?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
This systematic review and meta-analysis of randomized controlled trials examined whether dietary nitrate supplementation attenuates exercise-induced muscle damage (EIMD) and is reported according to the PRISMA guidelines. Medline and SPORTDiscus databases were searched from inception to June 2020. Inclusion criteria were studies in adult humans consuming inorganic nitrate before and after exercise and that measured markers implicated in the etiology of EIMD (muscle function, muscle soreness, inflammation, myocellular protein efflux, oxidative stress, range of motion) <168 h post. The Cochrane Collaboration risk of bias two tool was used to critically appraise the studies; forest plots were generated with random-effects models and standardized mean differences (SMD). Nine studies were included in the systematic review and six in the meta-analysis. All studies were rated to have some concerns for risk of bias. All trials in the meta-analysis provided nitrate as beetroot juice, which accelerated isometric strength recovery 72 h post-exercise (SMD: 0.54, p = 0.01) and countermovement jump performance 24–72 h post-exercise (SMD range: 0.75-1.32, p < 0.03). Pressure pain threshold was greater with beetroot juice 48 (SMD: 0.58, p = 0.03) and 72 h post-exercise (SMD: 0.61, p = 0.02). Beetroot juice had no effect on markers of oxidative stress and creatine kinase (p > 0.05), but c-reactive protein was higher vs. placebo at 48 h post-exercise (SMD: 0.55, p = 0.03). These findings suggest that nitrate-rich beetroot juice may attenuate some markers of EIMD, but more large-scale controlled trials in elite athletes are needed.
Introduction
Exercise-induced muscle damage (EIMD) is defined as a set of potentially deleterious symptoms that develop in the hours and days following exercise (Hyldahl and Hubal Citation2014; Owens et al. Citation2019). Common symptoms include reduced force generating capacity, increased muscle soreness, swelling, and a reduced range of motion (Howatson and Van Someren Citation2008; Paulsen et al. Citation2012; Hyldahl and Hubal Citation2014). These symptoms can be evoked by resistance training (Burt et al. Citation2014), prolonged running and cycling (Howatson et al. Citation2010; Bell et al. Citation2016), intermittent, high-intensity exercise (Leeder et al. Citation2015) and eccentric exercise (Paulsen et al. Citation2005), and can last for several days, depending on age, sex, training status, and genetics (Paulsen et al. Citation2012; Hyldahl and Hubal Citation2014).
While the etiology of EIMD is not completely understood, the current consensus is that it stems from physical damage to myofibrils and disruption of excitation-contraction coupling (Hyldahl and Hubal Citation2014). Subsequently, these perturbations evoke proteolysis and inflammation that can exacerbate the initial injury and delay recovery (Warren et al. Citation2002; Paulsen et al. Citation2012; Hyldahl and Hubal Citation2014). Strategies to attenuate EIMD typically attempt to mitigate the inflammatory response and/or oxidative stress (defined as a state where the production of reactive oxygen species (ROS) outweighs their clearance and macromolecule damage ensues) (Betteridge Citation2000; Howatson and Van Someren Citation2008; Owens et al. Citation2019). In this regard, numerous interventions have been administered including cold water immersion, compression garments, non-steroidal-anti-inflammatory drugs, and nutritional supplements with anti-inflammatory or antioxidant effects (e.g., vitamins C and E or (poly)phenols) (Howatson and Van Someren Citation2008; Owens et al. Citation2019). Such interventions may be beneficial in situations where rapid recovery is essential, such as during multi-day events (e.g., fixture congestion in team-sports, stage races, heptathlon/decathlon, or competitions comprising heats, semifinals, and finals).
Supplementation with nitrate, a precursor for the multifunctional signaling molecule nitric oxide (NO) and other reactive nitrogen intermediates, has been shown to reduce inflammation (Jädert et al. Citation2012; Raubenheimer et al. Citation2019; Peleli et al. Citation2020), oxidative stress (Carlström et al. Citation2011; Clifford et al. Citation2015), and stimulate muscle-resident satellite cells (Anderson et al. Citation2018). In addition, nitrate is most often administered in the form of beetroot juice, which also contains various bioactive compounds known to have antioxidant and anti-inflammatory effects, such as betalains and (poly)phenols (Clifford et al. Citation2015; Esatbeyoglu et al. Citation2015; Clifford, Constantinou et al. Citation2017). Such effects suggest nitrate or nitraterich vegetable supplements with other bioactive compounds like beetroot juice, hold promise as recovery aids following EIMD (Clifford et al. Citation2015). To date, human studies examining the effects of nitrate-rich supplements on markers of EIMD such as muscle soreness and muscle function have produced mixed findings. Two studies reported that nitrate-rich beetroot juice attenuated muscle pain and expedited the recovery of countermovement (CMJ) performance in the 72 h following exercise (Clifford, Bell et al. Citation2016; Clifford, Berntzen et al. Citation2016), but others found no such effects (Clifford, Allerton et al. Citation2017; Larsen et al. Citation2019), or only benefits on specific symptoms such as muscle soreness (Clifford, Howatson et al. Citation2017). Findings from studies exploring the effects of nitrate or nitrate-rich beetroot juice on the underlying mechanisms of EIMD (e.g., inflammation) have produced similarly mixed effects (Clifford, Allerton et al. Citation2017; Clifford et al. Citation2018; Menezes et al. Citation2019). The current discrepancy in findings could be due to the wide heterogeneity in study designs (e.g., type of exercise and participant cohort studied), nitrate source administered (e.g., with or without bioactive compounds in beetroot juice), and low sample sizes employed. Irrespective of the precise reasons, consensus on the effects of nitrate as a recovery aid following EIMD is lacking.
Meta-analysis of the available research could help resolve the ambiguity surrounding the effects of dietary nitrate on EIMD and identify factors that could account for the divergent results reported. Results could then be used to help inform athletes, coaches, and practitioners of the factors that impact the efficacy of BR ingestion to improve recovery following EIMD and the EIMD markers that are more and less likely to be positively impacted by BR ingestion. To this end, the aim of the present systematic review and meta-analysis was to examine the effects of dietary nitrate supplementation on common markers of EIMD, including muscle function, muscle soreness, creatine kinase, inflammation, oxidative stress, and range of motion. A secondary aim was to determine if these outcomes were influenced by age, sex, type of exercise or source of nitrate.
Methods
The study protocol for this review was preregistered on the International Prospective Register of Systematic Reviews (PROSPERO) database (CRD42020201187) and is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Moher et al. Citation2009). We did not deviate from our pre-registered protocol.
Search strategy
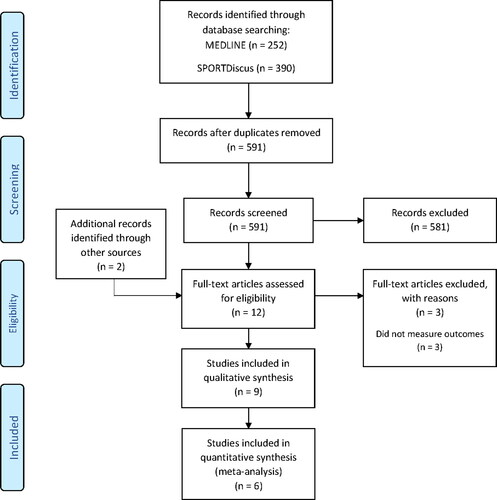
Medline and SPORTDiscus databases were searched to identify eligible studies from inception until June 2020. The search strategy was based on our Population, Intervention, Comparator, Outcome, Study design (PICOS) methodology. The full search terms and strategies of this review are displayed in the Online Supplementary Material. The search results were downloaded and filtered in the Mendeley v1.19.4 (Mendeley Ltd, UK) software. The study screening and selection process was carried out as outlined in the study selection process below. The reference lists of the full-text articles included were also searched manually for any additional studies. A flow diagram of the search strategy is displayed in .
