Abstract
The global scientific community is striving to understand the pathophysiological mechanisms and develop effective therapeutic strategies for COVID-19. Despite overwhelming data, there is limited knowledge about the molecular mechanisms involved in the prominent cytokine storm syndrome and multiple organ failure and fatality in COVID-19 cases. The aim of this work is to investigate the possible role of of α-lipoic acid (ALA) and palmitoylethanolamide (PEA), in countering the mechanisms in overproduction of reactive oxygen species (ROS), and inflammatory cytokines. An in vitro model of lipopolysaccharide (LPS)-stimulated human epithelial lung cells that mimics the pathogen-associated molecular pattern and reproduces the cell signaling pathways in cytokine storm syndrome has been used. In this model of acute lung injury, the combination effects of ALAPEA, administered before and after LPS injury, were investigated. Our data demonstrated that a combination of 50 µM ALA + 5 µM PEA can reduce ROS and nitric oxide (NO) levels modulating the major cytokines involved on COVID-19 infection when administered either before or after LPS-induced damage. The best outcome was observed when administered after LPS, thus reinforcing the hypothesis that ALA combined with PEA to modulate the key point of cytokine storm syndrome. This work supports for the first time that the combination of ALA with PEA may represent a novel intervention strategy to counteract inflammatory damage related to COVID-19 by restoring the cascade activation of the immune response and acting as a powerful antioxidant.
Introduction
There is a global scientific pursuit to elucidate the pathophysiological spectrum of COVID-19 pandemic, caused by SARS-CoV-2, the virulent human β-coronavirus. Although much is known about the clinical features and mortality rates of this clinical disease, the molecular mechanisms involved in its pathobiology remains elusive (Citation1–3), due to limited access to autopsy specimens and biopsies (Citation4). The severity of COVID-19 is triggered by "cytokine storm syndrome"; however, strategies to combat this uncontrolled inflammation are limited (Citation5). Activation of coagulation pathways during COVID-19 infection is concomitant with an overproduction of proinflammatory cytokines leading to multiorgan lesions, often accompanied by edema, gas exchange dysfunction, acute respiratory distress syndrome (ARDS), acute heart damage, and secondary infection (Citation6). The cytokine storm includes several host factors involved in adaptive immunity, proinflammatory cytokines and interleukins, and anti-inflammatory cytokines (Citation6). Numerous studies have reported abnormal levels of specific cytokines and chemokines in moderate and severe infection stages in Covid-19 patients, which include: IL-1, IL-2, IL-4, IL-6, IL-7, IL-10, IL-12, IL-13, IL-17, macrophage colony-stimulating factor (M-CSF), granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), interferon-gamma inducible protein 10 kD (IP10), monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein 1-α (MIP 1-α), IFN-γ, hepatocyte growth factor (HGF), TNFα, and vascular endothelial growth factor (VEGF) (Citation6–9). Therefore, the exhaustion of antiviral defenses associated with innate immune response and elevated synthesis of inflammatory cytokines in SARS-CoV-2 infection are key therapeutic targets to control early stages of COVID-19 (Citation10).
Based on early studies on SARS-CoV-2 infection, the disease can be divided into three phases corresponding to different clinical stages (Citation11). The first phase is an asymptomatic state in which the inhaled SARS-CoV-2 virus likely binds to epithelial cells in the nasal cavity and starts replicating (Citation12). Therefore, the ciliated cells are the primary cells infected in the conducting airways (Citation3). In the next stage, the virus propagates and migrates down along the conducting airways and trigger a robust innate immune response. At this point, the COVID-19 disease is clinically manifested (Citation4). Pulmonary epithelia interact with immune cells to control the viral spread. These interactions are largely mediated by cytokine signaling and cell-cell interactions. After proper viral clearance, it is necessary to restore homeostasis through controlled elimination of activated immune cells to avoid overactivation of the immune system and consequent severe tissue damage (Citation13). The disease is mild and mostly restricted to the upper and conducting airways in about 80% of the infected patients (Citation11). About 20% of infected patients progress to stage 3 and develop pulmonary infiltrates, some of which lead to a severe stage of the disease. Elderly population is at particular risk due to compromised immune system and reduced ability to repair damaged epithelium, which facilitates the virus to spread more easily to the lung gas exchange area (Citation14). Several studies have investigated the relationship between infection and the innate immune response of differentiated primary human lung cells using culture models that mimic the air-liquid interface and preserve key features of the in vivo airway epithelium that viruses attack. This basic research on SARS-CoV-2 is essential to understand its intricate pathophysiology and identify the best drug targets (Citation15). Studies on human airway epithelia, the first line of defense against respiratory pathogens, air pollution and allergens, is therefore particularly relevant (Citation16). In humans, airway epithelial cells abundantly express angiotensin-converting enzyme 2 (ACE2), the putative SARS-CoV-2 entry receptor, along with the transmembrane serine proteinase 2 (TMPRSS2), the host enzyme virus uses to prime the S protein (spike protein of SARS-CoV-2) (Citation12). SARS-CoV-2 infection experiments with primary human airway epithelial cells show cytopathic effects 96 h after virus first contact (Citation17). The air epithelia line up the conduction airways and maintain air flow in and out of the alveoli. Furthermore, these cells also, regulate the innate and adaptive immune system against airborne pathogens through the combined function of ciliated and secretory cells. Accordingly, the SARS-CoV-2 virus is shown to infect and replicate in primary human airway epithelial cultures isolated from the nasal and tracheobronchial regions (Citation3). Furthermore, organotypic culture of well-differentiated human airway epithelial (HAE) cells have been successfully used to isolate SARS-CoV-2, since they support virus replication compared to standard immortalized cells (Citation3). Therefore, HAE cell can be used as an in vitro physiological model of human lung to investigate the morphogenesis and pathogenesis of SARS-CoV-2 (Citation18). Pharmacological agents may be directed specifically against a viral or cellular target element or may aim to nonspecifically alleviate the symptomatic burden associated with COVID-19. Overall, there is an urgent need for effective early COVID-19 therapies, with fewest side effects (Citation19). Basic research and clinical trials have suggested that certain treatments, including dietary supplements and phytochemicals, may have the potential to help fight coronavirus infection (Citation20). In addition, a recent report on the possible role played by SARS-CoV-2 in weakening mitochondrial functions suggests the need to consider these organelles as targets for specific adjuvant therapies (Citation21). In this context, the use of several antioxidants has been proposed to mitigate the prooxidant state (oxidative stress) in COVID-19.
Recent comprehensive reviews have focused on the roles and on the prospective potential clinical administration of alpha-lipoic acid (ALA) (Citation21) to reduce reactive oxygen species (ROS) production. Viral infection could induce ROS production, which plays an important role in virus replication and invasion (Citation22), organ damage (Citation23), and systemic inflammatory response. Several clinical studies have been conducted to support the use of ALA (Citation24,Citation25). The protective efficiency of ALA in critically ill COVID-19 patients could be explained by antioxidant and anti-inflammatory actions (Citation26). First, ALA could prevent virus-induced organ dysfunction by counteracting ROS, reduce plasma inflammatory cytokine levels and improve symptoms of some severe patients (such as acute coronary syndrome, etc.) (Citation26).
Drugs currently in use are designed to counteract the symptoms of infection and are based on anti-inflammatory and immunomodulatory effects, and on antiviral actions observed in in vitro studies (Citation27). For this reason, it could be useful to combine traditional drugs with an adjuvant treatment consisting of natural bioactive compounds such as the afore mentioned ALA or another bioactive substance such as palmitoylethanolamide (PEA) with known anti-inflammatory properties. PEA is considered a food supplement with immunomodulatory, anti-inflammatory, neuroprotective and pain-relieving properties (Citation28). The main ultra-micronized-PEA immunomodulatory effect seems to be related to mast cells (Citation29) which are typically involved in respiratory infections caused by coronavirus and/or other influenza viruses (Citation30). In Italy, a clinical study is currently in progress (approved by the Ethical Committee of Policlinico Tor Vergata Hospital, Rome, protocol number R.S. 73.20) to evaluate the possible beneficial physiological effects of um-PEA on inflammatory indices in asymptomatic and pauci-symptomatic COVID-19 patients (Citation31).
