Abstract
Spirulina (Arthrospira platensis) is a cyanobacterium associated with multiple health benefits. Cardiometabolic diseases such as cardiovascular disease, nonalcoholic fatty liver disease, and diabetes are prevalent yet usually preventable non-communicable diseases. Modifiable risk factors for cardiometabolic diseases include excessive body weight, body inflammation, atherogenic lipid profile, and imbalanced glucose metabolism. This review explores the effects of spirulina on cardiometabolic diseases risk factors. Spirulina was effective in reducing body weight, body mass index, and waist circumference, with a potential dose-dependent effect. It also decreased interleukin 6, an important biomarker of body inflammation, by inhibiting NADPH oxidase enzyme, and lowering insulin resistance. spirulina supplementation also reduced triglycerides, low-density lipoprotein cholesterol, and increased high-density lipoprotein cholesterol. Additionally, spirulina reduced fasting blood sugar and post-prandial blood sugar and increased insulin sensitivity, but no effect was observed on glycated hemoglobin A1c. The diverse nutrients, such as phycocyanin, gamma-linolenic acid, and vitamin B12, present in spirulina contribute to its cardiometabolic benefits. The doses used are heterogeneous for most studies, ranging from 1 to 8 grams daily, but most studies administered spirulina for 3 months to observe an effect. The collective evidence suggests that spirulina supplements may help improve risk factors for cardiometabolic diseases, thus, preventing its development. However, due to the heterogeneity of the results, more randomized clinical trials are needed to draw robust conclusions about spirulina’s therapeutic potential in ameliorating risk factors for cardiometabolic diseases and fully elucidate the mechanisms by which it exerts its effects.
Introduction
Spirulina (Arthrospira platensis) is a filamentous cyanobacterium, a type of blue-green algae originating from lagoons in Latin America and Africa. However, because of its capacity to survive in extreme conditions, it has spread to other hot regions on the planet. Spirulina has been traditionally used in food for many years, being consumed by Kanembous civilizations (Africa) and Aztecs (Mexico) for centuries (Lupatini et al. Citation2017). Microalgae cultivation has become the interest of scientists as a source of single-cell protein in the 1960s and 1970s as researchers pointed at the exponential increase in the world’s population and the possibility of a food shortage (Ugalde and Castrillo Citation2002). Currently, spirulina accounts for around one third of the total microalgal biomass produced worldwide (Costa et al. Citation2019) mostly produced in the united states (Belay Citation2007). Currently, a very large facility in inner Mongolia established since 2001 produces great amounts of spirulina (Lu et al. Citation2011). The common spirulina species used as food supplements are Spirulina platensis (Arthrospira platensis), Spirulina maxima (Arthrospira maxima) and Spirulina fusiformis (Arthrospira fusiformis). Spirulina has been gaining attention recently due to its unique chemical and nutritional composition. In fact, Spirulina has been recognized and recommended as a food supplement by NASA and the European Space Agency for long-term space trips (Maddiboyina et al. Citation2023). Spirulina is also favored due to being a low-cost nutritional supplement and for having no established side effects (Karkos et al. Citation2011).
Spirulina has been utilized for nutrition for a long time due to its high protein content, high digestibility, and its balanced essential amino acids profile (Demir and Tükel Citation2010). The protein content in spirulina is significant, along with the various vitamins, carotenoids, minerals, essential fatty acids and polysaccharides, among others (Tomaselli Citation1997; Babadzhanov et al. Citation2004). Specifically, spirulina is high in B vitamins, particularly B12, and minerals including calcium, zinc, iron, magnesium, potassium, and manganese (Kennedy Citation2016). Additionally, gamma-linolenic acid (GLA) is abundant in spirulina, which is an essential fatty acid that must be obtained from the diet. The phytocomplex of spirulina is rather rich in pigments, specifically chlorophyl and phycobilins, which includes phycocyanin and allophycocyanin (Babadzhanov et al. Citation2004; Wells et al. Citation2017). However, it is important to mention that the amount of nutrients in spirulina varies depending on the region of production, the climate, and the salt content of the water in which spirulina grew. The harvesting techniques may also affect the quantity of vitamins, minerals, and Phyto-derivatives present in spirulina (Tomaselli Citation1997). The nutrients spirulina offers to the human body after its ingestion is also highly absorbable, which is an efficient approach to restore deficient levels of nutrients in the body to its physiological levels (Wells et al. Citation2017).