Eligibility criteria
Inclusion criteria were based on our PICOS framework (see Supplementary material ) and included: (1) adult participants (≥18 years); (2) inorganic nitrate/nitrite or nitrate-rich vegetable supplementation with a supervised EIMD protocol; (3) a comparator group that received a control supplement (placebo) or no supplement but completed the same EIMD protocol; (4) reporting of changes in at least one of the following markers of EIMD pre and up to 168 h post-exercise: muscle function, muscle soreness, creatine kinase, myoglobin, inflammation, oxidative stress, and range of motion; (5) randomized controlled trials performed in humans. These outcomes were based on previous studies that outline the most frequent and valid markers of EIMD and its underlying causes (Hyldahl and Hubal Citation2014; Paulsen et al. Citation2012; Warren et al. Citation1999). The 168 h cut off for follow up data was adapted from a similarly designed review (Ranchordas et al. Citation2017). Crossover and parallel study designs were eligible. Studies were excluded if other nutrients were taken alongside the nitrate supplement or if insufficient information was provided on the type, dose, formulation, frequency, or route of administration. Studies were excluded if the intervention was not combined with a supervised exercise protocol and if the full text was not available in English.
Table 1. Summary of studies included in the systematic review and meta-analysis.
Study selection
The study screening and selection process was completed independently by two authors (LJ and TC). After removing duplicate articles, the title and abstracts of all retrieved articles were screened to exclude studies that did not meet the eligibility criteria. Full texts of studies deemed eligible were retrieved and compared against our PICOS criteria. All eligible articles were included in the systematic review, but meta-analyses were only conducted on outcomes with ≥2 studies. Both investigators agreed on the articles to be included in the systematic review and meta-analysis; any disagreements were resolved with the remaining authors.
Data extraction
Two investigators (LJ and NA) independently extracted data from the studies and recorded them in a Microsoft Excel Spreadsheet that has previously been used by one of the authors (Clifford et al. Citation2020). The spreadsheet was adapted to ensure all relevant material was captured, including study design, type of participants, interventions and comparators (including dose and duration), and continuous outcomes (mean ± SD) at baseline and any post-intervention time points up to 168 h. The 30 min post-exercise time point represents any measure taken ≤30 min post-exercise. The list of extracted outcomes is presented in . None of the included studies measured myoglobin. Data were transferred into the Review Manager 5.4 (Cochrane Collaboration, UK) software for meta-analysis. A second author verified the transferred data (TC). When numerical data were missing, the study authors were contacted (Carriker et al. Citation2018; Larsen et al. Citation2019; Menezes et al. Citation2019; Daab et al. Citation2021) but none replied. Therefore, means and SDs not available in articles were extracted using online software (WebPlotDigitizer, Version 4.3). This was completed by two authors (LJ and NA) to ensure reliability.
Risk of bias assessment
Two review authors (OS and NA) evaluated risk of bias for each included study by using the Cochrane Collaboration risk of bias tool version 2 (ROB2) (Higgins, Savović et al. Citation2019). This tool addresses five specific domains relating to the randomization process; deviations from intended interventions; missing outcome data; measurement of the outcome; selection of the reported result. A score for overall bias was also provided for each study. The risk of bias of the three studies that employed a crossover design were evaluated on the same ROB2 worksheet for consistency, but as recommended (Higgins, Eldridge et al. Citation2019) these studies were also evaluated for bias relating to carry over and period effects. Disparities in judgements between reviewers were resolved through discussion. Publication bias was assessed by visualizing Funnel plot asymmetry.
Assessment of heterogeneity
Assessments of heterogeneity were calculated using the Review Manager v5.4 software (Cochrane Collaboration, UK). Statistical heterogeneity was assessed by visually inspecting forest plots to assess for obvious differences in study results, and by using I2 and Chi2 statistical tests; p ≤ 0.10 indicates significant heterogeneity for Chi2, and ≥75% suggests considerable heterogeneity for I2 (Higgins and Green Citation2011b).
Sensitivity and sub-group analysis
Where possible, sensitivity analyses were performed to investigate causes of statistical heterogeneity. We planned to explore heterogeneity through sub-group analysis (Higgins and Green Citation2011b); specifically, whether outcomes were influenced by the type of nitrate/nitrite supplementation (e.g., beetroot juice vs. nitrate salts), type of exercise, and age and sex of the participants. We also performed a sensitivity analysis to assess whether the only trial in the meta-analysis using a crossover design altered the results.
Statistical analysis
The meta-analysis was conducted using the Review Manager v5.4 software. The following outcomes: maximal isometric voluntary contraction (MIVC), pressure pain threshold (PPT), countermovement jump (CMJ) height, creatine kinase (CK), and C-reactive protein (CRP), were analyzed as percentage of baseline because we could not obtain the raw data for all eligible studies. Data on subjective muscle soreness, interleukin-6 (IL-6), interleukin-8 (IL-8), and tumor necrosis factor-alpha (TNF-α) were inputted as raw values. To enable PPT and muscle soreness data to be combined for analysis, we multiplied the muscle soreness data by −1 so that both scales were in same direction (higher values = reduced muscle soreness) (Higgins, Li et al. Citation2019). We calculated standardized mean differences (SMDs) and 95% confidence intervals (CI) with forest plots for the outcome measures. SMDs were employed because of the wide heterogeneity in designs, equipment used, and scales for each outcome measure (Higgins and Green Citation2011b). We anticipated some degree of heterogeneity for most outcomes so employed random-effects models using The DerSimonian-Laird method (DerSimonian and Laird Citation1986). As there was only one study suitable for meta-analysis that employed a crossover design, we were unable to perform a separate meta-analysis for trials with parallel and crossover designs. Instead, we took a conservative approach and incorporated the data from this study into our meta-analysis by treating it as if the two groups were independent (Higgins, Savović et al. Citation2019). We performed a sensitivity analysis to assess whether removing this study influenced our overall findings.
Results
Study selection
Results from the search strategy are presented in . A total of 642 articles were identified, which was reduced to 591 after removing duplicates. After the initial screening, 10 full-texts were retrieved and two were identified through other sources. Of the 12 full-texts, three were excluded because they did not measure any of the eligible outcomes. Nine articles were included in the qualitative synthesis and six of these were eligible for inclusion in the meta-analysis. Three of the nine articles were not included in the meta-analysis because the markers of EIMD they measured were not available in at least two separate studies.