In this context, it seems a very promising strategy to investigate the effectiveness of ALA combined with PEA, in an in vitro lipopolysaccharide-stimulated (LPS) human epithelial pulmonary cell model which mimics the pathogen-associated molecular pattern and signaling seen in the “cytokine storm syndrome” (Citation32,Citation33). In LPS-induced acute lung injury (ALI) in vitro model, the positive effects on the main mechanisms involved in pulmonary epithelial cells injury of the combination before and after LPS-damage have been investigated to hypothesize a new human formulation with side effects-free therapy.
Methods
Pulmonary and macrophages cell culture
Primary Human Small Airway Epithelial Cells (HSAEC) purchased from American Type Culture Collection (Manassas, VA, USA), are patho-physiologically relevant for respiratory diseases affecting the distal airways/lung including COVID-19 since they are positive to ACE2 and KRT5 receptors (Citation34). The cells were cultured in small airway epithelial basal medium (PromoCell, Heidelberg, Germany) with Supplement Mix (PromoCell, Heidelberg, Germania) containing Epidermal Growth Factor (recombinant human) 10 ng/ml, Insulin (recombinant human) 5 µg/ml, Hydrocortisone 0.5 µg/ml, Epinephrine 0.5 µg/ml, Triiodo-L-thyronine 6.7 ng/ml, Transferrin (recombinant human) 10 µg/ml, Retinoic Acid 0.1 ng/ml, Bovine Serum Albumin-Fatty Acid Free (BSA-FAF) 2.5 mg/ml and 1% penicillin/streptomycin (P/S; 10,000 U/ml, Gibco Life technologies, USA) in incubator at 37 °C, 5% CO2.
Human monocytic THP-1 cells were maintained in culture in Roswell Park Memorial Institute medium (RPMI, Invitrogen, USA) culture medium containing 10% of heat inactivated fetal bovine serum (Invitrogen, USA) and supplemented with 10 mM Hepes (Gibco, Life technologies, USA), 1 mM pyruvate (Gibco, Life technologies, USA), 2.5 g/l D-glucose (Merck, Germany) and 50 pM ß-mercaptoethanol (Gibco; Life technologies, USA). THP-1 monocytes were differentiated to macrophages-like cells with 7.5 ng/ml of phorbol 12-myristate 13-acetate (PMA; Sigma-Aldrich, Milan, Italy) incubated for 48 h. Differentiated THP-1 cells were trypsinized and used in the experiments (Citation35).
Experimental protocol
Both cell types were used to investigate the effects of ALA and PEA, either alone or in combination to support the cellular mechanisms to prevent fibrosis. In addition, the role of inflammatory conditions mimicking COVID-19 syndrome caused by LPS as a side effect was investigated in presence of these substances tested alone or in combination to prevent or restore the damage. In the first set of experiments, on both HSAEC and THP-1 cells, the role of PEA and ALA under physiological condition was analyzed in a dose-dependent manner. In this step, cell viability test was performed with ALA (from 5 µM to 500 µM) (Citation36) and PEA (from 1 µM to 10 µM) (Citation28) to determine their optimal concentrations for 24 h, and these concentrations (ALA 50 µM and PEA 5 µM) were maintained in all successive experiments. The combination with ALA and PEA was investigated during 24 h to analyze cell viability without cytotoxicity on both cell types. In this part of the study a co-culture system was performed to analyze the effects of ALA and PEA, alone or in combination, using an in vitro model of the human air-blood barrier (Citation35). In a second set of experiment, the role of inflammatory network was investigated by pretreatment for 24 h with 1 µg/ml LPS, to mimic COVID-19 infection (Citation37). Particularly, the ability of ALA and PEA alone or in combination to restore COVID-19 infection was investigated by analyzing the cytokines panel, as IL-1b (Citation38), IL-6 (Citation39), IL-10 (Citation40) and TNFα (Citation6), involved during COVID-19 infection. Moreover, the evaluation of inflammation receptors, such as TLR4 (Citation41), and analysis of the principal molecular mechanisms involved during COVID-19 inflammation were performed at 24 h.
To evaluate the prophylactic effects of ALA and PEA against COVID-19 infection, a third set of experiments co-culture system was first treated with ALA and PEA, alone or in combination and later with LPS 1 µg/ml for 24 h (Citation33,Citation35). All test samples were analyzed for cytokine panel, TLR4 activation and the molecular mechanisms involved.
Co-culture model
For co-culture experiments, HSAEC cells were grown on collagen coated Transwell inserts under perfused conditions. Subsequently macrophages were allowed to adhere to the apical surface of the insert. In details HSAEC were trypsinized and seeded onto collagen (Corning collagen I, Rat tail)-coated 0.33 cm2 porous (0.4 µm) polyester membrane inserts with a seeding density of 1 × 105 cells per Transwell (Costar, Corning) (Citation42). The cells were grown to near confluence in submerged culture for 2–3 days and then differentiated by air-liquid interface for 21–28 days (Citation43). THP-1 macrophages (at passages 43 to 50) cells were seeded in 6-well plates at a density of 105 cells/well in RPMI 1640 medium with GlutaMAX supplemented with 10% FBS. Monocytes were differentiated into macrophages using phorbol-12-myristate-12-acetate (PMA; Cayman Chemicals) at a concentration of 20 ng ml−1 for 24 h. After this time, THP-1 was seeded to achieve a final ratio of 3:1 following what is required for alveolar epithelial cells (Citation35). The co-culture was cultivated for 24 h at 37 °C, 5% CO2 and then stimulated (Citation35,Citation44).
MTT test
MTT-based In Vitro Toxicology Assay Kit (Sigma-Aldrich, Milan, Italy) was performed on a 96-well plate to determine cell viability after each stimulation on monolayer culture, following a standard technique (Citation35,Citation36). Briefly, purple formazan crystals obtained from cells incubated with 1% MTT dye for 2 h at 37 °C in an incubator, 5% CO2, and 95% humidity, were solubilized in equal volume of MTT Solubilization Solution and measured by spectrometer (VICTOR X4, multilabel plate reader) at 570 nm with correction at 690 nm. Results were expressed as means (%) compared to control (100% viable cells).
ROS production and measurement
After 24 h of stimulation with ALA and PEA on the coculture system, cytochrome C (Sigma-Aldrich) was added to each sample for 30 min in an incubator at 37 °C with 5% CO2. At the end of stimulations, 100 µL of supernatant was measured at 550 nm using a spectrometer (VICTOR X4, multilabel plate reader). Oxygen (O2) was expressed as the mean ± SD (%) of nanomoles per reduced cytochrome C per micrograms of protein compared to control (Citation45).
NO detection
After treatment, supernatants obtained from the co-culture system were used to detect NO production using Griess assay (Promega) according to the manufacture instruction. Briefly, supernatant was mixed with equal volumes of Griess reagent and incubated in the dark, at room temperature for 10 min. The absorbance was measured using VICTORX4 at 570 nm. NO production was quantified by comparing the samples to a standard curve, expressed as a percentage (Citation46).
IL-1β assay
Co-culture basolateral medium was collected for IL-1β quantification with IL-1β ELISA kit (R&D systems, MN) according to the manufacturer’s instructions. In brief, IL-1β-containing culture medium was added to a microplate strip, 100 μl/well, incubated for 2 h at room temperature, mixed with IL-1β conjugate, and then incubated for another 2 h at room temperature. Thorough washes were performed between and after the two incubations. 100 μl of substrate solution was applied to generate chemiluminescence. Chemiluminescent absorbance was determined using a microplate reader at 450 nm with correction at 570 nm. The IL-1β was quantified by relating the sample readings to the generated standard curve (Citation47).
IL-6 assay
IL-6 concentration in basolateral co-culture media was analyzed with IL-6 ELISA kit (eBioscience, USA). Briefly, 40 µl of the sample were added to the plate and then 10 µl of the biotin-labeled antibody were added. The plate was sealed and incubated at 37 °C for 30 min. The plate was washed and 50 µl enzyme-labeled solution was added to each well; the plate was sealed again and incubated at 37 °C for 60 min. The plate was washed again for five times, and a total of 100 µl/well horseradish peroxidase (HRP) marker was added before sealing the plate and incubated in the dark under 37 °C for 15 min. Another 100 µl/well chromogenic substrate TMB was added, and incubated in the dark at room temperature for 20 min. Finally, a 50 µl/well stop buffer was added, and the maximum absorption wavelength of 450 nm was measured (Citation48).
