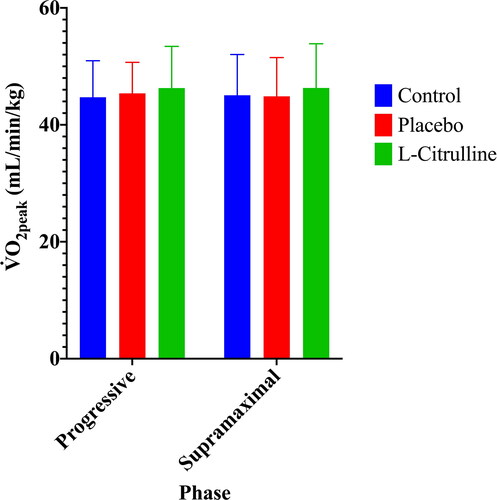
VO2peak was determined as the highest achieved rate of oxygen consumption prior to exhaustion during the EXH and SUP phases of exercise. VO2peak There were no significant differences between any trials for VO2peak during either the Progressive Phase or the Supramaximal Phase of exercise ( and ).
Lactate kinetics
Lactate removal kinetics were determined by sampling blood lactate concentration from a finger six times during the AR phase of exercise (minutes 0, 2, 4, 6, 8, 10). Mean trial values for blood lactate concentration were graphed and analyzed with a linear regression equation, providing a line of best fit and a slope value. The resulting slope values for each trial were statistically compared. Mean blood lactate concentration values are in and . Mean blood lactate concentration values with associated line of best fit are displayed in . The slope values for mean blood lactate concentration values are displayed in . The were no significant differences found between any of the trials for each of blood-lactate sample times, or the lactate slopes from each trial.
Figure 3. Blood lactate concentration ([lactate]bl.) during active recovery for all three trials (control, placebo, and L-citrulline). Data represents mean values and standard deviation for all participants (n = 10) during each trial. Statistical significance is indicated as *p < 0.05.
![Figure 3. Blood lactate concentration ([lactate]bl.) during active recovery for all three trials (control, placebo, and L-citrulline). Data represents mean values and standard deviation for all participants (n = 10) during each trial. Statistical significance is indicated as *p < 0.05.](/cms/asset/5fa3e264-0d9c-41fd-b311-1a0524d4c5a7/ijds_a_1926392_f0003_c.jpg)
Figure 4. Blood lactate concentration ([lactate]bl.) during active recovery for all three trials (control, placebo, and L-citrulline) with line of best fit. Data represents mean values for all participants (n = 10) during each trial with associated line of best fit. Statistical significance is indicated as *p < 0.05.
![Figure 4. Blood lactate concentration ([lactate]bl.) during active recovery for all three trials (control, placebo, and L-citrulline) with line of best fit. Data represents mean values for all participants (n = 10) during each trial with associated line of best fit. Statistical significance is indicated as *p < 0.05.](/cms/asset/f5421f5f-893c-47fd-914b-f55837df209d/ijds_a_1926392_f0004_c.jpg)
Table 3. Mean values for blood lactate concentration during the active recovery phase of exercise.
Time to exhaustion during supramaximal exercise
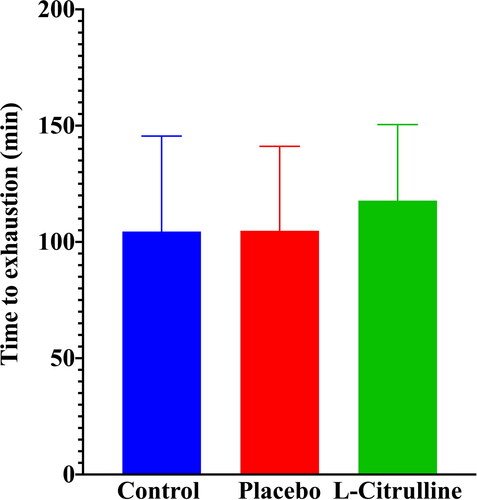
Time to exhaustion during supramaximal-intensity exercise was determined during the SUP phase of the exercise protocol. Time to exhaustion was defined as the duration from the start of the SUP phase of exercise until the point at which the participant could no longer maintain pedaling cadence above 60 rpm. Mean data for time to exhaustion are displayed in and . There were no significant between-trial differences.