Study characteristics
A summary of the included references is presented in . Most studies employed a parallel design (n = 6), with the remaining studies adopting a crossover design (n = 3). All studies were randomized and contained a placebo group that undertook the same exercise as their respective intervention group. One study used a nitrate depleted beetroot juice as a placebo (Carriker et al. Citation2018); six studies used a non-taste matched but isocaloric (added maltodextrin and protein) nitrate and (poly)phenol free fruit flavored drink (Clifford, Bell et al. Citation2016; Clifford, Berntzen et al. Citation2016; Clifford, Allerton et al. Citation2017; Clifford, Howatson et al. Citation2017; Clifford et al. Citation2018; Daab et al. Citation2021); one study used a fruit flavored water drink (Larsen et al. Citation2019); one study supplied sodium chloride as capsules (Menezes et al. Citation2019). The sample sizes of included studies were modest, with the number of participants per group ranging from 9 to 17. None of the participants were elite athletes, with most reported as being healthy and recreationally or physically active. The range of mean ages of participants was 21 to 45 years.
One study administered nitrate as sodium nitrate (Menezes et al. Citation2019); the rest provided nitrate as beetroot juice. One study (Clifford, Bell et al. Citation2016) included two arms, differentiated by a high-dose and low-dose: data from these arms were pooled together in the meta-analysis using the Review Manager Calculator to generate a single mean, SD and sample size (Higgins and Green Citation2011a). One study included a beetroot juice and sodium nitrate arm (Clifford, Howatson et al. Citation2017). However, because beetroot juice and sodium nitrate contain different bioactive compounds (Clifford, Howatson et al. Citation2017), it was deemed inappropriate to combine these two arms. We also could not include them in the same meta-analysis as separate effect sizes because they would not be statistically independent and could introduce bias (López-López et al. Citation2018). As such, we excluded the sodium nitrate arm from our meta-analysis. Thus, all study data included in the meta-analysis were obtained following beetroot juice supplementation.
The nitrate content of the supplements varied, with the most common dosage being between 210 mg (∼3.4 mmol) and 250 mg (∼4.0 mmol) per serving. Six studies provided nitrate chronically as 7–11 total servings over 3 to 7 days. One study provided an acute beetroot juice dose 2.5 h pre-exercise and one study implemented both acute and chronic (5 days) supplementation of sodium nitrate. One study (Larsen et al. Citation2019) provided an acute dose of beetroot juice 46 h post-exercise which differed from the chronic supplementation methods used in the other studies in the meta-analysis. This study was included in the meta-analysis because its exclusion did not alter the overall pooled effect.
Six studies measured MIVC, five studies measured CMJ and CK, four studies measured muscle soreness as PPT and two with a subjective visual analogue scale (VAS), four studies measured CRP, and two studies measured IL-6, IL8, and TNF-α. Four studies measured markers of oxidative stress, but these were not included in the meta-analysis because the same markers were not measured in at least two independent studies. Only one study measured reactive strength index (Clifford, Berntzen et al. Citation2016) so this is not discussed.
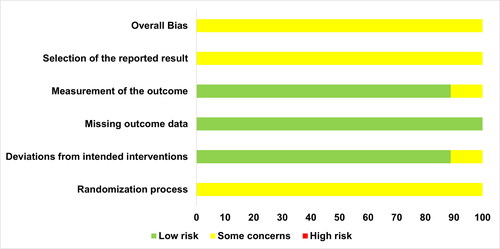
Risk of bias
A summary of the risk of bias is presented in and (Supplementary material). Risk of bias arising from period and carry over effects was deemed to be low in the studies by Carriker et al. (Citation2018) and Daab et al. (Citation2021). However, some concerns were identified for the study by Menezes et al. (Citation2019) as period effects were not accounted for in the analysis (e.g., by the inclusion of a time-by-treatment interaction term). Overall bias for all studies was considered to have some concerns, and this was mainly due to insufficient information provided on the randomization procedures and any deviations from a pre-specified protocol and analysis plan.
Publication bias
A funnel plot is presented in the Supplementary material (). We did not observe plot asymmetry suggesting little evidence of publication bias; however, tests of publication bias are not recommended when a meta-analysis contains fewer than 10 studies (Higgins and Green Citation2011b), so these plots should be interpreted cautiously.
Meta-analysis
Muscle function
Outcomes related to muscle function were MIVC and CMJ ( and ). There were no group differences in MIVC following dietary nitrate supplementation vs. placebo at 30 min (SMD: 0.48, 95% CI: −0.20 to 1.15, p = 0.17; n = 70 vs. 60), 24 h (SMD: 0.90, 95% CI: −0.23 to 2.02, p = 0.12; n = 70 vs. 60), or 48 h post-exercise (SMD: 0.49, 95% CI: −0.18 to 1.15, p = 0.15; n = 80 vs. 70) and there was significant heterogeneity at these time points (Chi2 ≥ 13.52; I2 ≥ 70%, p ≤ 0.009). Dietary nitrate improved MIVC recovery at 72 h post-exercise (SMD: 0.54, 95% CI: 0.12 to 0.96, p = 0.01; n = 53 vs. 43) and there was no heterogeneity between studies (Chi2 = 0.31; I2 = 0%, p = 0.96).
Figure 3. Forest plot demonstrating maximal isometric contractions (MIVC) in groups receiving nitrate or placebo. Plots are separated by follow up times post-exercise: <30 min, 24, 48, and 72 h. Higher standardized mean difference favors nitrate. 95% CI, 95% confidence interval; IV, inverse variance.
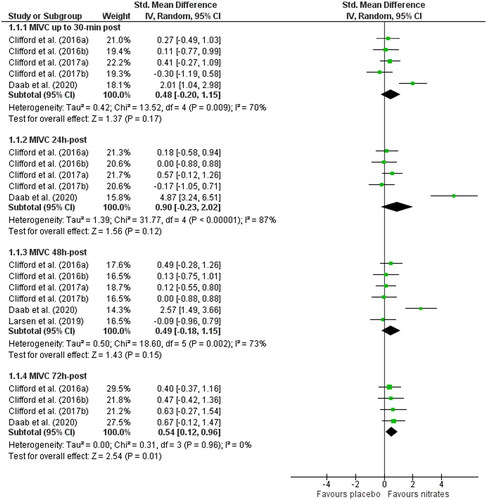
Figure 4. Forest plot demonstrating countermovement jump (CMJ) performance in groups receiving nitrate or placebo. Plots are separated by follow up times post-exercise: <30 min, 24, 48, and 72 h. Higher standardized mean difference favors nitrate. 95% CI, 95% confidence interval; IV, inverse variance.
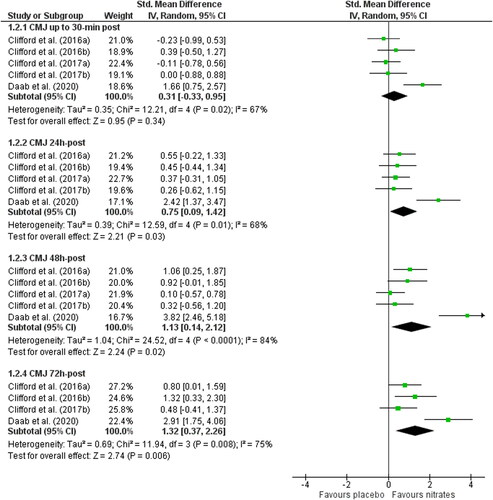
CMJ recovery at 30 min post-exercise was no different for nitrate vs. placebo (SMD: 0.31, 95% CI: −0.33 to 0.95, p = 0.34; n = 70 vs. 60) but nitrate supplementation enhanced the recovery of CMJ at 24 h (SMD: 0.75, 95% CI: 0.09 to 1.42, p = 0.03; n = 70 vs. 60), 48 h (SMD: 1.13, 95% CI: 0.14 to 2.12, p = 0.02; n = 70 vs. 60) and 72 h post-exercise (SMD: 1.32, 95% CI: 0.37 to 2.26, p = 0.006; n = 53 vs. 43). Heterogeneity was moderate to high at all time points post-exercise (Chi2 ≥ 11.94; I2 ≥ 67%, p < 0.02).