IL-8 assay
The amount of IL-8 on the basolateral co-culture media was determined using the human IL-8 (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions. Briefly, 100 μl/well were incubated for 2 h at room temperature and, after washing, mixed with IL-8 conjugate. The plate was incubated for another 2 h at room temperature. After washing 100 μl of substrate solution was applied to generate chemiluminescence. Optical density was read by using a microplate reader at 450 nm and corrected with 540 nm. The amounts of IL-8 (pg/mL) were calculated from a standard curve (Citation49).
TNF-α assay
TNFα concentration on the basolateral environment was determined using the TNF-α ELISA kit (Sigma, Milan, Italy) according to the manufacturer’s instructions. Briefly, 100 μL of samples were added to each well of a 96-well ELISA plate. The plate was incubated at room temperature for 2 h followed by overnight incubation at 4 °C. At the end of the incubation periods, wells were washed five times with a washing buffer and 100 μL of biotinylated anti-TNFα was added into each well. After 2 h incubation at room temperature, the solution in each well was aspirated and the wells were washed five times. Then, 100 μL Streptavidin-HRP was added to each well and incubated at room temperature for 1 h. After washing the plate from Streptavidin-HRP solution, 100 μL of chromogen solution was added to each well and incubated for 30 min at room temperature and in the dark. The absorbance of each well was measured after the addition of stop solution at 450 nm using a plate reader (VICTORX4 multilabel plate reader) (Citation50).
Western blot
Cell layers were washed twice with sterile PBS, scraped off the Transwell inserts using sterile cell scrapers (20 mm wide; Bedalab) (Citation51) suspended in Complete Tablet buffer (Roche, Basel, Swiss) supplemented with 1 mM phenylmethanesulfonyl fluoride (PMSF; Sigma-Aldrich, Milan, Italy), 1:100 mix Protease Inhibitor Cocktail (Sigma-Aldrich, Milan, Italy) and 2 mM sodium orthovanadate. Subsequently, the protein concentration was measured with a BCA Protein Assay. From each lysate, 35 μg proteins were resolved into 8% and 15% SDS-PAGE gels, and polyvinylidene difluoride (PVDF) membranes (GE Healthcare Europe GmbH, Milan, Italy) were incubated overnight at 4 °C with specific primary antibody: anti-TLR4 (1:250, Santa Cruz, CA, USA), and the protein expression was normalized and verified through β-actin detection (1:5000; Sigma-Aldrich, Milan, Italy) and expressed as a mean ± SD (% vs. control).
Statistical analysis
At least four independent experiments were run for each experimental protocol; the results are expressed as means ± SD of independent experiments performed on four technical replicates using One-way ANOVA followed by Bonferroni post hoc test for statistical analysis and expressed as percentage (%) vs control (untreated sample). p values < 0.05 were considered statistically significant.
Results
Dose response study on HSAEC and TPH1 cells
To exclude cytotoxicity and choose a better concentration of ALA (ranging from 5 µM to 500 µM) and PEA (ranging from 1 µM to10 µM), in the first set of experiments a dose response study was carried out by analyzing cell viability by MTT test at 24 h of stimulation. As reported in on both HSAEC and TPH1 cells all concentrations of ALA and PEA could induce a significant increase in cell viability compared to control (p < 0.05), indicating a mitochondrial balance, and excluding negative effects caused by dosage used. In , main effects (p < 0.05) were observed using 50 µM ALA compared to control (about 22.33% on HSAEC and about 30% on THP1 cells, respectively) and to other concentrations tested (p < 0.05) on both cell types. In addition, 5 µM PEA was able to induce a greater effect (p < 0.05) compared to control (about 14.7% on HSAEC and about 25% on THP1 cells, respectively) and to other concentrations tested on both cell types (p < 0.05). 50 µM ALA and 5 µM PEA were chosen and maintained on all subsequent experiments both alone and combined.
Figure 1. Cell viability on HSAEC and TPH1 cells. In (a) and (b) dose-response study on HSAEC and TPH1 cells, respectively, for ALA (5 µM–500 µM) and PEA (1 µM–10 µM). In (c) and (d), the effects of the combination of 50 µM ALA and 5 µM PEA alone and combined (ALA50 µM + 5 µMPEA) on HSAEC and TPH1 cells, respectively. The results are expressed as means ± SD (%) vs control (0% line) of 4 independent experiments each performed in triplicate. * p < 0.05 vs control (baseline); the bars p < 0.05 between different stimulations.
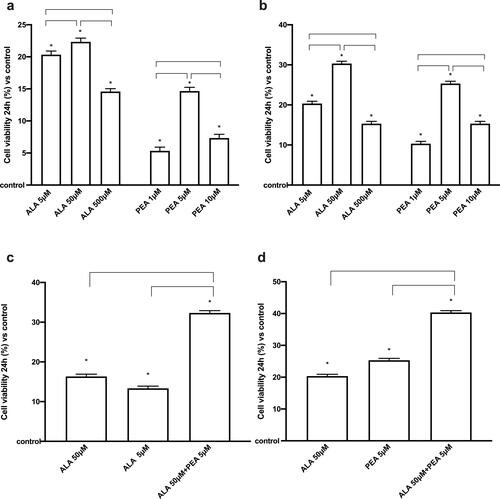
Indeed, the importance of the 50 µM ALA + 5 µM PEA combination was also investigated to minimize the risk of adverse effects of the individual components. As reported in , the analysis of cell viability on both cells showed similar effects; the combined effect of 50 µM ALA+ 5 µM PEA was able to induce an increase (p < 0.05) compared to 50 µM ALA (about two times on HSAEC and 1.9 times on THP1 cells, respectively) and 5 µM PEA (about 2.Four times on HSAEC and 1.Six times on THP1 cells, respectively) alone. These preliminary data support the possible beneficial effects of the combination of ALA + PEA which amplifies the effects of the individual components on mitochondrial balance.
Co-culture analysis for combination effects of ALA + PEA
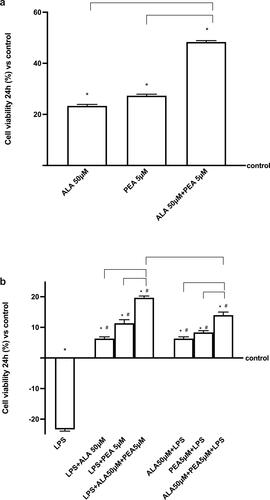
In the second set of experiments, the co-culture system (composed by HSAEC and THP1 cells) was treated with 50 µM ALA and 5 µM PEA alone and combined (50 µM ALA+ 5 µM PEA) to verify the effectiveness of the combination and to exclude any adverse effects. As reported in , all substance alone and combined can induce a significant increase on cell viability compared to control (p < 0.05) and main effects were observed in presence of 50 µM ALA+ 5 µM PEA compared to 50 µM ALA (about 50%; p < 0.05) and 5 µM PEA alone (about 70%; p < 0.05). These data support the beneficial effects of the substances as hypothesized. Since the main topic application of this combination may be before or after COVID injury, successive experiments were carried out on air-liquid interface condition under 1 µg/ml LPS stimulations (ALI condition). As illustrated in , 1 µg/ml LPS confirmed its negative consequence decreasing cell viability (about 23%) compared to control (p < 0.05) when used alone. The post-stimulation with 50 µM ALA and 5 µM PEA added alone, were able to revert the negative effects inducing cell viability which increased compared to control (p < 0.05; about 6% with 50 µM ALA and about 11% with 5 µM PEA alone, respectively). The co-stimulation with 50 µM ALA + 5 µM PEA confirmed its positive effects reverting the negative consequence of LPS better than ALA and PEA alone (about three times vs ALA and about 1.7 times vs PEA), supporting the hypothesis of a synergistic effect of the combination. To explore a possible role to prevent the damage caused by LPS mimic COVID-19 conditions, additional experiments were carried out analyzing the effects of the same agents added before the damage induced by LPS. As reported in , both 50 µM ALA and 5 µM PEA alone can prevent LPS-injury compared to control (p < 0.05) and compared to LPS alone (p < 0.05). In addition, the pretreatment with the combination 50 µM ALA+ 5 µM PEA increased the preventive capacity on damage induced by LPS compared to ALA alone (p < 0.05), supporting the importance of the combined antioxidant and anti-inflammatory effect on the prevention of COVID syndrome in vitro. Finally, between the combined effects observed before or after LPS-injury, the main effects are observed on post-stimulation (p < 0.05; about 40%), confirming the importance of the biological properties of substances to restore the injury.