Discussion
The purpose of this study was to assess the efficacy of nutritional supplementation with L-citrulline as an ergogenic aid that would enhance supramaximal intensity exercise performance and the recovery after exhaustive exercise. We hypothesized that five days of supplementation with L-citrulline would increase the rate at which lactate disappears from the blood following exhaustive exercise and during active recovery. Based on the results of this study, it can be concluded that five days of supplementation with L-citrulline does not impact that rate at which lactate is cleared from the blood during active recovery. While prior research indicates that supplementation with L-citrulline augments vasodilatory responses in the body (Gonzales et al. Citation2017; Safi et al. Citation2017) and other research suggests that augmenting vasodilation facilitates greater lactate removal from the blood (Gladden Citation2000; Citation2004), the results of the current investigation do not support this relationship. Research up to this point on the effects of L-citrulline supplementation and exercise-induced blood lactate are mixed. Research utilizing L-citrulline supplementation over a 4-week athletic training period reported a reduction in blood lactate concentration at the end of exercise but not during recovery (Kiyici et al. Citation2017). Despite the reduction in blood lactate concentration following exercise, this research supports our conclusion that L-citrulline supplementation is insufficient to improve lactate removal kinetics during recovery. Furthermore, a recent meta-analysis of research (Rhim et al. Citation2020) reported that an acute dose of L-citrulline does not significantly affect blood lactate concentration following endurance-based exercise, further supporting our conclusion.
Similarly, previous research reports that dietary supplementation with nitrate and L-arginine decreases the oxygen cost of submaximal exercise (Larsen et al. Citation2007; Bailey et al. Citation2009; Citation2010; Vanhatalo et al. Citation2010; Jones Citation2014). It was therefore hypothesized in the current investigation that supplementation with L-citrulline would lower the oxygen cost of submaximal exercise, due to its ability to augment L-arginine and NOx (nitric oxide, nitrite, nitrate) concentrations in the blood (Moncada and Higgs Citation1993; Greenwood et al. Citation2008; Menzies et al. Citation2010). However, the current study did not yield supporting results, as there were no significant effects of L-citrulline supplementation on the oxygen cost of submaximal exercise.
While the mechanisms for L-citrulline’s actions are relatively clear and well supported, the current study’s results raise questions about the efficacy of L-citrulline for augmenting the performance of cellular systems which may likely already be maximally engaged during exercise. For example, when considering the rate of lactate disappearance from the blood (, and ) it is important to recognize that lactate removal from the blood is dependent on its metabolism by metabolically active tissues throughout the body, capacities and limitations to transport, and the degree of blood flow which facilitates the transport of lactate to those metabolically active tissues. It is clear that blood vessel radius is a key determinant of peripheral blood flow, and thus a primary determinant of the degree of lactate removal from the blood should be vasodilatory control of blood vessel radius. Therefore, from a theoretical perspective, there is certainly a basis for L-citrulline or any supplement that augments in vivo nitric oxide concentration, to increase the rate of lactate disappearance from the blood. However, the results of this study suggest that the previously documented vasodilatory effects of L-citrulline ingestion may not extend to being relevant to the magnitude of physiological responses and reserves already activated and called upon in response to supramaximal intensity exercise. The results of this study suggest that there may be a physiological limit to the potential for L-citrulline supplementation to ameliorate the rate of lactate removal, the oxygen cost of exercise, and VO2peak.