Muscle soreness
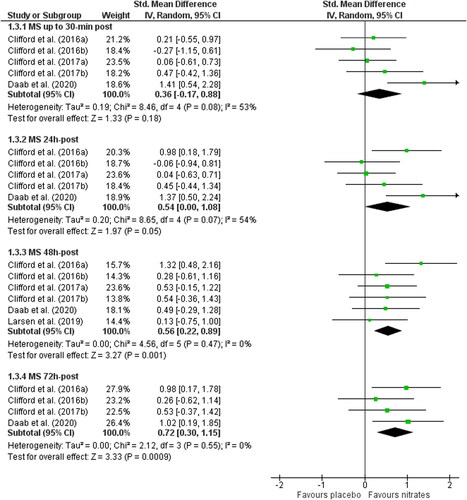
Four studies measured muscle soreness as PPT (Supplementary material ) and three studies used subjective soreness scales. Of the three studies using a VAS, data from one study was excluded because we could not accurately determine the standard deviations from the figure and the authors did not provide us the raw data (Larsen et al. Citation2019) (Supplementary material). There were no differences in PPT for nitrate vs. placebo at 30 min (SMD: 0.14, 95% CI: −0.34 to 0.62, p = 0.57; n = 40 vs. 30), or 24 h post-exercise (SMD: 0.48, 95% CI: −0.12 to 1.08, p = 0.12; n = 40 vs. 30). However, PPT was significantly improved following dietary nitrate supplementation at 48 h (SMD: 0.58, 95% CI: 0.04 to 1.11, p = 0.03; n = 50 vs. 40) and 72 h post-exercise (SMD: 0.61, 95% CI: 0.12 to 1.11, p = 0.02; n = 40 vs. 30). Heterogeneity was not high at any follow up measure (Chi2 ≤ 4.51; I2 ≤ 33%, p ≥ 0.21). Nitrate had no effect on subjective muscle soreness 30 min (SMD: −0.71, 95% CI: −2.03 to 0.62, p = 0.30; n = 30 per group) or 24 h post-exercise (SMD −0.68, 95% CI: −1.98 to 0.63, p = 0.31; n = 30 per group) but appeared to improve symptoms at 48 h post-exercise (SMD −0.52, 95% CI: −1.03 to 0.00, p = 0.05; n = 30 per group). High heterogeneity was observed 30 min and 24 h post-exercise (Chi2 ≥ 5.751; ≥ I2 82%, p < 0.05).
After combining PPT and subjective soreness data (), we found no differences in muscle soreness for nitrate vs. placebo at 30 min (SMD: 0.06, 95% CI: −0.61 to 0.73, p = 0.18; n = 70 vs. 60), but at 24 h post-exercise the effect size was moderate (SMD: 0.54, 95% CI: 0.00 to 1.08, p = 0.05; n = 70 vs. 60). At 48 h and 72 h post-exercise nitrate significantly reduced muscle soreness (48 h: SMD: 0.56, 95% CI: 0.22 to 0.89, p = 0.001); n = 80 vs. 70, 72 h: SMD: 0.72, 95% CI: 0.30 to 1.15, p = 0.0009). Heterogeneity was moderate 30 min and 24 h post-exercise (Chi2 ≥ 8.46; I2 ≥ 53%, p ≤ 0.07).
Creatine kinase
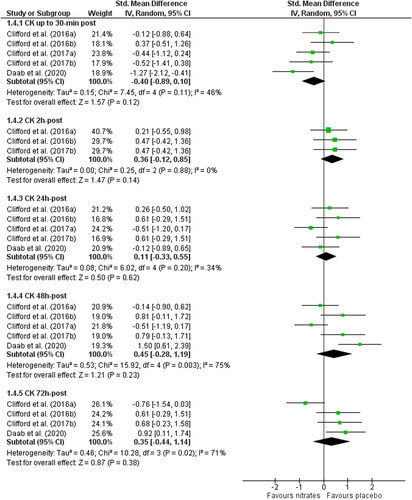
There were no differences in CK between nitrate and placebo at 30 min (SMD: −0.40, 95% CI: −0.89 to 0.10, p = 0.12; n = 70 vs. 60), 2 h (SMD: 0.36, 95% CI: −0.12 to 0.85, p = 0.14; n = 40 vs. 30), 24 h (SMD: 0.11, 95% CI: −0.33 to 0.55, p = 0.62; n = 70 vs. 60), 48 h (SMD: 0.45, 95% CI: −0.28 to 1.19, p = 0.23), or 72 h post-exercise (SMD: 0.35, 95% CI: −0.44 to 1.14, p = 0.38; n = 40 per group). There was high heterogeneity at 48 and 72 h post-exercise (Chi2 ≥ 10.28; I2 ≥ 71%, p ≤ 0.02) ().
Inflammation
Inflammatory markers included were IL-6, IL-8, CRP, and TNF-α. Figures for these data are available in the Supplementary material (Figures S4–S7). Only two studies measured IL-6, IL-8 and TNF-α. There were no group differences in IL-6 at 30 min (SMD: 0.28, 95% CI: −0.35 to 0.91, p = 0.38; n = 36 vs. 26), 24 h (SMD: 0.15, 95% CI: −0.58 to 0.88, p = 0.69; n = 36 vs. 26) and 48 h post-exercise (SMD: 0.20, 95% CI: −1.26 to 1.65, p = 0.79; n = 36 vs. 26). Significant heterogeneity was identified at 48 post (Chi2 = 7.44; I2 = 87%, p = 0.006). IL-8 did not differ between nitrate and placebo 30 min (SMD: 0.31, 95% CI: −0.32 to 0.95, p = 0.34; n = 36 vs. 26), 24 h (SMD: 0.23, 95% CI: −0.29 to 0.75, p = 0.38; n = 36 vs. 26) and 48 h post-exercise (SMD: 0.20, 95% CI: −0.31 to 0.71, p = 0.44; n = 36 vs. 26). TNF-α did not differ between the two groups at 30-min (SMD: 0.37, 95% CI: −0.15 to 0.88, p = 0.16; n = 36 vs. 26), 24 h (SMD: 0.20, 95% CI: −0.31 to 0.71 p = 0.45; n = 36 vs. 26) or 48 h post-exercise (SMD 0.00, 95% CI: −0.51 to 0.52 p = 0.99; n = 36 vs. 26).