Figure 2. Cell viability measured in a co-culture system. In (a), the effects of 50 µM ALA and 5 µM PEA alone and combined (ALA 50 µM+ 5 µM PEA) and in (b) the same agents added after 1 µg/ml LPS and before 1 µg/ml LPS administration. The results are expressed as means ± SD (%) vs control (0% line) of 4 independent experiments each performed in triplicate. * p < 0.05 vs control (baseline); # p < 0.05 vs LPS; the bars p < 0.05 between different stimulations.
Analysis of oxidative stress under LPS conditions
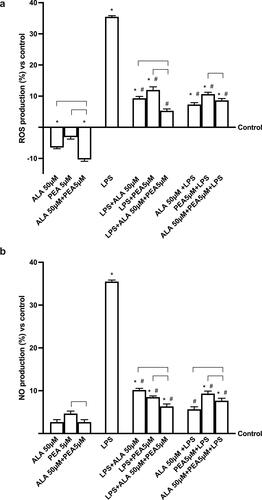
Since two important aspects as ROS production and inflammatory patterns are involved in pulmonary damage, additional experiments were carried out to analyze ROS production under LPS stimulation before and after the administration of ALA and PEA alone or in combination. As shown in , 50 µM ALA and 5 µM PEA alone confirmed their antioxidant properties compared to control and in combination (50 µM ALA + 5 µM PEA) maintained the ROS production under control values (p < 0.05). LPS (1 µg/ml) exerts negative effects improving the ROS production compared to control (p < 0.05) which is significantly reduced by the post-stimulation with 50 µM ALA and 5 µM PEA alone (about 3.Six times and about 2.9 times respectively; p < 0.05) and prevented by the pre-stimulation with 50 µM ALA and 5 µM PEA alone (about 4.8 times and about 3.Three times respectively; p < 0.05). The combination of 50 µM ALA + 5 µM PEA both post and pre-administered may improve the antioxidant effects (p < 0.05), but the post-stimulation is more effectiveness than the pre-stimulation (about 62%; p < 0.05). In addition, NO production was observed by the Griess test. As reported in , 1 µg/ml of LPS increases NO production compared to the control (p < 0.05), supporting the hypothesis of cell loss previously observed via the apoptotic pathway. The noxious action of LPS was counteracted by both pre- and post-stimulation with combined 50 µM ALA + 5 µM PEA (p < 0.05). However, the post-stimulation effect was found to be significantly more effective in reducing NO production (p < 0.05) than pretreatment (approximately 21%). All these data confirm the importance of limiting oxidative stress, which we know to be important in cytokine storm syndrome, and the ability of ALA and PEA to induce beneficial effects.
Figure 3. Analysis of oxidative stress. In (a) ROS production and in (b) NO production is measured on a co-culture system in presence or absence of 1 µg/ml LPS. 50 µM ALA, 5 µM PEA alone and combined (50 µM ALA + 5 µM PEA) are added as post-stimulation or as pretreatment. The results are expressed as means ± SD (%) vs control (0% line) of 4 independent experiments each performed in triplicate. * p < 0.05 vs control (baseline); # p < 0.05 vs LPS; the bars p < 0.05 between different stimulations.
In vitro analysis of the main cytokines involved in “cytokine storm syndrome”
Air-liquid interface cultures are helpful because they preserve key features of the airway epithelium that viruses target, as well as an in vitro biologically in vitro model relevant for investigating the pathogenesis of SARS-CoV-2. Further experiments were conducted to explore, in a co-culture model subjected to conditions that simulate what happens in COVID-19, the influence of the combination 50 µM ALA + 5 µM PEA on different molecules involved in the "storm syndrome of cytokines." As reported in , 1 µg/ml of LPS can induce a significant increase in Il-β (Citation38,Citation52,Citation53). This negative condition is significantly reduced (p < 0.05) by post stimulation with 50 µM of ALA and 5 µM of PEA, individually (approximately 1.7 times and approximately 2.Six times, respectively), supporting the antioxidant/anti-inflammatory roles of these agents. Furthermore, the 50 µM ALA + 5 µM PEA combination amplifies these effects (p < 0.05) compared to ALA (about 2.97 times) and PEA (about 1.98 times) alone, indicating that the combination may be better than the single ones agents used. Similar data are observed when alone and combined agents are added prior to LPS damage. In particular, the main effect is observed in the presence of 50 µM ALA + 5 µM PEA (p < 0.05) compared to ALA (about 1.08 times) and PEA (about 1.62 times) alone. However, when analyzing the effects of the combination added before or after 1 µg/ml of LPS, a significant difference is observed (p < 0.05); in fact, post stimulation can induce a greater reduction in IL-β compared to pretreatment (about 50%).
Figure 4. Analysis of main cytokines involved in storm syndrome. In (a) IL-β, in (b) IL-6, in (c) TNFα and in (d) IL-10. The data are obtained by ELISA test in presence or absence of 1 µg/ml LPS. 50 µM ALA, 5 µM PEA alone and combined (50 µM ALA + 5 µM PEA) are added in post-stimulation or in pretreatment. The results are expressed as means ± SD (%) vs control (0% line) of 4 independent experiments each performed in triplicate. * p < 0.05 vs control (baseline); # p < 0.05 vs LPS; the bars p < 0.05 between different stimulations.
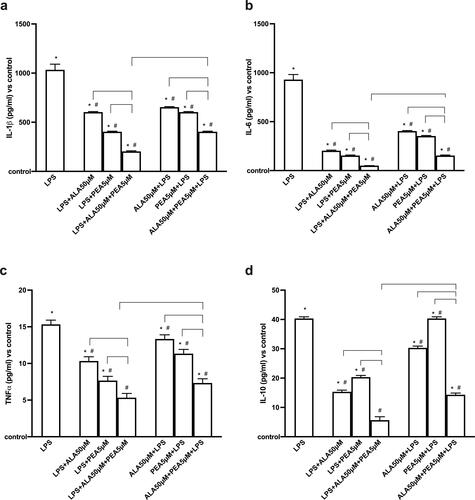
The activation of IL-1β by SARS-CoV-2 in turn activates IL-6 (Citation6) which its high expression in COVID-19 patients can accelerate the inflammatory process, contributing to the cytokine storm syndrome. As reported in , 1 µg/ml of LPS can induce a significant increase in IL-6 compared to the control (p < 0.05) which is reduced by post-stimulation with 50 µM ALA (approximately 4.Six times) and 5 µM PEA (about six times) alone. The main effect is observed after co-stimulation with 50 µM ALA + 5 µM PEA, which can significantly reduce IL-6 production (p < 0.05) compared to single agents (approximately four times vs ALA and approximately three times vs PEA alone, respectively). This supports the hypothesis of a synergistic effect represented by antioxidant and anti-inflammatory properties. Similarly, the data are reported in pretreatment conditions in which a main effect is obtained with 50 µM ALA + 5 µM PEA (p < 0.05) compared to ALA (about three times) and PEA (about 2.Three times) alone. Between post-stimulation and pre-stimulation with 50 µM ALA + 5 µM PEA in conditions of LPS damage, post-stimulation exerts a better effect (about 200%, p < 0.05).
A consequence of the presence of IL-1β and IL-6 is the activation of TNFα and its increase in the serum of COVID-19 patients correlates with a poor prognosis. As shown in , 1 µg/ml LPS induced a significant increase in TNFα level compared to control (p < 0.05). This is reduced by the post-stimulation with 50 µM ALA (about 53%) and 5 µM PEA (about 114%) alone. The co-stimulation with 50 µM ALA + 5 µM PEA can significantly boost up this reduction (p < 0.05) compared to the single agents (about two times vs ALA and about 1.Four times vs PEA alone, respectively) and this effect is higher (p < 0.05) than what obtained by the pre-stimulation with 50 µM ALA + 5 µM PEA (about 37%). In addition, the pre-stimulation with 50 µM ALA+ 5 µM PEA was confirmed to be able to reduce TNFα (p < 0.05) better than 50 µM ALA (about 1.1 times) and 5 µM PEA (about 1.8 times) alone.