Cardiometabolic diseases include diseases of the heart such as coronary heart disease, heart failure, stroke, myocardial infarction along with diseases of disrupted glucose metabolism such as insulin resistance and type 2 diabetes mellitus, as well as nonalcoholic fatty liver disease, all of which are prevalent yet usually preventable non-communicable diseases. According to the World Health Organization (WHO), non-communicable diseases claims the lives of 41 million people each year, equivalent to 74% of all deaths globally, and cardiovascular diseases account for 17.9 million deaths annually (World Health Organization Citation2022). Cardiometabolic health is influenced by risk factors that intricately shape cardiovascular and metabolic health, such as overweight and obesity, impaired glucose metabolism, dyslipidemia, and hypertension and body inflammation (Mezhal et al. Citation2023). Obesity and body composition is tightly related to metabolic health, as it affects the body’s inflammatory state, which influences how the body metabolizes nutrients and stores them (Piché et al. Citation2020; Drozdz et al. Citation2021; Powell-Wiley et al. Citation2021). This in turn increases the risk of a disrupted cellular response to insulin, leading to disrupted blood sugar control, a condition known as insulin resistance (Piché et al. Citation2020). Additionally, dyslipidemia, referring to the imbalance of lipids in the bloodstream, contributes to atherosclerotic risk and cardiovascular complications (Powell-Wiley et al. Citation2021). Elevated levels of low-density lipoprotein cholesterol (LDL-C), total cholesterol (TC), and triglycerides (TG), accompanied by reduced levels of high-density lipoprotein cholesterol (HDL-C) mark an atherogenic blood lipid profile (Powell-Wiley et al. Citation2021). Numerous health benefits of spirulina, including its antiviral, antitumor, anti-diabetic, antioxidant, anti-inflammatory, and cardioprotective effects have sparked interest in the scientific field (Capelli and Cysewski Citation2010; Serban et al. Citation2016). Preclinical trials were conducted to assess the cardioprotective properties of nutraceutical formulations based on spirulina and was found to be protective against highly toxic drugs such as doxorubicin (Quagliariello et al. Citation2022). The positive effects of spirulina were also seen in a randomized, active-control field trial involving infants through an enhancement in gross and fine motor development, language proficiency, and social skills after spirulina supplementation during the first 1000 days of life (Masuda and Chitundu Citation2019). The primary aim of this article is to review the effects of spirulina on cardiometabolic diseases risk factors including excess body weight, disrupted glucose metabolism, atherogenic blood lipid profile, and elevated blood pressure ().
Table 1. Summary of human studies investigating the effect of spirulina supplementation on risk factors for cardiometabolic diseases.
Methods
Scientific databases were searched including Cochrane Library, EMBASE, PubMed (Medline), and SCOPUS for scientific papers published until August 2023. The following MeSH terms were utilized in various combinations: (‘Spirulina’ OR ‘Arthrospira’) AND (‘Cardiometabolic risk factors’ OR ‘Cardiovascular risk factors’ OR ‘Obesity’ OR ‘Insulin resistance’ OR ‘Diabetes’ OR ‘Dyslipidemia’ OR ‘Lipid profile’ OR ‘Blood pressure’ OR ‘Glucose metabolism’ OR ‘Inflammation’ OR ‘Antioxidants’). Inclusion criteria encompassed; spirulina given as an oral supplement and was not administered with other interventions that cannot be differentiated from each other, studies with adult participants (≥18 years old) that were either healthy or have at least one risk factor for cardiometabolic diseases.