Levels of CRP were no different at 30-min (SMD: 0.22, 95% CI: −0.62 to 1.05, p = 0.61; n = 49 per group), 2 h (SMD: 0.27, 95% CI: −0.35 to 0.90, p = 0.39); n = 20 per group), 24 h (SMD: 0.40, 95% CI: −0.24 to 1.04, p = 0.22; n = 49 per group), or 72 h post-exercise (SMD: 0.16, 95% CI: −0.40 to 0.73, p = 0.57; n = 33 per group) but they were lower with placebo at 48 h post (SMD: 0.55, 95% CI: 0.06 to 1.04, p = 0.03; n = 49 per group). There was high heterogeneity at 30-min post-exercise (Chi2 = 11.99; I2 = 75%, p = 0.007) and moderate heterogeneity 24 h-post-exercise (Chi2 = 7.19; I2 = 58%, p = 0.07).
Sensitivity and sub-group analysis
Due to insufficient studies we could not perform our planned sub-group analysis (Higgins and Green Citation2011b). Our sensitivity analysis revealed that most of the statistical heterogeneity could be removed by excluding one outlying study that unlike the others employed a crossover design (Daab et al. Citation2021). Excluding this study removed the heterogeneity for MIVC <48 h post-exercise (Chi2 ≤ 1.98; I2 = 0%, p ≥ 0.58), CMJ at all time points (Chi2 = ≤ 4.06; I2 ≤ 26%, p ≥ 0.25) and CRP at 30-min and 24 h post-exercise (Chi2 ≤ 0.07; I2 = 0%, p ≥ 0.71) without markedly changing the pooled SMD or level of significance. Removing this study, due to the fact it employed a crossover design, did not markedly change the results for any outcomes apart from CRP at 48 h post, which was no longer statistically significant, and the effect size reduced to small (SMD: 0.35, 95% CI: −0.12 to 0.83, p = 0.15). We did not perform the same sensitivity analysis in data sets that only contained two studies.
Oxidative stress
We did not include oxidative stress in the meta-analysis because no two studies measured the same marker (). Of the four studies that included markers of oxidative stress, only one of these studies suggested that nitrate supplementation can reduce exercise-induced oxidative stress (Menezes et al. Citation2019).
Discussion
Our results suggest that dietary nitrate, specifically in the form of beetroot juice, may accelerate the recovery of muscle function and reduce muscle soreness following strenuous exercise. In contrast, the effects of nitrate supplementation on some of the underlying determinants of EIMD, such as inflammation and oxidative stress, were largely inconclusive, and thus the mechanisms to explain our findings are unclear. There was insufficient data available to determine the mediating role of age, sex, exercise, and type nitrate of the markers of EIMD. Overall, we retrieved few studies (n = 9), the first of which was published in 2016, suggesting research evaluating the effects of dietary nitrate as a recovery aid in humans is still in its infancy.
This is the first review to evaluate the effects of dietary nitrate supplementation on EIMD and therefore direct comparisons with previous systematic reviews and meta-analyses are not possible. However, our findings for muscle soreness and muscle function recovery are broadly in line with a recent review of fruit supplements on EIMD (Doma et al. Citation2021), which like nitrate based supplements, are purported to attenuate markers of EIMD via antioxidant and/or anti-inflammatory effects. These authors reported that supplements derived from fruit (poly)phenols accelerated the recovery of isometric strength and reduced muscle soreness following exercise. In contrast to our study, they found that fruit supplements attenuated systemic markers of inflammation and CK. In the present study, except a possible increase of CRP 48 h post-exercise (SMD: 0.55), nitrate had no effect on other inflammatory markers (IL-8, IL-6, TNF-α). These disparate findings might be attributed to the fact that the analysis by Doma et al. (Citation2021) contained twice as many studies and thereby provided greater statistical power to detect effects compared to the present analysis. Indeed, the lack of effect of nitrate (in the form of beetroot juice) on the cytokines in the present analysis should be interpreted cautiously given only two studies measured these markers using differing experimental designs (Clifford, Bell et al. Citation2016; Clifford, Allerton et al. Citation2017). An alternative explanation is that fruit-based supplements are more effective than nitrate or beetroot juice supplements at attenuating exercise-induced inflammation; however, we are not aware of any original research that has directly compared these two interventions. More research is needed to explore the effects of nitrate-rich supplements on inflammation resulting from EIMD.
Strenuous exercise generates potentially damaging ROS such as hypochlorous acid and the hydroxyl radical (Powers and Jackson Citation2008). If the generation of these radicals outweighs their clearance, oxidative stress ensues and muscle function is depressed (Cheng et al. Citation2015; Place et al. Citation2015; Reid Citation2016). Hence, abating oxidative stress is proposed to reduce muscle fatigue and, in the context of EIMD, expedite the recovery of muscle function (Close et al. Citation2005). Another beneficial effect of lowering oxidative stress might be a reduction in muscle soreness (Close et al. Citation2005; Lee et al. Citation2002). Oxidative stress and inflammation propagate pain by sensitizing nociceptors (Aparicio-Soto et al. Citation2013; Zhang and An Citation2007); hence, attenuating either post-exercise could, theoretically, mitigate exercise-induced muscle pain and soreness. Accordingly, lowering oxidative stress, inflammation, and its subsequent damage to cellular constituents, is one of the mechanisms by which nitrate supplementation could aid aspects of recovery. The antioxidant effects of dietary nitrate are supported by several animal studies (reviewed by Clifford et al. (Citation2015)), but have scarcely been considered in humans (Ashor et al. Citation2016). Animal studies have shown that the amount of nitrate required to attenuate oxidative stress is equivalent to a high dietary intake of nitrate-rich vegetables in humans (e.g., 100–300 g per day) (Carlström et al. Citation2011; Gheibi et al. Citation2018). Of the available human studies, which are often in clinical populations (Ashor et al. Citation2016; Basaqr et al. Citation2021), the nitrate dose shown to modulate markers of oxidative stress is broadly comparable to the doses used in the studies in the present meta-analysis (∼6.4–11.0 mmol/day). In the present review, markers of oxidative stress were only measured by four studies and none of them used the same markers, meaning a meta-analysis was deemed inappropriate. Of these four studies, only one suggested nitrate supplementation (sodium nitrate) could attenuate markers of oxidative stress following exercise (Menezes et al. Citation2019). Their main findings were that nitrate reduced salivary thiobarbituric acid reactive substances (TBARS), a marker of lipid oxidation, and increased ferric reducing antioxidant power (FRAP), a marker of antioxidant capacity, in the 2.5 h following high intensity cycling exercise. These findings should be interpreted cautiously, however, as both TBARS and FRAP have been criticized as nonspecific markers of oxidative stress (Powers et al. Citation2010; Cobley et al. Citation2017). While the remaining studies included more valid markers of oxidative stress such as 8-isoprostanes (Carriker et al. Citation2018) and lipid hydroperoxides (Clifford, Berntzen et al. Citation2016), neither were altered by nitrate-rich beetroot juice. Yet, the lack of an effect on these markers of lipid peroxidation does not exclude the possibility of protection of other cellular constituents such as proteins and DNA. Future studies are needed to determine whether the beneficial effects of nitrate supplementation on muscle function recovery observed in the present study are, at least partly, associated with a reduction in oxidative stress.