Since a unique feature of the COVID-19-induced cytokine storm is the dramatic elevation of IL-10 (Citation40), this cytokine was also assessed in the same conditions reported before. As reported in , 1 µg/ml LPS can significantly increase (p < 0.05) IL-10 compared to the control confirming that the "cytokine storm syndrome" typical of COVID-19 has been fully reproduced. The post-stimulation (p < 0.05) with 50 µM ALA and 5 µM PEA alone reduced the IL-10 level compared to LPS (about 2.6 time and two times, respectively) and the main effect is obtained by the co-stimulation with 50 µM ALA+ 5 µM PEA which induced a better decrease (p < 0.05) compared to ALA and PEA alone (about 2.Five times and 3.Three times, respectively). Similarly, the pre-stimulation with 50 µM ALA+ 5 µM PEA is able to induce a greater reduction compared to ALA and PEA alone (about 2.1 times and 2.7 times, respectively). Furthermore, the main effects are obtained using 50 µM ALA and 5 µM PEA combined in post-stimulation (p < 0.05, about 64%), as observed for all other cytokines.
All these data support the beneficial role exerted by the antioxidant and anti-inflammatory properties of combined ALA and PEA to help and restore the cytokine balance reducing the conditions leading to the storm. It is therefore possible to hypothesize that, if this combination is used in the initial phase of the disease, which is equivalent to a pre-stimulation, it could be able to prevent the excessive production of cytokines.
Action on the intracellular mechanisms involved in inflammation
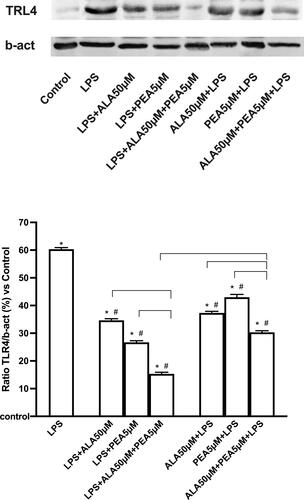
TLR4 modulates a wide range of inflammatory responses and has notably been reported to be a mediator of lung damage caused by COVID-19. The Western blot and densitometric analyzes reported in confirmed the ability to increase the expression of TRL4 after 1 µg/ml LPS confirming the presence of the damage. Post-stimulation with 50 µM ALA and 5 µM PEA alone reduced this increase (approximately 1.4-fold and 1.87-fold, respectively; p < 0.05) and this effect is better than that induced by the same agents used before of the damage (approximately 50% and 59%, respectively; p < 0.05). Co-stimulation with 50 µM ALA + 5 µM PEA added after LPS can amplify the reduction (p < 0.05) compared to ALA (about 57%) and PEA (about 44%) alone and this effect is better than when was added before LPS (p < 0.05, approximately 50%), supporting the cytokine data shown above.
Figure 5. Western blot and densitometric analysis of TRL4. Data are obtained after 50 µM ALA, 5 µM PEA alone and combined (50 µM ALA + 5 µM PEA) added as post-stimulation or as pretreatment in presence or absence of 1 µg/ml LPS. Densitometric analysis is expressed as means ± SD (%) of 4 independent experiments normalized and verified by β-actin detection. The images shown are an example of TRL4 from 4 independent experiments. * p < 0.05 vs control (baseline); # p < 0.05 vs LPS; the bars p < 0.05 between different stimulations.
Discussion
In recent years, the scientific community has focused its attention on the use of phytochemicals/natural products and nutraceuticals for the treatment of various diseases including infectious diseases (Citation54). Underlying this interest is the ability of various natural products and herbal remedies against some important viral pathogens, including RSV, measles virus, dengue virus, influenza virus, human immunodeficiency virus (HIV), HSV, HCV, hepatitis B virus, enterovirus 71, coxsackievirus and coronavirus (Citation55). Therefore, today there are numerous food supplements containing natural ingredients, with the main aim of modulating or improving the functions of the immune system. These easy-to-use and low-cost remedies play an important role as an adjunct approach in the fight against infectious diseases, including COVID-19. They are also effective in counteracting acute respiratory complications (Citation56). As regards COVID-19, the inflammatory response of the airways due to the excessive activity of immune cells can induce oxidative stress and tissue damage through the production of ROS induced by inflammatory cytokines (Citation57). Acute pathological conditions display well-established links with oxidative stress (Citation21) and the use of pharmacological and immunological tools, as adjuvant agents for mitigating the inflammatory conditions in relevant therapeutic strategies (Citation21). Several in vitro and in vivo studies and clinical trials have recently explored the possibility of using nutraceuticals to support COVID therapy, since they are useful in mitigating the manifestations of COVID-19 by acting as immune boosters (Citation58). For example, to mitigate the prooxidant state typical of COVID-19, the use of several antioxidants has been proposed, such as: vitamin C (Citation59), vitamin D (Citation60), resveratrol (Citation61), and lipoic acid (ALA) (Citation62). ALA is a potent antioxidant able to reduce oxidative stress inhibiting nuclear factor-kappa B (NF-kB) and the subsequent cytokine release (Citation62). Furthermore, it is known to have antiviral effects by relieving redox reactions and increasing the level of intracellular glutathione which leads to a strengthening of the human host defense even against COVID-19 (Citation63). These results were obtained from several clinical studies which observed that, after 30 days of follow-up, the mortality rate was two times lower in the ALA group than in the placebo group (Citation64). ALA appears to work by reducing viral load (Citation62), preventing upregulation of the ACE2 receptor and increasing intracellular pH and antioxidant levels, thus leading to a decrease in oxidative stress (Citation62). These data demonstrates that ALA may strengthen the human host defense against SARS-CoV-2 playing a crucial role in the treatment of patients with critically ill COVID-19.
Currently, new strategies can act to counteract the symptoms of infection, reduce oxidative stress, and improve anti-inflammatory and immunomodulatory functions; in this context PEA is very interesting, which is an amide of palmitic acid with known anti-inflammatory properties (Citation31). The main reason for using PEA is related to its ability to mitigate "cytokine storm syndrome," which is a critical consequence of COVID-19 infection. In general, patients experience uncontrolled inflammation with overproduction of cytokines on the plasma sample followed by edema, gas exchange dysfunction, acute respiratory syndrome, acute heart injury, and secondary infection (Citation6). In the inflammatory process triggered by coronavirus infection, the Toll-Like four receptor pathway (TLR4) plays an important role, generating numerous proinflammatory cytokines such as IL-1, IL-6 and TNFα; however, anti-inflammatory cytokines such as IL-10 also participate in the development of the clinical picture (Citation41).
In this scenario, PEA can explain its effects, which depend on its ability to act as a mast cell stabilizer (Citation65). PEA has multitarget action related to several signaling pathways, which include TLR, nitric oxide, IL-6 and IL-1b by binding TLRs (Citation66).