The effect of spirulina on body weight
The effect of spirulina supplementation on body weight parameters have been explored in multiple clinical trials. Zeinalian et al. (Citation2017) in a randomized, double-blinded, placebo-controlled clinical trial, studied 62 obese subjects after administering 1 g spirulina for 12 wk and observed a significant reduction in appetite by −4.16% (p = 0.008), Body Mass Index (BMI) by −1.9% (p < 0.001), and in body weight by −1.79% (p < 0.001) (Zeinalian et al. Citation2017). Another randomized, double-blinded, placebo-controlled clinical trial investigated the effect of spirulina supplementation at a dose of 2 grams per day for 12 wk on 50 obese patients with diagnosed hypertension and observed a statistically significant improvements (mean ± SD) in body weight (92.96 ± 18.58 kg to 88.97 ± 17.13 kg), BMI (33.5 ± 6.7 kg/m2 to 31.7 ± 5.8 kg/m2), and waist circumference (105.2 ± 15.3 to 103.4 ± 14.1 cm) at the end of the experiment. These improvements were not seen in the placebo group that was given only pure microcrystalline cellulose capsule (Szulinska et al. Citation2017). A recent meta-analysis aimed to assess the impact of spirulina oral supplementation on obesity indices showed a significant reduction in body weight (weighted mean difference (WMD) = −1.85 Kg; 95% CI: −2.44, −1.26; p < 0.001), and waist circumference (WMD = −1.09 cm; 95% CI: −2.16, −0.01; p = 0.046), while no significant effect was observed on BMI (SMD = −0.53 Kg/m2; 95% CI: −1.25, 0.19; p = .149). Notably, spirulina more effectively reduced BMI in studies conducted for ≥12 wk (SMD = −1.25 Kg/m2; 95% CI: −2.21, −0.28; p = .011) (Zarezadeh et al. Citation2021). A randomized controlled trial by Shariat et al. (Citation2019) tested the effect of 1 g/day spirulina supplement on 56 obese patients and found a significant reduction in macrophage inhibitory cytokine 1 concentrations and appetite (p < 0.05). A significant decline in inflammation, measured by superoxide dismutase concentrations, and in body weight, BMI, and WC were also seen in both groups but was more substantial in the spirulina group (Shariat et al. Citation2019). A double-blind, placebo-controlled, randomized trial by Miczke et al. (Citation2016) supplemented 2 grams of spirulina maxima for 3 months to 40 hypertensive patients without evidence of cardiovascular disease. They perceived a significant reduction in BMI (26.9 ± 3.1 vs 25.0 ± 2.7 kg/m2) and body weight (75.5 ± 11.8 kg vs 70.5 ± 10.3 kg) compared to baseline measurements in the treatment group but not in the control group (Miczke et al. Citation2016). The effect of spirulina supplementation was first investigated by Ramamoorthy and Premakumari (Citation1996) in a clinical trial involving ischemic heart patients with hypercholesterolemia (serum cholesterol levels >250 mg/dL), where either 2 grams or 4 grams per day of spirulina was given for 3 months. At the end of the supplementation period, there was a significant weight loss for both dosages compared to the controls (−2.2 kg vs +0.7 kg; p < 0.01) (Ramamoorthy and Premakumari Citation1996). Additionally, a randomized controlled trial conducted on 52 obese patients showed a significant body weight, TG and high sensitivity c-reactive protein levels reduction after supplementation of 2 grams of spirulina with a personalized low-calorie diet compared to only low-calorie diet (Yousefi et al. Citation2018). The addition of a systematic physical exercise also enhances the weight-lowering effects of spirulina. A double-blind, randomized, and crossover controlled trial was conducted on 52 overweight and obese sedentary male subjects supplemented with 4 grams daily of spirulina for 6 wk showed a significantly lower body fat percentages and body weight compared to the control (Hernández-Lepe et al. Citation2018). Notably, although the evidence thus far is pointing at a beneficial weight-lowering effect across all studies, it is limited by not testing for multiple hypotheses.