All studies included in the meta-analysis administered nitrate as beetroot juice. This is in line with previous reviews of nitrate supplementation and exercise performance that found beetroot juice is the most frequently examined nitrate-rich supplement (McMahon et al. Citation2017; Calvo et al. Citation2020). However, beetroot juice is not merely a nitrate vehicle but contains other bioactive compounds that could influence EIMD (Clifford et al. Citation2015; Clifford, Howatson et al. Citation2017). Indeed, beetroot is rich in betalains, which, like (poly)phenols, are potent antioxidants and anti-inflammatories in vitro (Clifford et al. Citation2015; Esatbeyoglu et al. Citation2015; Khan Citation2016) and, in a recent study, were found to attenuate CK levels 24 h following exercise (Montenegro et al. Citation2017). Although human studies with betalains are still limited, the fact they might influence EIMD suggests the favorable effects observed with beetroot juice in the present study cannot necessarily be ascribed to nitrate. Instead, they might be the result of an additive or synergistic effect between nitrate and betalains or possibly just the betalains and other (poly)phenolic constituents in beetroot juice. We wanted to compare studies that used nitrate salts and beetroot juice to try and shed some light on this, but insufficient data was available. Interestingly, one of the included studies (Clifford, Howatson et al. Citation2017) directly compared the effects of a nitrate matched sodium nitrate and beetroot juice supplement on EIMD and found greater decreases in muscle soreness with beetroot juice, suggesting betalains may be more potent than nitrate, at least in this context. But given this is only one study, the influence of nitrate should not be discounted, especially when accounting for the superior bioavailability of nitrate compared to betalains and (poly)phenols (Clifford, Constantinou et al. Citation2017), and the wealth of human evidence indicating that nitrate has a multitude of physiological and metabolic effects (Lundberg et al. Citation2018). Unraveling the independent effects of nitrate, betalains, and beetroot juice on EIMD requires more research.
The eligible studies had several limitations. Firstly, many did not verify that the nitrate supplementation protocols were sufficient to raise nitrate and nitrite concentrations (Clifford, Bell et al. Citation2016; Clifford, Allerton et al. Citation2017; Clifford, Bell et al. Citation2017; Clifford et al. Citation2018; Daab et al. Citation2021). Secondly, only three studies reported a priori power analysis for the primary outcome (Clifford, Berntzen et al. Citation2016; Clifford, Allerton et al. Citation2017; Clifford, Howatson et al. Citation2017), so it is unclear whether all studies were sufficiently powered to detect significant between group changes. Many studies did not attempt to measure the mechanisms by which nitrate supplementation might attenuate EIMD, and those that did relied on systemic markers, with no study analyzing changes in skeletal muscle. In addition, most of the studies only included young recreationally active male participants, so these findings are unlikely to be generalizable to females, older adults, or elite athletes.
We acknowledge several limitations of the current analysis. Firstly, our analysis was limited to a small number of studies and therefore our findings should be treated as preliminary rather than definitive. In addition, 5/9 studies were from the same research group and were led by the corresponding author of this research. To minimize any bias arising from this authors involvement, they were not involved in the ROB2 assessments and all authors agreed on the interpretations of the overall findings. The limited number of eligible studies meant we could not perform any sub-group analysis to identify any variables (e.g., dose, age) capable of modifying the effectiveness of nitrate supplementation on EIMD. Another limitation is that we observed high statistical heterogeneity for many outcomes, ostensibly because of the low number of included studies. However, our findings can be considered robust as excluding one outlier study removed the heterogeneity for many outcomes without affecting the pooled effect sizes.
Conclusion
This systematic review and meta-analysis suggests that beetroot juice may attenuate post-exercise deficits in muscle function, and increases in muscle pain/soreness. Nonetheless, these findings should be interpreted cautiously given the limited number of studies included in the review and some of their methodological limitations. In addition, although effect sizes for the recovery of CMJ were large, for all other variables they were moderate or small; thus, beetroot juice may only confer a small advantage. Future well designed, adequately powered studies are required, including studies with other population groups, especially elite athletes, to further understanding in this field. Furthermore, there are many questions that remain unanswered. Firstly, the mechanisms for the potential improvements in the recovery in muscle function and muscle pain/soreness following EIMD after nitrate supplementation are not clear as there was little evidence that nitrate modified markers of oxidative stress or inflammation; in fact, there was evidence that CRP may have been increased with nitrate, albeit this was removed when a study with a crossover design was removed. These results should be interpreted cautiously though, as few studies measured markers of inflammation and oxidative stress, and therefore more research is required to determine whether nitrate and/or beetroot juice can modify these processes after exercise. Because our quantitative analysis only included studies using beetroot juice, it is unclear whether nitrate, other bioactive compounds in beetroot juice, or their additive/synergistic effects, are responsible for the influence of beetroot juice on EIMD recovery. Future studies are required to resolve these shortcomings in our understanding of the potential efficacy and mechanistic bases of nitrate and beetroot juice supplementation recovery following EIMD.
Supplemental Material
Download MS Word (492.4 KB)Disclosure statement
This study received no funding and declares no conflicts of interest.
Additional information
Notes on contributors
Louise Jones
Louise Jones is a MSc student from Loughborough University.
Stephen J. Bailey
Stephen J. Bailey is a Senior Lecturer at Loughborough University.
Samantha N. Rowland
Samantha N. Rowland is a PhD candidate at Loughborough University.
Nehal Alsharif
Nehal Alsharif is a PhD candidate at Loughborough University.
Oliver M. Shannon
Oliver M. Shannon is a Research Associate at Newcastle University.
Tom Clifford
Tom Clifford is a Lecturer at Loughborough University.
References
- Anderson JE, Zhu A, Mizuno TM. 2018. Nitric oxide treatment attenuates muscle atrophy during hind limb suspension in mice. Free Radical Biol Med. 115:458–470. doi:10.1016/j.freeradbiomed.2017.12.021.
- Aparicio-Soto M, de la Lastra CA, Cardeno A, Sanchez-Fidalgo S, Sanchez-Hidalgo M. 2013. Melatonin exhibits anti-inflammatory effect in LPS-induced murine macrophages via NFKB suppression and NRF-2 and HO-1 induction. United Eur Gastroenterol J. 1(1 SUPPL. 1):A231. doi:10.1177/2050640613502900.
- Ashor AW, Chowdhury S, Oggioni C, Qadir O, Brandt K, Ishaq A, Mathers JC, Saretzki G, Siervo M. 2016. Inorganic nitrate supplementation in young and old obese adults does not affect acute glucose and insulin responses but lowers oxidative stress. J Nutr. 146(11):2224–2232. doi:10.3945/jn.116.237529.
- Basaqr R, Skleres M, Jayswal R, Thomas DT. 2021. The effect of dietary nitrate and vitamin C on endothelial function, oxidative stress and blood lipids in untreated hypercholesterolemic subjects: a randomized double-blind crossover study. Clin Nutr. 40(4):1851–1860. doi:10.1016/j.clnu.2020.10.012.
- Bell PG, Stevenson E, Davison GW, Howatson G. 2016. The effects of montmorency tart cherry concentrate supplementation on recovery following prolonged, intermittent exercise. Nutrients. 8(7):441. doi:10.3390/nu8070441.
- Betteridge DJ. 2000. What is oxidative stress? Metab Clin Exp. 49(2):3–8. doi:10.1016/S0026-0495(00)80077-3.
- Burt DG, Lamb K, Nicholas C, Twist C. 2014. Effects of exercise-induced muscle damage on resting metabolic rate, sub-maximal running and post-exercise oxygen consumption. Eur J Sport Sci. 14(4):337–344. doi:10.1080/17461391.2013.783628.