Limitations
We acknowledge that a lack of measurement of NO or NOx is a study limitation. Our study design and hypothesis were based upon the previously mentioned findings that both acute (Raghavan and Dikshit Citation2001; Sureda et al. Citation2009; Mori et al. Citation2015; Le Roux-Mallouf et al. Citation2017) and prolonged supplementation (i.e. 7+ days) (Schwedhelm et al. Citation2008; Alsop and Hauton Citation2016; Gonzales et al. Citation2017; Safi et al. Citation2017) with L-citrulline have been previously reported to enhance vasodilation by facilitating production of nitric oxide. Thus, while the measurement of NOx would have provided information as to the magnitude of the effect provided by L-citrulline, we are very confident that NOx levels were sufficiently elevated and we do not believe that the lack of this measurement undermines the validity of the study. Nevertheless, whether or not NO was elevated sufficiently by our protocol, our conclusion that 5 days of supplementation with L-citrulline does not enhance blood lactate clearance remains sound.
Conclusion
Five days of dietary supplementation with L-citrulline does not affect the rate of lactate disappearance from the blood following exhaustive exercise during active recovery. Furthermore, five days of supplementation with L-citrulline does not affect VO2peak, the oxygen cost of exercise, and time-to-exhaustion during supramaximal intensity exercise. The findings of this study suggest that L-citrulline is likely not an effective ergogenic aid for the acceleration of recovery after exhaustive exercise in young, healthy adults.
Ethics approval and consent to participate
The study design and procedures were approved by the University of Toronto Research Ethics Board. All participants completed an informed consent form prior to their participation in the study.
Acknowledgements
The authors acknowledge the support of NOW Canada for supplying the L-citrulline for this study.
Declaration of interest
The authors declare that they have no competing interests.
Data availability statement
All data pertaining to the results and conclusions of this study are found within the article. The corresponding data set is available upon reasonable request.
Additional information
Funding
Notes on contributors
Benjamin Divito
B. Divito is a Bachelor of Kinesiology graduate of the Faculty of Kinesiology & Physical Education at the University of Toronto. This research was conducted in partial fulfillment of degree requirements.
Mackenzie McLaughlin
M. McLaughlin is a PhD Candidate in the Faculty of Kinesiology & Physical Education at the University of Toronto. His research interests include exercise and drug interactions, and nutritional ergogenic aids.
Ira Jacobs
I. Jacobs is a professor and Dean of the Faculty of Kinesiology & Physical Education at the University of Toronto. His research interests include nutritional and pharmacological interventions affecting exercise performance, and human thermal physiology.
References
- Alderton WK, Cooper CE, Knowles RG. 2001. Nitric oxide synthases: structure, function and inhibition. Biochem J 357(3):593–615. http://www.ncbi.nlm.nih.gov/pubmed/11463332. doi:10.1042/bj3570593.
- Alsop P, Hauton D. 2016. Oral nitrate and citrulline decrease blood pressure and increase vascular conductance in young adults: a potential therapy for heart failure. Eur J Appl Physiol. 116(9):1651–1661. doi:10.1007/s00421-016-3418-7.
- Bailey SJ, Winyard P, Vanhatalo A, Blackwell JR, Dimenna FJ, Wilkerson DP, Tarr J, Benjamin N, Jones AM. 2009. Dietary nitrate supplementation reduces the O2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. J Appl Physiol 107(4):1144–1155.
- Bailey SJ, Winyard PG, Vanhatalo A, Blackwell JR, DiMenna FJ, Wilkerson DP, Jones AM. 2010. Acute L -arginine supplementation reduces the O 2 cost of moderate-intensity exercise and enhances high-intensity exercise tolerance. J Appl Physiol. 109(5):1394–1403. http://jap.physiology.org/content/jap/109/5/1394.full.pdf. doi:10.1152/japplphysiol.00503.2010.
- Barbul A. 1986. Arginine: biochemistry, physiology, and therapeutic implications. JPEN J Parenter Enteral Nutr. 10(2):227–238. doi:10.1177/0148607186010002227.