Several clinical studies available in the literature have confirmed these mechanisms. For example, the Phase two clinical trial sponsored by the FDA that evaluated the effects of ultra-micronized PEA on hospitalized COVID-19 patients: the clinical study underway in Italy (Policlinico Tor Vergata, Rome) with the aim of evaluating the possible beneficial physiological effects of ultra-micronized PEA on inflammatory indices in COVID-19 patients (Citation31). Although clinical studies have presented significant data, more information on biological mechanisms is needed. In this context, in vitro cell models mimicking the typical alterations of COVID-19 have been developed to study this disease. Basic research on SARS-CoV-2 is essential to support the rapid development of new therapeutic strategies. An in vitro cell model is important for the preclinical evaluation of molecules with therapeutic potential, and therefore it is essential to choose a correct cell model to preserve the complex morphological and functional characteristics of human airways in vivo. In humans, airway epithelial cells highly express the putative SARS-CoV-2 entry receptor, angiotensin converting enzyme 2 (ACE2) and transmembrane serine proteinase 2 (TMPRSS2), the host enzyme virus uses to cleave protein S (SARS-CoV-2 spike protein) (Citation12). Air-liquid interface cultures are advantageous because they preserve key characteristics of the in vivo airway epithelium targeted by viruses (Citation3). In these cells, LPS-stimulation mimics the pathogen-associated molecular pattern (PAMP) and DAMP signaling seen in the end stage of disease where excessive viral loads and tissue damage trigger excessive immune response (Citation32). Based on this information, the study explores for the first time a possible synergistic effect of an antioxidant molecule, ALA, and an anti-inflammatory, PEA, to reduce the molecular effects that characterize the cytokine storm syndrome typical of COVID-19. An in vitro model with LPS-stimulated HSAC and TPH1 cells was used. In this experimental model, our results demonstrate that the combination of ALA and PEA (50 µM + 5 µM, respectively) exerts an effect of increasing cell viability, indicating a new possible strategy to prevent and/or restore damage resulting from SARS-CoV-2 infection. Furthermore, our experiments have shown that the combination of 50 µM ALA + 5 µM PEA is able to reduce ROS and NO levels both when administered before and after induction of damage by LPS. The best effect was observed when the active ingredients were administered after LPS, supporting the ability of ALA and PEA to modulate oxidative stress, which is a key point in the pathogenesis of cytokine storm syndrome. Since the massive production of inflammatory cytokines is a critical consequence of COVID-19, further experiments have been carried out on ALI cultures on air-liquid interface. Further analyzes were carried out to explore the influence of the 50 µM ALA + 5 µM PEA combination on a co-culture system under conditions equivalent to those predicted in COVID-19. Our results demonstrated that the immunological reaction triggered by the LPS-dependent lesion mobilizes the main cytokines involved during COVID-19 but 50 µM of ALA + 5 µM of PEA can attenuate its levels, with better effects when used as therapy, i.e. after appearance of the damage. These data support the beneficial role exerted by the antioxidant and anti-inflammatory properties of ALA and PEA combined in promoting and restoring the balance of cytokines. Finally, in our in vitro experimental model, we found that reproducing the typical conditions of COVID-19 not only triggers the activation of the oxidative stress mechanism, but also induces the upregulation of TLR4. Indeed, our data revealed that 50 µM ALA + 5 µM PEA can directly control cytokine production by modulating the severity of TLR4 expression-dependent damage. Thus, the acute onset of proinflammatory immune responses and severe COVID-19 infection, caused by the LPS-related injury, depends on the activation of the oxidative stress mechanism that couples with innate immunity, but 50 µM ALA + 5 µM PEA may restore even this condition if used preventively.
Conclusions
In conclusion, this work supports for the first time the possibility of 50 µM ALA + 5 µM PEA to become a new therapeutic strategy to treat COVID-19 by restoring the cascade activation of the immune response and acting as a powerful antioxidant, thanks also to the synergistic effect exerted by the two combined substances. All these results confirm for the first time the ability of ALA to act in synergistical manner with PEA in a new combination of 50 µM ALA + 5 µM PEA modulating the pathways involved in the damage observed in COVID-19. Therefore, this new formulation could be used to protect patients from the development of the serious consequences observed in this disease.
Authors’ contributions
Conceptualization, FU; methodology, SR and RG; formal analysis, SR, RG and MF; investigation, FU and CM; writing—original draft preparation, MF and SR; supervision, FU; project administration, FU and CM; funding acquisition, FU and CM. All authors have read and agreed to the published version of the manuscript.
About the authors
Francesca Uberti, Researcher in Physiology on “Aging Project” funding at the University of Eastern Piedmont (Italy). During her scientific activity she has dealt with the extraskeletal effects of vitamin D, as well as with mechanisms related to degenerative processes also in the field of neurophysiology. He is also co-founder of the university spin-off noiVita s.r.l.s., and co-owner of patents in the field of natural extracts suitable for the preparation of food supplements. Member of the Italian Society of Physiology and of the American Physiological Society. Editor of numerous peer reviewed scientific journals. He is a member of the Governance group of the AGING project.
Sara Ruga, Second year PhD student in “Food, Health and factor promoting longevity” in the translational medicine department of the Eastern Piedmont University (Italy). His scientific activity focuses on Aging and the mechanisms involving metabolic dysregulation and hormones related to aging; in particular, the line of research focuses on biological mechanisms that act directly on muscle and bone. In addition, he is co-author of numerous articles in the nutraceutical field.
Mahitab Farghali, Third year PhD student in “Biological and Biotechnological Sciences” in the translational medicine department of the University of Eastern Piedmont (Italy). During her scientific activity she has been involved in neuroscience at the University of Alexandria in Egypt. His research topics currently focus on the study of targeted antioxidants to protect brain aging. He is also co-author of numerous articles in the nutraceutical field.
Rebecca Galla, Master’s Degree in Medical Biotechnology at the University of Eastern Piedmont (Italy). Winner of the “Fondazione Goria e Fondazione CRT, Master dei talenti della Società Civile 2020” in which she is carrying out in-depth studies on the problems related to hypolactasia. In addition, he is co-author of articles in the nutraceutical field.
Claudio Molinari, Associate Professor of Physiology at the University of Eastern Piedmont (Italy). Over the years he has also dealt with regulatory neurophysiology, heart rate variability and extraskeletal effects of vitamin D. He is co-founder of the university spin-off noiVita srls, and co-owner of patents in the field of natural extracts suitable for the preparation of food supplements. Member of numerous scientific societies of Physiology, Italian and foreign. Member of the Italian Sports Medicine Federation. Member of the Piedmont Region Commission for Complementary Medicines. It has an intense activity of public engagement and scientific dissemination. He is a member of the Governance group of the Aging Project and of the editorial board of the site.
| Abbreviations: | ||
| M-CSF | = | macrophage colony-stimulating factor |
| G-CSF | = | granulocyte colony-stimulating factor |
| GM-CSF | = | granulocyte-macrophage colony-stimulating factor |
| TNF-α | = | tumor necrosis factor |
| ALI | = | acute lung injury |
| HAE | = | human airway epithelial cells |
| ALA | = | α-lipoic acid |
| ROS | = | reactive oxygen species |
| PEA | = | palmitoylethanolamide |
| LPS | = | lipopolysaccharide-stimulated |
Acknowledgements
Authors thank Mariangela Fortunato for reviewing the language. Authors also thank Laborest spa which donated the substances.
Declaration of interest
The authors declare that they have no competing interests. FU and CM are co-founder of noivita srls start up at University of Eastern Piedmont.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–2. doi:10.1016/S2213-2600(20)30076-X.
- Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, Ren R, Leung KSM, Lau EHY, Wong JY, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382(13):1199–207. doi:10.1056/NEJMoa2001316.
- Zhu N, Wang W, Liu Z, Liang C, Wang W, Ye F, Huang B, Zhao L, Wang H, Zhou W, et al. Morphogenesis and cytopathic effect of SARS-CoV-2 infection in human airway epithelial cells. Nat Commun. 2020;11(1):3910. doi:10.1038/s41467-020-17796-z.
- Mason RJ. Pathogenesis of COVID-19 from a cell biology perspective. Eur Respir J. 2020;55(4):2000607. http:// doi:10.1183/13993003.00607-2020.
- Ahmadpoor P, Rostaing L. Why the immune system fails to mount an adaptive immune response to a COVID-19 infection. Transpl Int. 2020;33(7):824–5. doi:10.1111/tri.13611.
- Costela-Ruiz VJ, Illescas-Montes R, Puerta-Puerta JM, Ruiz C, Melguizo-Rodríguez L. SARS-CoV-2 infection: the role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020;54:62–75. doi:10.1016/j.cytogfr.2020.06.001.
- Wang W, Liu X, Wu S, Chen S, Li Y, Nong L, Lie P, Huang L, Cheng L, Lin Y, et al. Definition and risks of cytokine release syndrome in 11 critically ill COVID-19 patients with pneumonia: analysis of disease characteristics. J Infect Dis. 2020;222(9):1444–51. doi:10.1101/2020.02.26. 20026989.
- Liu Y, Zhang C, Huang F, Yang Y, Wang F, Yuan J, Zhang Z, Qin Y, Li X, Zhao D, et al. Novel coronavirus (2019-nCoV) infections trigger an exaggerated cytokine response aggravating lung injury. 2020. http://www.chinaxiv.org/abs/202002.00018.