- Calvo JL, Alorda-Capo F, Pareja-Galeano H, Jiménez SL. 2020. Influence of nitrate supplementation on endurance cyclic sports performance: a systematic review. Nutrients. 12(6):1796. doi:10.3390/nu12061796.
- Carlström M, Persson AEG, Larsson E, Hezel M, Scheffer PG, Teerlink T, Weitzberg E, Lundberg JO. 2011. Dietary nitrate attenuates oxidative stress, prevents cardiac and renal injuries, and reduces blood pressure in salt-induced hypertension. Cardiovascular Research. 89(3):574–585. doi:10.1093/cvr/cvq366.
- Carriker CR, Rombach P, Stevens BM, Vaughan RA, Gibson AL. 2018. Acute dietary nitrate supplementation does not attenuate oxidative stress or the hemodynamic response during submaximal exercise in hypobaric hypoxia. Appl Physiol Nutr Metab. 43(12):1268–1274. http://search.ebscohost.com/login.aspx?direct=true&db=s3h&AN=133235200&site=ehost-live. doi:10.1139/apnm-2017-0813.
- Cheng AJ, Bruton JD, Lanner JT, Westerblad H. 2015. Antioxidant treatments do not improve force recovery after fatiguing stimulation of mouse skeletal muscle fibres. J Physiol. 593(2):457–472. doi:10.1113/jphysiol.2014.279398.
- Clifford T, Allerton DM, Brown MA, Harper L, Horsburgh S, Keane KM, Stevenson EJ, Howatson G. 2017. Minimal muscle damage after a marathon and no influence of beetroot juice on inflammation and recovery. Appl Physiol Nutr Metab. 42(3):263–270. doi:10.1139/apnm-2016-0525.
- Clifford T, Bell O, West DJ, Howatson G, Stevenson EJ. 2016. The effects of beetroot juice supplementation on indices of muscle damage following eccentric exercise. Eur J Appl Physiol. 116(2):353–362. doi:10.1007/s00421-015-3290-x.
- Clifford T, Bell O, West DJ, Howatson G, Stevenson EJ. 2017. Antioxidant-rich beetroot juice does not adversely affect acute neuromuscular adaptation following eccentric exercise. J Sports Sci. 35(8):812–819. http://search.ebscohost.com/login.aspx?direct=true&db=s3h&AN=120931961&site=ehost-live. doi:10.1080/02640414.2016.1192670.
- Clifford T, Berntzen B, Davison GW, West DJ, Howatson G, Stevenson EJ. 2016. Effects of beetroot juice on recovery of muscle function and performance between bouts of repeated sprint exercise. Nutrients. 8(8):506. doi:10.3390/nu8080506.
- Clifford T, Bowman A, Capper T, Allerton DM, Foster E, Birch-Machin M, Lietz G, Howatson G, Stevenson EJ. 2018. A pilot study investigating reactive oxygen species production in capillary blood after a marathon and the influence of an antioxidant-rich beetroot juice. Appl Physiol Nutr Metab. 43(3):303–306. http://search.ebscohost.com/login.aspx?direct=true&db=s3h&AN=128202692&site=ehost-live. doi:10.1139/apnm-2017-0587.
- Clifford T, Constantinou CM, Keane KM, West DJ, Howatson G, Stevenson EJ. 2017. The plasma bioavailability of nitrate and betanin from Beta vulgaris rubra in humans. Eur J Nutr. 56(3):1245–1254. doi:10.1007/s00394-016-1173-5.
- Clifford T, Howatson G, West DJ, Stevenson EJ. 2015. The potential benefits of red beetroot supplementation in health and disease. Nutrients. 7(4):2801–2822. doi:10.3390/nu7042801.
- Clifford T, Howatson G, West DJ, Stevenson EJ. 2017. Beetroot juice is more beneficial than sodium nitrate for attenuating muscle pain after strenuous eccentric-bias exercise. Appl Physiol Nutr Metab. 42(11):1185–1191. http://search.ebscohost.com/login.aspx?direct=true&db=s3h&AN=125928183&site=ehost-live. doi:10.1139/apnm-2017-0238.
- Clifford T, Jeffries O, Stevenson EJ, Davies KAB. 2020. The effects of vitamin C and E on exercise-induced physiological adaptations: a systematic review and meta-analysis of randomized controlled trials. Crit Rev Food Sci Nutr. 60(21):3669–3679. doi:10.1080/10408398.2019.1703642.
- Close GL, Ashton T, McArdle A, MacLaren DPM. 2005. The emerging role of free radicals in delayed onset muscle soreness and contraction-induced muscle injury. Comp Biochem Physiol A Mol Integr Physiol. 142(3):257–266. doi:10.1016/j.cbpa.2005.08.005.
- Cobley JN, Close GL, Bailey DM, Davison GW. 2017. Exercise redox biochemistry: conceptual, methodological and technical recommendations. Redox Biol. 12:540–548. doi:10.1016/j.redox.2017.03.022.
- Daab W, Bouzid MA, Lajri M, Bouchiba M, Saafi MA, Rebai H. 2021. Chronic beetroot juice supplementation accelerates recovery kinetics following simulated match play in soccer players. J Am Coll Nutr. 40(1):61–69. doi:10.1080/07315724.2020.1735571.
- DerSimonian R, Laird N. 1986. Meta-analysis in clinical trials. Controlled Clin Trials. 7(3):177–188. doi:10.1016/0197-2456(86)90046-2.
- Doma K, Gahreman D, Connor J. 2021. Fruit supplementation reduces indices of exercise-induced muscle damage: a systematic review and meta-analysis. Eur J Sport Sci. 21(4):562–579. doi:10.1080/17461391.2020.1775895.
- Esatbeyoglu T, Wagner AE, Schini-Kerth VB, Rimbach G. 2015. Betanin–a food colorant with biological activity. Mol Nutr Food Res. 59(1):36–47. doi:10.1002/mnfr.201400484.
- Gheibi S, Jeddi S, Carlström M, Gholami H, Ghasemi A. 2018. Effects of long-term nitrate supplementation on carbohydrate metabolism, lipid profiles, oxidative stress, and inflammation in male obese type 2 diabetic rats. Nitric Oxide. 75:27–41. doi:10.1016/j.niox.2018.02.002.
- Higgins JP, Eldridge S, Li T. 2019. Including variants on randomized trials. In Cochrane handbook for systematic reviews of interventions. doi:10.1002/9781119536604.ch23.
- Higgins JPT, Green S. 2011a. How to include multiple groups from one study. In Cochrane handbook for systematic reviews of interventions v 5.1.0.
- Higgins JPT, Green S, editors. 2011b. Cochrane handbook for systematic reviews of interventions version 5.1.0.
- Higgins JPT, Li T, Deeks JJ. 2019. Choosing effect measures and computing estimates of effect. In Cochrane handbook for systematic reviews of interventions. doi:10.1002/9781119536604.ch6.
- Higgins JPT, Savović J, Page MJ, Sterne JAC, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng H-Y, Corbett MS, et al. 2019. RoB 2: a revised Cochrane risk-of-bias tool for randomized trials. Br Med J. 366:l4898.