- Cutrufello PT, Gadomski SJ, Zavorsky GS. 2015. The effect of l-citrulline and watermelon juice supplementation on anaerobic and aerobic exercise performance. J Sports Sci. 33(14):1459–1466. doi:10.1080/02640414.2014.990495.
- Figueroa A, Wong A, Jaime SJ, Gonzales JU. 2017. Influence of L-citrulline and watermelon supplementation on vascular function and exercise performance. Curr Opin Clin Nutr Metab Care. 20(1):92–98. doi:10.1097/MCO.0000000000000340.
- Flam BR, Eichler DC, Solomonson LP. 2007. Endothelial nitric oxide production is tightly coupled to the citrulline-NO cycle. Nitric Oxide Biol Chem. 17(3–4):115–121. doi:10.1016/j.niox.2007.07.001.
- Geller D. a, Billiar TR. 1998. Molecular biology of nitric oxide synthases. Cancer Metastasis Rev. 17(1):7–23. doi:10.1023/A:1005940202801.
- Gladden LB. 2000. Muscle as a consumer of lactate. Med Sci Sports Exerc. 32(4):764–771. doi:10.1097/00005768-200004000-00008.
- Gladden LB. 2004. Lactate metabolism: A new paradigm for the third millennium. J Physiol. 558(1):5–30. doi:10.1113/jphysiol.2003.058701.
- Gonzales JU, Raymond A, Ashley J, Kim Y. 2017. Does l-citrulline supplementation improve exercise blood flow in older adults? Exp Physiol. 102(12):1661–1671. doi:10.1113/EP086587.
- Greenwood JD, Moses GE, Bernardino FM, Gaesser GA, Weltman A. 2008. Intensity of exercise recovery, blood lactate disappearance, and subsequent swimming performance. J Sports Sci. 26(1):29–34. doi:10.1080/02640410701287263.
- Jobgen WS, Fried SK, Fu WJ, Meininger CJ, Wu G. 2006. Regulatory role for the arginine-nitric oxide pathway in metabolism of energy substrates. J Nutr Biochem. 17(9):571–588. doi:10.1016/j.jnutbio.2005.12.001.
- Jones AM. 2014. Dietary nitrate supplementation and exercise performance. Sport Med. 44(SUPPL.1):S35–S45.
- Kiyici F, Eroğlu H, Kishali NF, Burmaoglu G. 2017. The effect of citrulline/malate on blood lactate levels in intensive exercise. Biochem Genet. 55(5–6):387–394. doi:10.1007/s10528-017-9807-8.
- Klausen K, Knuttgen HG, Forster HV. 1972. Effect of pre-existing high blood lactate concentration on maximal exercise performance. Scand J Clin Lab Invest. 30(4):415–419. doi:10.3109/00365517209080279.
- Larsen FJ, Weitzberg E, Lundberg JO, Ekblom B. 2007. Effects of dietary nitrate on oxygen cost during exercise. Acta Physiol. 191(1):59–66. doi:10.1111/j.1748-1716.2007.01713.x.
- Le Roux-Mallouf T, Vibert F, Doutreleau S, Verges S. 2017. Effect of acute nitrate and citrulline supplementation on muscle microvascular response to ischemia–reperfusion in healthy humans. Appl Physiol Nutr Metab. 42(9):901–908. http://www.nrcresearchpress.com/doi/10.1139/apnm-2017-0081.
- Madias NE. 1986. Lactic acidosis. Kidney Int. 29(3):752–774. doi:10.1038/ki.1986.62.
- Menzies P, Menzies C, McIntyre L, Paterson P, Wilson J, Kemi OJ. 2010. Blood lactate clearance during active recovery after an intense running bout depends on the intensity of the active recovery. J Sports Sci. 28(9):975–982. doi:10.1080/02640414.2010.481721.