- Chen C, Zhang XR, Ju ZY, He WF. [Advances in the research of mechanism and related immunotherapy on the cytokine storm induced by coronavirus disease 2019]. Zhonghua Shao Shang Za Zhi. 2020;36(6):471–5. Chinese. doi:10.3760/cma.j.cn501120-20200224-00088.
- Blanco-Melo D, Nilsson-Payant BE, Liu W-C, Uhl S, Hoagland D, Møller R, Jordan TX, Oishi K, Panis M, Sachs D, et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181(5):1036–45. doi:10.1016/j.cell.2020.04.026.
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–42. doi:10.1001/jama.2020.2648.
- Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu N-H, Nitsche A, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–80.e8. doi:10.1016/j.cell.2020.02.052.
- Chua RL, Lukassen S, Trump S, Henning BP, WEndis D, Pott F, Debnath O, Thurmann, Kurth F, Kazmierski J, et al. Cross-talk between the airway epithelium and activated immune cells defines severity in COVID-19. medRxiv. 2020. doi:10.1101/2020.04.29.20084327.
- Jeffers SA, Tusell SM, Gillim-Ross L, Hemmila EM, Achenbach JE, Babcock GJ, Thomas WD, Thackray LB, Young MD, Mason RJ, et al. CD209L (L-SIGN) is a receptor for severe acute respiratory syndrome coronavirus. Proc Natl Acad Sci USA. 2004;101(44):15748–53. doi:10.1073/pnas.0403812101.
- Takayama K. In vitro and animal models for SARS-CoV-2 research. Trends Pharmacol Sci. 2020;41(8):513–7. doi:10.1016/j.tips.2020.05.005.
- Zhou J, Alvarez-Elizondo MB, Botvinick E, George SC. Local small airway epithelial injury induces global smooth muscle contraction and airway constriction. J Appl Physiol. 2012;112(4):627–37. doi:10.1152/japplphysiol.00739.2011.
- Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, et al. China novel coronavirus investigating and research team. A novel coronavirus from patients with pneumonia in China. N Engl J Med. 2020;382(8):727–33. 2020doi:10.1056/NEJMoa2001017.
- Sungnak W, Huang N, Bécavin C, Berg M, Queen R, Litvinukova M, Talavera-López C, Maatz H, Reichart D, Sampaziotis F, et al. HCA lung biological network. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26(5):681–7. doi:10.1038/s41591-020-0868-6.
- De Pellegrin ML, Rohrhofer A, Schuster P, Schmidt B, Peterburs P, Gessner A. The potential of herbal extracts to inhibit SARS-CoV-2: a pilot study. Clin Phytosci. 2021;7(1):29. doi:10.1186/s40816-021-00264-6.
- Mrityunjaya M, Pavithra V, Neelam R, Janhavi P, Halami P M Ravindra P V., Immune-boosting, antioxidant and anti-inflammatory food supplements targeting pathogenesis of COVID-19. Front Immunol. 2020;11(570122).
- Pagano G, Manfredi C, Pallardó FV, Lyakhovich A, Tiano L, Trifuoggi M. Potential roles of mitochondrial cofactors in the adjuvant mitigation of proinflammatory acute infections, as in the case of sepsis and COVID-19 pneumonia. Inflamm Res. 2021;70(2):159–70. doi:10.1007/s00011-020-01423-0.
- Lin X, Wang R, Zou W, Sun X, Liu X, Zhao L, Wang S, Jin M. The influenza virus H5N1 infection can induce ROS production for viral replication and host cell death in A549 cells modulated by human Cu/Zn superoxide dismutase (SOD1) overexpression. Viruses. 2016;8(1):13. doi:10.3390/v8010013.
- Vlahos R, Stambas J, Selemidis S. Suppressing production of reactive oxygen species (ROS) for influenza A virus therapy. Trends Pharmacol Sci. 2012;33(1):3–8. doi:10.1016/j.tips.2011.09.001.
- Su R, Wang H, Xiao C, Tao Y, Li M, Chen Z. Venetoclax nanomedicine alleviates acute lung injury via increasing neutrophil apoptosis. Biomater Sci. 2021;9(13):4746–54. doi:10.1039/d1bm00481f.
- McCarty MF, DiNicolantonio JJ, Lerner A. Review - Nutraceuticals can target asthmatic bronchoconstriction: NADPH oxidase-dependent oxidative stress, RhoA and calcium dynamics. J Asthma Allergy. 2021;14:685–701. doi:10.2147/JAA.S307549.
- Li R-J, Ji W-Q, Pang J-J, Wang J-L, Chen Y-G, Zhang Y. Alpha-lipoic acid ameliorates oxidative stress by increasing aldehyde dehydrogenase-2 activity in patients with acute coronary syndrome. Tohoku J Exp Med. 2013;229(1):45–51. doi:10.1620/tjem.229.45.
- Esakandari H, Nabi-Afjadi M, Fakkari-Afjadi J, Farahmandian N, Miresmaeili S-M, Bahreini E. A comprehensive review of COVID-19 characteristics. Biol Proced Online. 2020;22:19. doi:10.1186/s12575-020-00128-2.
- Morsanuto V, Galla R, Molinari C, Uberti F. A new palmitoylethanolamide form combined with antioxidant molecules to improve its effectivess on neuronal aging. Brain Sci. 2020;10(7):457. doi:10.3390/brainsci10070457.
- Fusco R, Cordaro M, Genovese T, Impellizzeri D, Siracusa R, Gugliandolo E, Peritore AF, D’Amico R, Crupi R, Cuzzocrea S, et al. Adelmidrol: a new promising antioxidant and anti-inflammatory therapeutic tool in pulmonary fibrosis. Antioxidants (Basel). 2020;9(7):601. doi:10.3390/antiox9070601.
- Gralinski LE, Sheahan TP, Morrison TE, Menachery VD, Jensen K, Leist SR, Whitmore A, Heise MT, Baric RS. Complement activation contributes to severe acute respiratory syndrome coronavirus pathogenesis. mBio. 2018;9(5):e01753–18. doi:10.1128/mBio.01753-18.
- Noce A, Albanese M, Marrone G, Di Lauro M, Pietroboni Zaitseva A, Palazzetti D, Guerriero C, Paolino A, Pizzenti G, Di Daniele F, et al. Ultramicronized palmitoylethanolamide (um-PEA): a new possible adjuvant treatment in COVID-19 patients. Pharmaceuticals. 2021;14(4):336. doi:10.3390/ph14040336.
- Thomas G, Frederick E, Hausburg M, Goldberg L, Hoke M, Roshon M, Mains C, Bar-Or D. The novel immunomodulatory biologic LMWF5A for pharmacological attenuation of the "cytokine storm" in COVID-19 patients: a hypothesis. Patient Saf Surg. 2020;14:21. doi:10.1186/s13037-020-00248-4.
- Thorley AJ, Ford PA, Giembycz MA, Goldstraw P, Young A, Tetley TD. Differential regulation of cytokine release and leukocyte migration by lipopolysaccharide-stimulated primary human lung alveolar type II epithelial cells and macrophages. J Immunol. 2007;178(1):463–73. doi:10.4049/jimmunol.178.1.463.
- Vanderheiden A, Ralfs P, Chirkova T, Upadhyay AA, Zimmerman MG, Bedoya S, Aoued H, Tharp GM, Pellegrini KL, Manfredi C, et al. Type I and Type III interferons restrict SARS-CoV-2 infection of human airway epithelial cultures. J Virol. 2020;94(19):e00985-20. doi:10.1128/JVI.00985-20.
- Kletting S, Barthold S, Repnik U, Griffiths G, Loretz B, Schneider-Daum N, de Souza Carvalho-Wodarz C, Lehr C-M. Co-culture of human alveolar epithelial (hAELVi) and macrophage (THP-1) cell lines. ALTEX. 2018;35(2):211–22. doi:10.14573/altex.1607191.
- Molinari C, Morsanuto V, Ghirlanda S, Ruga S, Notte F, Gaetano L, Uberti F. Role of combined lipoic acid and vitamin D3 on astrocytes as a way to prevent brain ageing by induced oxidative stress and iron accumulation. Oxid Med Cell Longev. 2019;2019:2843121. doi:10.1155/2019/2843121.