- Howatson G, McHugh MP, Hill JA, Brouner J, Jewell AP, Van Someren KA, Shave RE, Howatson SA. 2010. Influence of tart cherry juice on indices of recovery following marathon running Howatson et al. Cherry juice supplementation and Marathon running. Scand J Med Sci Sports. 20(6):843–852. http://search.ebscohost.com/login.aspx?direct=true&db=s3h&AN=54503169&site=ehost-live. doi:10.1111/j.1600-0838.2009.01005.x.
- Howatson G, Van Someren KA. 2008. The prevention and treatment of exercise-induced muscle damage. Sports Med. 38(6):483–503. doi:10.2165/00007256-200838060-00004.
- Hyldahl RD, Hubal MJ. 2014. Lengthening our perspective: morphological, cellular, and molecular responses to eccentric exercise. Muscle Nerve. 49(2):155–170. doi:10.1002/mus.24077.
- Jädert C, Petersson J, Massena S, Ahl D, Grapensparr L, Holm L, Lundberg JO, Phillipson M. 2012. Decreased leukocyte recruitment by inorganic nitrate and nitrite in microvascular inflammation and NSAID-induced intestinal injury. Free Radical Biol Med. 52(3):683–692. doi:10.1016/j.freeradbiomed.2011.11.018.
- Khan MI. 2016. Plant betalains: safety, antioxidant activity, clinical efficacy, and bioavailability. Compr Rev Food Sci Food Saf. 15(2):316–330. doi:10.1111/1541-4337.12185.
- Larsen RG, Thomsen JM, Hirata RP, Steffensen R, Poulsen ER, Frøkjaer JB, Graven‐Nielsen T. 2019. Impaired microvascular reactivity after eccentric muscle contractions is not restored by acute ingestion of antioxidants or dietary nitrate. Physiol Rep. 7(13):e14162. doi:10.14814/phy2.14162.
- Lee J, Goldfarb AH, Rescino MH, Hegde S, Patrick S, Apperson K. 2002. Eccentric exercise effect on blood oxidative-stress markers and delayed onset of muscle soreness. Med Sci Sports Exer. 34(3):443–448. doi:10.1097/00005768-200203000-00010.
- Leeder JDC, Van Someren KA, Bell PG, Spence JR, Jewell AP, Gaze D, Howatson G. 2015. Effects of seated and standing cold water immersion on recovery from repeated sprinting. J Sports Sci. 33(15):1544–1552. doi:10.1080/02640414.2014.996914.
- López-López JA, Page MJ, Lipsey MW, Higgins JPT. 2018. Dealing with effect size multiplicity in systematic reviews and meta-analyses. Res Syn Meth. 9(3):336–351. doi:10.1002/jrsm.1310.
- Lundberg JO, Carlström M, Weitzberg E. 2018. Metabolic effects of dietary nitrate in health and disease. Cell Metab. 28(1):9–22. doi:10.1016/j.cmet.2018.06.007.
- McMahon NF, Leveritt MD, Pavey TG. 2017. The effect of dietary nitrate supplementation on endurance exercise performance in healthy adults: a systematic review and meta-analysis. Sports Med. 47(4):735–756. doi:10.1007/s40279-016-0617-7.
- Menezes EF, Peixoto LG, Teixeira RR, Justino AB, Puga GM, Espindola FS. 2019. Potential benefits of nitrate supplementation on antioxidant defense system and blood pressure responses after exercise performance. Oxid Med Cell Longevity. 2019:1–10. doi:10.1155/2019/7218936.
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 6(7):e1000097. doi:10.1371/journal.pmed.1000097.
- Montenegro CF, Kwong DA, Minow ZA, Davis BA, Lozada CF, Casazza GA. 2017. Betalain-rich concentrate supplementation improves exercise performance and recovery in competitive triathletes. Appl Physiol Nutr Metab. 42(2):166–172. doi:10.1139/apnm-2016-0452.
- Owens DJ, Twist C, Cobley JN, Howatson G, Close GL. 2019. Exercise-induced muscle damage: what is it, what causes it and what are the nutritional solutions? Eur J Sport Sci. 19(1):71–85. doi:10.1080/17461391.2018.1505957.
- Paulsen G, Benestad HB, Strøm-Gundersen I, Mørkrid L, Lappegård KT, Raastad T. 2005. Delayed leukocytosis and cytokine response to high-force eccentric exercise. Med Sci Sports Exer. 37(11):1877–1883. doi:10.1249/01.mss.0000177064.65927.98.
- Paulsen G, Mikkelsen UR, Raastad T, Peake JM. 2012. Leucocytes, cytokines and satellite cells: what role do they play in muscle damage and regeneration following eccentric exercise? Exer Immunol Rev. 18:42–97.
- Peleli M, Ferreira DMS, Tarnawski L, McCann Haworth S, Xuechen L, Zhuge Z, Newton PT, Massart J, Chagin AS, Olofsson PS, et al. 2020. Dietary nitrate attenuates high-fat diet-induced obesity via mechanisms involving higher adipocyte respiration and alterations in inflammatory status. Redox Biol. 28:101387. doi:10.1016/j.redox.2019.101387.
- Place N, Ivarsson N, Venckunas T, Neyroud D, Brazaitis M, Cheng AJ, Ochala J, Kamandulis S, Girard S, Volungevičius G, et al. 2015. Ryanodine receptor fragmentation and sarcoplasmic reticulum Ca2+ leak after one session of high-intensity interval exercise. Proc Natl Acad Sci USA. 112(50):15492–15497. doi:10.1073/pnas.1507176112.
- Powers SK, Jackson MJ. 2008. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev. 88(4):1243–1276. doi:10.1152/physrev.00031.2007.
- Powers SK, Smuder AJ, Kavazis AN, Hudson MB. 2010. Experimental guidelines for studies designed to investigate the impact of antioxidant supplementation on exercise performance. Int J Sport Nutr Exercise Metab. 20(1):2–14. doi:10.1123/ijsnem.20.1.2.
- Ranchordas MK, Rogerson D, Soltani H, Costello JT. 2017. Antioxidants for preventing and reducing muscle soreness after exercise. Cochrane Database Syst Rev. 12:CD009789. doi:10.1002/14651858.CD009789.pub2.
- Raubenheimer K, Bondonno C, Blekkenhorst L, Wagner KH, Peake JM, Neubauer O. 2019. Effects of dietary nitrate on inflammation and immune function, and implications for cardiovascular health. Nutr Rev. 77(8):584–599. doi:10.1093/nutrit/nuz025.
- Reid MB. 2016. Redox interventions to increase exercise performance. J Physiol. 594(18):5125–5133. doi:10.1113/JP270653.
- Warren GL, Ingalls CP, Lowe DA, Armstrong RB. 2002. What mechanisms contribute to the strength loss that occurs during and in the recovery from skeletal muscle injury? J Orthop Sports Phys Ther. 32(2):58–64. doi:10.2519/jospt.2002.32.2.58.
- Warren GL, Lowe DA, Armstrong RB. 1999. Measurement tools used in the study of eccentric contraction-induced injury. Sports Med. 37:43–59. doi:10.2165/00007256-199927010-00004.
- Zhang JM, An J. 2007. Cytokines, inflammation, and pain. Int Anesthesiol Clin. 45(2):27–37. doi:10.1097/AIA.0b013e318034194e.