- Moncada S, Higgs A. 1993. The L-arginine-nitric oxide pathway. N Engl J Med. 329(7):2002–2012.
- Mori A, Morita M, Morishita K, Sakamoto K, Nakahara T, Ishii K. 2015. L-Citrulline dilates rat retinal arterioles via nitric oxide- and prostaglandin-dependent pathways in vivo. J Pharmacol Sci 127(4):419–423.
- Morita M, Hayashi T, Ochiai M, Maeda M, Yamaguchi T, Ina K, et al. 2014. Oral supplementation with a combination of L-citrulline and L-arginine rapidly increases plasma L-arginine concentration and enhances NO bioavailability. Biochem Biophys Res Commun. 454(1):53–57. doi:10.1016/j.bbrc.2014.10.029.
- Mota MR, Dantas RAE, Oliveira-Silva I, Sales MM, Sotero RDC, Venâncio PEM, Teixeira Júnior J, Chaves SN, de Lima FD. 2017. Effect of self-paced active recovery and passive recovery on blood lactate removal following a 200 m freestyle swimming trial. Open Access J Sports Med. 8:155–160. doi:10.2147/OAJSM.S127948.
- Raghavan SAV, Dikshit M. 2001. L-Citrulline mediated relaxation in the control and lipopolysaccharide-treated rat aortic rings. Eur J Pharmacol. 431(1):61–69. doi:10.1016/S0014-2999(01)01407-8.
- Rhim HC, Kim SJ, Park J, Jang K-M. 2020. Effect of citrulline on post-exercise rating of perceived exertion, muscle soreness, and blood lactate levels: a systematic review and meta-analysis. J Sport Health Sci. 9(6):553–561. doi:10.1016/j.jshs.2020.02.003.
- Safi M, Mahjoob MP, Nateghi S, Khaheshi I, Akbarzadeh MA, Naderian M. 2017. The Assessment of short-term effect of L-Citrulline on endothelial function via FMD to NMD ratio in known CAD patients: A randomized, cross-over clinical trial (Clinical trial number: NCT02638727). Rom J Intern Med. 55(1):23–27.
- Schoenfeld D. Statistical considerations for a cross-over study where the outcome is a measurement. 2010. http://hedwig.mgh.harvard.edu/sample_size/js/js_crossover_quant.html.
- Schwedhelm E, Maas R, Freese R, Jung D, Lukacs Z, Jambrecina A, Spickler W, Schulze F, Böger RH. 2008. Pharmacokinetic and pharmacodynamic properties of oral L-citrulline and L-arginine: Impact on nitric oxide metabolism. Br J Clin Pharmacol. 65(1):51–59. doi:10.1111/j.1365-2125.2007.02990.x.
- Solomonson LP, Flam BR, Pendleton LC, Goodwin BL, Eichler DC. 2003. The caveolar nitric oxide synthase/arginine regeneration system for NO production in endothelial cells. J Exp Biol. 206(Pt 12):2083–2087. doi:10.1242/jeb.00361.
- Sureda A, Cordova A, Ferrer MD, Tauler P, Perez G, Tur JA, Pons A. 2009. Effects of L-citrulline oral supplementation on polymorphonuclear neutrophils oxidative burst and nitric oxide production after exercise. Free Radic Res 43(9):828–835. https://pubmed.ncbi.nlm.nih.gov/19585317/.
- Vanhatalo A, Bailey SJ, Blackwell JR, DiMenna FJ, Pavey TG, Wilkerson DP, Benjamin N, Winyard PG, Jones AM. 2010. Acute and chronic effects of dietary nitrate supplementation on blood pressure and the physiological responses to moderate-intensity and incremental exercise. Am J Physiol Regul Integr Comp Physiol. 299(4):R1121–R1131. http://www.ncbi.nlm.nih.gov/pubmed/20702806. doi:10.1152/ajpregu.00206.2010.