Possible mechanisms of action
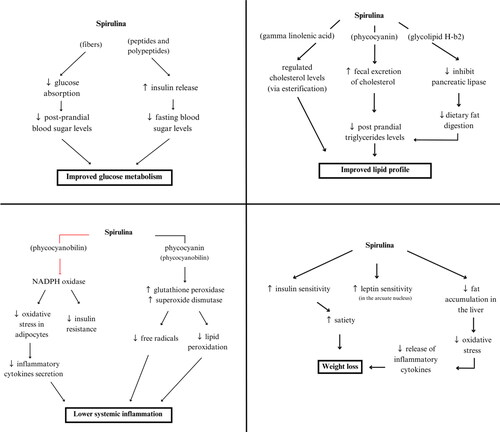
Spirulina could contribute to weigh loss through the following: prevention of liver lipid buildup, reduction of oxidative stress, and inhibition of macrophage infiltration into visceral adipose tissue (Fujimoto et al. Citation2012). This leads to less production of inflammatory cytokines and lowering overall oxidative stress (Karkos et al. Citation2011; Yang et al. Citation2020). Spirulina also contains a lot of essential amino acids, such phenylalanine, which triggers the release of cholecystokinin. This molecule decreases body weight by inhibiting the appetite centers in the central nervous system (Mazokopakis et al. Citation2014). Furthermore, ingestion of spirulina was shown to improve insulin sensitivity and increases satiety, which leads to lower food consumption and body weight reduction (Bobescu et al. Citation2020) ().
The effects of spirulina on inflammation
An increased body adiposity status is linked closely to higher inflammation, hyperlipidemia, and insulin resistance (Park et al. Citation2005; Shah et al. Citation2008). This is due to the release of adipokines and chemokines from adipose tissue, which in turn induces inflammation and contribute to the development of atherosclerosis (Skrypnik et al. Citation2017). All of which increase the risk of developing cardiometabolic diseases (Furukawa et al. Citation2017). The effect of spirulina supplementation on inflammation and oxidative stress is debatable in human clinical trials. A recent meta-analysis included 11 studies and 465 subjects showed that spirulina was able to significantly increase IL-2 concentrations (SMD= 2.69 pg/ml; 95% CI: 0.26, 5.11; p = 0.03) but such significance disappeared when sensitivity analysis was applied. The sensitivity analysis was done using the leave-one-out method, which assesses the effect of omitting a study on the effect size. Lower levels of IL-2 indicate lower body inflammation. IL-6 and thiobarbituric acid reactive substances, which are pro-inflammatory biomarkers, decreased only marginally with spirulina ingestion. Meanwhile, no effect was seen on TNF-α and malondialdehyde, which indicate inflammation (Mohiti et al. Citation2021). Another meta-analysis included 9 studies and 415 subjects found that supplementing spirulina marginally increased total antioxidant capacity (SMD = 0.49; 95% CI: −0.001, 0.98; p = .05) and superoxide dismutase (SMD = 0.72; 95% CI: −0.03, 1.46; p = 0.06), while no effect was seen on glutathione peroxidase (Naeini et al. Citation2021). A clinical trial by Szulinska et al. (Citation2017) also showed that, along with significant weight loss, spirulina supplementation was able to significantly lower IL-6 from 4.3 ± 0.6 mmol/L to 3.9 + 0.4 mmol/L, and improve the total oxidative stress statud from 8 ± 0.3 to 2.2 ± 1.0 mmol/L (Szulinska et al. Citation2017). Another human clinical trial on 37 Korean diabetic patients revealed that 8 grams per day of spirulina for 12 wk was able to significantly reduce plasma levels of malonaldehyde from 2.57 µM/L to 1.85 µM/L (p < 0.01) and slightly increase adiponectin levels from 5.52 µg/ml to 6.62 µg/ml (p < 0.1), indicating an improved oxidative stress status (Lee et al. Citation2008). Urinary isoprostane is a reliable marker for oxidative stress and it reflects the peroxidation of PUFAs in cell membranes (Graille et al. Citation2020), while oxidized LDL/total cholesterol ratio reflects increased oxidative damage to LDL-C particles which are particularly detrimental and increases inflammation and risk for atherosclerosis (Matsuura et al. Citation2008). A clinical trial by Koite et al. (Citation2022) tested the effect of the spirulina’s liquid extract ‘Spirulysat®’ on oxidative stress markers, urinary isoprostane and oxidized LDL/total cholesterol ratio, in 20 patients with metabolic syndrome. After the intervention period, a significant decrease in the urinary isoprostane concentrations in the intervention group was found (p = 0.014), while no significant difference in oxidized LDL/total cholesterol ratio between the intervention and control groups was detected (Koite et al. Citation2022).