- MD Biosciences. LPS-induced lung injury and fibrosis models for COVID-19 therapeutic developments. 2020. https://www.mdbiosciences.com/newsevents/news-archives/lps-induced-lung-injury-and-fibrosis-models-for-covid-19-therapeutic-developments.
- Conti P, Ronconi G, Caraffa A, Gallenga C, Ross R, Frydas I, Kritas S. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J Biol Regul Homeost Agents. 2020;34(2):327–31. doi:10.23812/CONTI-E.
- Grifoni E, Valoriani A, Cei F, Lamanna R, Gelli AMG, Ciambotti B, Vannucchi V, Moroni F, Pelagatti L, Tarquini R, et al. Interleukin-6 as prognosticator in patients with COVID-19. J Infect. 2020;81(3):452–82. doi:10.1016/j.jinf.2020.06.008.
- Lu L, Zhang H, Dauphars DJ, He Y-W. A potential role of interleukin 10 in COVID-19 pathogenesis. Trends Immunol. 2021;42(1):3–5. doi:10.1016/j.it.2020.10.012.
- Brandão SCS, Ramos JdOX, Dompieri LT, Godoi ETAM, Figueiredo JL, Sarinho ESC, Chelvanambi S, Aikawa M. Is Toll-like receptor 4 involved in the severity of COVID-19 pathology in patients with cardiometabolic comorbidities?Cytokine Growth Factor Rev. 2021;58:102–10. S1359-6101(20)30205-7. doi:10.1016/j.cytogfr.2020.09.002.
- Chandorkar P, Posch W, Zaderer V, Blatzer M, Steger M, Ammann CG, Binder U, Hermann M, Hörtnagl P, Lass-Flörl C, et al. Fast-track development of an in vitro 3D lung/immune cell model to study Aspergillus infections. Sci Rep. 2017;7(1):11644. doi:10.1038/s41598-017-11271-4.
- Jonsdottir HR, Dijkman R. Coronaviruses and the human airway: a universal system for virus-host interaction studies. Virol J. 2016;13:24. doi:10.1186/s12985-016-0479-5.
- Chua RL, Lukassen S, Trump S, Hennig BP, Wendisch D, Pott F, Debnath O, Thürmann L, Kurth F, Völker MT, et al. COVID-19 severity correlates with airway epithelium-immune cell interactions identified by single-cell analysis. Nat Biotechnol. 2020;38(8):970–9. doi:10.1038/s41587-020-0602-4.
- Uberti F, Morsanuto V, Ruga S, Galla R, Farghali M, Notte F, Bozzo C, Magnani C, Nardone A, Molinari C. Study of magnesium formulations on intestinal cells to influence myometrium cell relaxation. Nutrients. 2020;12(2):573. doi:10.3390/nu12020573.
- Uberti F, Lattuada D, Morsanuto V, Nava U, Bolis G, Vacca G, Squarzanti DF, Cisari C, Molinari C. Vitamin D protects human endothelial cells from oxidative stress through the autophagic and survival pathways. J Clin Endocrinol Metab. 2014;99(4):1367–74. doi:10.1210/jc.2013-2103.
- Xu X, Zhang A, Li N, Li P-L, Zhang F. Concentration-dependent diversifcation effects of free cholesterol loading on macrophage viability and polarization. Cell Physiol Biochem. 2015;37(2):419–31. doi:10.1159/000430365.
- Huang K, Li W, Chen Y, Zhu J. Effect of PM2.5 on invasion and proliferation of HeLa cells and the expression of inflammatory cytokines IL-1 and IL-6. Oncol Lett. 2018;16(6):7068–73. doi:10.3892/ol.2018.9516.
- Hu D-N, Bi M, Zhang DY, Ye F, McCormick SA, Chan C-C. Constitutive and LPS-induced expression of MCP-1 and IL-8 by human uveal melanocytes in vitro and relevant signal pathways. Invest Ophthalmol Vis Sci. 2014;55(9):5760–9. doi:10.1167/iovs.14-14685.
- Qosa H, Lichter J, Sarlo M, Markandaiah SS, McAvoy K, Richard J-P, Jablonski MR, Maragakis NJ, Pasinelli P, Trotti D, et al. Astrocytes drive upregulation of the multidrug resistance transporter ABCB1 (P-Glycoprotein) in endothelial cells of the blood-brain barrier in mutant superoxide dismutase 1-linked amyotrophic lateral sclerosis. Glia. 2016;64(8):1298–313. doi:10.1002/glia.23003.
- De Rudder C, Calatayud Arroyo M, Lebeer S, Van de Wiele T. Dual and triple epithelial coculture model systems with donor-derived microbiota and THP-1 macrophages to mimic host-microbe interactions in the human sinonasal cavities. mSphere. 2020;5(1):e00916-19. doi:10.1128/mSphere.00916-19.
- Hui DSC, Zumla A. Severe acute respiratory syndrome: historical, epidemiologic, and clinical Features. Infect Dis Clin North Am. 2019;33(4):869–89. doi:10.1016/j.idc.2019.07.001.
- Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, HLH Across Speciality Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–4. doi:10.1016/S0140-6736(20)30628-0.
- Altemimi A, Lakhssassi N, Baharlouei A, Watson DG, Lightfoot DA. Phytochemicals: Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants. 2017;6(4):42. doi:10.3390/plants6040042.
- Tahir A, Javed M, Hussain Z. Nutraceuticals and herbal extracts: A ray of hope for COVID-19 and related infections (Review). Int J Funct Nutr. 2020;1(2):1. doi:10.3892/ijfn.2020.6.
- Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. Jama. 2020;323(11):1061–1069. doi:10.1001/jama.2020.1585.
- Sahebnasagh A, Saghafi F, Avan R, Khoshi A, Khataminia M, Safdari M, Habtemariam S, Ghaleno HR, Nabavi SM. The prophylaxis and treatment potential of supplements for COVID-19. Eur J Pharmacol. 2020;887:173530. doi:10.1016/j.ejphar.2020.173530.
- Subedi L, Tchen S, Gaire BP, Hu B, Hu K. Adjunctive nutraceutical therapies for COVID-19. Int J Mol Sci. 2021;22(4):1963. doi:10.3390/ijms22041963.
- Carr AC. A new clinical trial to test high-dose vitamin C in patients with COVID-19. Crit Care. 2020;24(1):133. doi:10.1186/s13054-020-02851-4.
- Grant WB, Lahore H, McDonnell SL, Baggerly CA, French CB, Aliano JL, Bhattoa HP. Evidence that vitamin D sup- plementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020;12:E988. doi:10.3390/nu12040988.
- Lin S-C, Ho C-T, Chuo W-H, Li S, Wang TT, Lin C-C. Effective inhibition of MERS-CoV infection by resveratrol. BMC Infect Dis. 2017;17(1):144. doi:10.1186/s12879-017-2253-8.
- Cure E, Cumhur Cure M. Alpha-lipoic acid may protect patients with diabetes against COVID-19 infection. Med Hypotheses. 2020;143:110185. doi:10.1016/j.mehy.2020.110185.
- Zhang L, Liu Y. Potential interventions for novel coronavirus in China: a systematic review. J Med Virol. 2020;92(5):479–490. doi:10.1002/jmv.25707.
- Zhong M, Sun A, Xiao T, Yao G, Sang L, Zheng X, Zhang J, Jin X, Xu L, Yang W, Wang P. A randomized, single-blind, group sequential, active-controlled study to evaluate the clinical efficacy and safety of α-lipoic acid for critically ill patients with coronavirus disease 2019 (COVID-19) MEDRXIV. 2020;4. doi:10.1101/2020.04.15.20066266.
- Pesce M, Seguella L, Cassarano S, Aurino L, Sanseverino W, Lu J, Corpetti C, Del Re A, Vincenzi M, Sarnelli G, et al. Phytotherapics in COVID19: Why palmitoylethanolamide?Phytother Res. 2020;9. doi:10.1002/ptr.6978.
- Esposito G, Capoccia E, Turco F, Palumbo I, Lu J, Steardo A, Cuomo R, Sarnelli G, Steardo L. Palmitoylethanolamide improves colon inflammation through an enteric glia/toll like receptor 4-dependent PPAR-α activation. Gut. 2014;63(8):1300–12. doi:10.1136/gutjnl-2013-305005.