Possible mechanisms of action
Tissue inflammation and damage are closely linked to reactive oxygen species (ROS) formation. C-phycocyanin in spirulina is the main protein proposed to be responsible for increasing the activity of glutathione peroxidase and superoxide dismutase, scavenging free radicals and inhibiting lipid peroxidation (Upasani and Balaraman Citation2003; Sharma et al. Citation2011). Furthermore, C-phycocyanin was shown to decrease the formation of nicotinamide adenine dinucleotide phosphate (NADPH) and nicotinamide adenine dinucleotide (NADH), as well as inhibit the expression of NADPH oxidase, potentially contributing to spirulina’s hypolipemic and antioxidant effects (Upasani and Balaraman Citation2003; Sharma et al. Citation2011). In relation to obesity, NADPH oxidase significantly contributes to oxidative stress in adipose tissue, leading to hypertrophy of adipocytes, insulin resistance and alterations in adipokine and cytokine secretion (Panday et al. Citation2015). Therefore, as spirulina suppresses adipocyte oxidative stress, it may lead to systemic anti-inflammatory effects and enhanced insulin sensitivity (Terry et al. Citation1993; Talior et al. Citation2005; Strasky et al. Citation2013; Zheng et al. Citation2013). Within phycocyanin, there is a water-soluble chromophore called phycocyanobilin, which represents the majority of the blue-green pigments found in spirulina. Phycocyanobilin has significant anti-inflammatory and antioxidant capabilities and shares structural similarities with bilirubin (Guo et al. Citation2022; Liu et al. Citation2022). It has been demonstrated that the amount of phycocyanin in spirulina directly correlates to its anti-oxidant action (Estrada et al. Citation2001) (). In addition, specific nutrients in spirulina such as resveratrol and quercetin were found to be cardioprotective through several inflammatory pathways (Wu and Hsieh Citation2011; Patel et al. Citation2018; Quagliariello et al. Citation2021).
The effect of spirulina on lipid profile
Components of the plasma lipid profile such as TG, TC, HDL-C, and LDL-C are important predictors and are causal factors of future cardiometabolic diseases (D’Agostino et al. Citation2008; Holmes et al. Citation2015; Hindy et al. Citation2018). The effect of spirulina on the lipid profile was demonstrated in multiple clinical trials. A randomized double-blinded clinical trial included 30 diabetic patients that were given 2 grams of spirulina daily for 3 months. The trial showed a significant reduction in TC by 41.36 mg/dL and LDL-C by 38.4 mg/dL (p < 0.001). Additionally, TG decreased by 38.25% and HDL-C increased by 7.2% from baseline, both of which were significantly different than the control group (Karizi et al. Citation2023). A clinical trial by Koite et al. (Citation2022) found a significant decrease in TG and an increase in HDL-C, but no effect on LDL-C and TC. This could be due to the small dose of spirulina administered within the liquid extract used (0.02 g/d), which was not able to affect all the blood lipids (Koite et al. Citation2022). A randomized double blinded placebo-controlled trial by Zeinalian et al. (Citation2017) observed a statistically significant reduction in TC by −4.67% and an elevation in HDL-C by 1.73% following one gram of spirulina supplementation for 3 months to 62 obese subjects. However, LDL-C was not changed by the end of the trial (Zeinalian et al. Citation2017). Another clinical trial on obese hypertensive patients showed that a dose of 2 grams per day for 12 wk significantly lowered LDL-C from 3.5 + 0.9 mmol/L to 3.0 ± 0.6 mmol/L (p < 0.001) (Szulinska et al. Citation2017). A recent meta-analysis of 23 studies and 1076 participants was conducted to assess the collective impact of spirulina supplementation on lipid profile. The analysis revealed a significant reduction in LDL-C (SMD: −0.6, 95% CI: −0.9, −0.2), TG (SMD: −0.6, 95% CI: −0.96, −0.25) and TC (SMD: −0.6, 95% CI: −0.9, −0.2) among participants who received spirulina supplementation. While HDL-C significantly increased by a standard mean difference of 0.3 (95% CI: 0.0008, 0.6) although the sensitivity analysis detected significant publication bias for these results. Furthermore, the dosage of spirulina at 4 grams, 5 grams and 10 grams/day had the highest effect on HDL-C, TG and TC levels, respectively. However, the studies selected in this meta-analysis were heterogenous for the health status of the participants and baseline BMI, which limits the ability to generalize the findings (Rahnama et al. Citation2023). Another prospective pilot study supplemented 1 gram of spirulina for 3 months to newly diagnosed dyslipidemia patients and found a statistically significant decrease in TG by 16.3%, LDL-C by 10.1%, TC by 8.9%, non-HDL-C by 10.8% and TC/HDL-C ratio by 11.5%. Additionally, HDL-C increased by 3.5% (Mazokopakis et al. Citation2014). Also, a randomized study by Lee et al. (Citation2008) on Korean diabetic patients showed a significant enhancement in lipid profile as indicated by lowered TG levels by 125.8–98.5 mg/dL (p < 0.05) although such reduction was more significant in patients with higher baseline TG levels. Similar observation was seen for TG and LDL-C serum levels (Lee et al. Citation2008). Moreover, in a human clinical trial, fifteen non-insulin dependent diabetic patients ingested 2 grams of spirulina per day for two months, which lead to a significant reduction in TG, TC, LDL-C, VLDL-C and LDL-C/HDL-C ratio (Mani et al. Citation2000). A study by Parikh et al. (Citation2001) also found that spirulina lowered TG by 6.4 mg, LDL-C by 7.1 mg, TC by 21.3 mg and an overall reduction in other atherogenic indices such as TC:HDL-C and LDL-C: HDL-C (from 5.4 ± 1.0 to 5.0 ± 1.0 and from 3.5 ± 0.8 to 2.9 ± 0.5, respectively) (Parikh et al. Citation2001). Ramamoorthy and Premakumari (Citation1996) also observed a decrease in TC by 22.4% and 33.5% and in LDL-C by 31% and 45% after supplementing 2 grams and 4 grams of spirulina for 3 months, respectively (p < 0.01 for both). They also observed an increase in HDL-C and a decrease in TG and very low density lipoprotein (VLDL) in the experimental groups compared to the control (Ramamoorthy and Premakumari Citation1996).
Possible mechanisms of action
The observed anti-atherogenic effect of spirulina could be explained through multiple mechanisms. A lower body inflammation helps achieve a balanced lipid profile by the pigment c-phycocyanin, which is abundant in spirulina, as it was able to increase cholesterol excretion when given as a residue in diet, increasing fecal excretion of cholesterol and bile (Nagaoka et al. Citation2005). Moreover, glycolipid H-b2 is present in spirulina and was shown to reduce pancreatic lipase activity in a dose-dependent manner, lowering dietary fat digestion and subsequent intestinal absorption (Han et al. Citation2006). Similar effect may also be observed due to phycocyanin pigment (Han et al. Citation2006). Additionally, the incorporation of Gamma-linolenic acid derived from spirulina can effectively regulate cholesterol levels via esterification processes (Karkos et al. Citation2011). On the other hand, a deficiency in gamma-linolenic acid was linked to worse cardiovascular outcomes such as increased arterial thickness, hypertension, and dyslipidemia, all of which increase the risk for cardiometabolic diseases (Horrobin Citation1992; Hornych et al. Citation2002). Spirulina is a low-fat, low-calorie protein source; therefore, its consumption may decrease hepatic triglyceride (TG) formation and enhance hepatic TG lipase and lipoprotein lipase activity, collectively contributing to an improved lipid profile (Westerbacka et al. Citation2005; Karkos et al. Citation2011). Furthermore, previous research showed a potential of algal supplementation to reduce cholesterol absorption and bile reabsorption in the ilium. Therefore, spirulina, a blue-green alga, may exert similar effects through this pathway (Ionov and Basova Citation2003; Kulshreshtha et al. Citation2008). Additionally, spirulina contains B3 vitamin, also known as niacin, which is established to improve dyslipidemia (Shah et al. Citation2013).
The effect of spirulina on glucose metabolism
The effect of spirulina supplementation on glucose metabolism biomarkers such as fasting blood sugar (FBS) and hemoglobin A1c (HbA1c) has been investigated through multiple clinical trials. The randomized, double-blind placebo-controlled trial by Karizi et al. (Citation2023) showed that after three–months of spirulina supplementation (2 g/day) a significant decrease in HbA1c (-1.43, p < 0.001) and FBS (-24.94 mg/dL, p < 001) levels were demonstrated in 30 patients with uncontrolled diabetes at baseline (Karizi et al. Citation2023). Another clinical trial administered a liquid extract of spirulina for three months to 20 metabolic syndrome patients found no change in glucose and insulin blood values (Koite et al. Citation2022). These results may be expected because the spirulina extract ‘Spirulysat®’ is mainly protein (2 g/L), 50% of which is phycocyanin (1 g/L) and also polysaccharides make 0.5 g/L. Many of the beneficial effects of spirulina previously reported studies used the whole algae supplement and not only the water extract. Finally, the dose of spirulina administered to patients in this study is around 0.02 g of spirulina per day, which is very low compared to other clinical trials and may explain their contrasting results (Koite et al. Citation2022). A single-blinded non-randomized controlled trial conducted on type 2 diabetes patients supplemented 7 grams of spirulina daily for 45 days and found that although FBS and post-prandial blood sugar was lowered, the difference was not significant when compared with the control group. Also, spirulina supplementation did not alter HbA1c and urine sugar levels in the experimental group (Alam et al. Citation2016). This indicates that such negative results are not likely due to the spirulina supplementation, but rather due to another confounder. A randomized clinical trial by Szulinska et al. (Citation2017) also found that insulin sensitivity ratio increased 3.2 ± 1.8 mg/kg/min to 4.3 ± 2.1 mg/kg/min (p < 0.001) by the end of the 3 months intervention period with 2 grams per day of spirulina (Szulinska et al. Citation2017). This implies that participants became more sensitive insulin, promoting efficient cellular glucose uptake and resulting in lower blood glucose levels. Another clinical trial by Beihaghi et al. (Citation2017) supplemented 8 grams of spirulina on a daily basis for 3 months to 20 diabetic patients. A significant decrease in FBS from 158.1 ± 44.2 mg/dl at baseline to 127.8 ± 36.7 mg/dl was demonstrated in the spirulina-supplemented group with a non-significant decrease in HbA1c levels (Beihaghi et al. Citation2017). A lower dose of 3 grams was administrated by Parikh et al. for 2 months to 25 diabetic patients was effective in lowering FBS by 19.3 mg/dl (p < 0.05), which is less impactful compared to Beihaghi’s study of −31 mg/dl. Parikh et al. also showed a decrease in postprandial blood sugar by 16.1 mg/dl (p < 0.05) and HbA1c by 1.0% (p < 0.05) compared with baseline concentrations in the study group (Parikh et al. Citation2001).
Possible mechanisms of action
The effect of spirulina on fasting blood sugar levels and post-prandial blood sugar levels may be explained through multiple mechanisms. Due to the rich content of high-quality proteins, spirulina helps in promoting the release of insulin into the bloodstream, as it is well established that proteins and amino acids consumption enhance insulin secretion (Westphal et al. Citation1990; Iyer Uma et al. Citation1999). This enhanced and healthier pattern of insulin secretion promotes an improved glucose tolerance. Moreover, it has been shown that spirulina affects pancreatic cells in multiple aspects, most importantly via Ca2+ ion and KATP channels (Billington et al. Citation2013; Wang et al. Citation2015) ().
Safety of spirulina supplements
Short- and long-term consumption of spirulina doesn’t induce toxicity or major complications (Sarubin-Fragakis Citation2007). A study supplementing 8 grams of spirulina for 3 months reported gastrointestinal side effects such as abdominal pain and diarrhea, however, the symptoms generally disappeared within days (Beihaghi et al. Citation2017). Notably, spirulina must be consumed with caution in patients with protein or amino acids abnormalities such as phenylketonuria or maple sirup urine disease, since spirulina is a high source of proteins and amino acids (Marles et al. Citation2011). Additionally, individuals with a history of allergy to algae should use spirulina with caution, as anaphylaxis, a severe and potentially life-threatening systemic allergic reaction, was reported in one study supplementing spirulina (Le et al. Citation2014). Of importance is the heavy metals and/or minerals concentrations in spirulina supplement preparation, which is mainly determined by the growth conditions (Al-Dhabi Citation2013). Nevertheless, a study by Al-Dhabi (Citation2013) showed that the heavy metals and/or minerals concentration in spirulina commercial supplements did not exceed the regulated upper limits (Pande et al. Citation1981; Al-Dhabi Citation2013).
Conclusion
Spirulina, a cyanobacterium rich in essential nutrients, emerges as a promising dietary intervention in addressing cardiometabolic risk factors. Multiple clinical trials showed consistent benefits in reducing body weight, enhancing lipid profiles, and improving inflammation markers and glucose metabolism. Spirulina’s composition of phycocyanin, gamma-linolenic acid, B vitamins, and powerful antioxidants are the reason for these positive outcomes. However, available evidence is heterogenous for spirulina dosage, intervention duration, and patient disease-status, which warrants further investigation. The small, reported effect sizes along with the small sample sizes mandates larger controlled clinical trials to reveal conclusive evidence regarding the protective of spirulina against cardiometabolic diseases. The integration of spirulina supplements into clinical practice still requires more long-term randomized clinical trials to establish the optimal usage of spirulina as a nutritional supplement and identify long-term safety profiles.
Acknowledgment
Open Access funding provided by the Qatar National Library.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Sara Sokary
Sara Sokary hold an MSc in Human nutrition from Qatar University.
Hiba Bawadi
Hiba Bawadi is a professor in human nutrition and dietetics and is the director of strategic development at QU-HEALTH. She is also the head of clinical training at the College of Health Sciences. She got her PhD from Louisiana State University in 2004 then moved to Jordan University of Science and Technology then joined Qatar University in In 2014. She published over 80 peer-reviewed articles.
Zain Zaki Zakaria
Zain Zaki Zakaria, MSc, PhD is the Section Head of Clinical Appointment. He holds an MSc in Medical Laboratory Science from the university of Jordan and a PhD in molecular biology from Qatar university.
Maha Al-Asmakh
Maha Al-Asmakh is an associate professor and is currently the Head of the Department of Biomedical Sciences at the College of Health Sciences at Qatar University. She has a bachelor’s degree in biomedical sciences (with honors) from Qatar University and a master’s degree in human reproductive biology from Imperial College London. She received her doctorate from Karolinska Institutet in Stockholm, Sweden and is certified by the American Board of Cytopathology from the American Society of Clinical Pathology. She is currently an educator specializing in teaching courses on embryology and developmental biology at the College of Health Sciences. Her expertise in the field of research has also resulted in many scientific publications.
References
- Alam A, Siddiqui M, Quamri A, Fatima S, Roqaiya M, Ahmad Z. 2016. Efficacy of Spirulina (Tahlab) in patients of type 2 diabetes mellitus (Ziabetus Shakri): a randomized controlled trial. J Diabetes Metab. 7(10):1–5. doi:10.4172/2155-6156.1000710.
- Al-Dhabi NA. 2013. Heavy metal analysis in commercial Spirulina products for human consumption. Saudi J Biol Sci. 20(4):383–388. doi:10.1016/j.sjbs.2013.04.006.
- Babadzhanov A, Abdusamatova N, Yusupova F, Faizullaeva N, Mezhlumyan L, Malikova MK. 2004. Chemical composition of spirulina platensis cultivated in Uzbekistan. Chem Nat Compd. 40(3):276–279. doi:10.1023/B:CONC.0000039141.98247.e8.
- Beihaghi M, Ghodrati Azadi H, Taherzadeh Z, Bahrami H. r 2017. The effects of oral administration of spirulina platensis (cultured Iranian) on blood glucose and glycosylated hemoglobin blood in type ii diabetes mellitus patients. Iran J Diabetes Lipid Disord. 16(3):183–190. http://ijdld.tums.ac.ir/article-1-5520-en.html.
- Belay A. 2007. Spirulina (Arthrospira): production and quality assurance. In: Amha Belay, editor. Spirulina in human nutrition and health. CRC Press; p. 15–40. https://www.taylorfrancis.com/chapters/edit/10.1201/9781420052572-4/spirulina-arthrospira-production-quality-assurance-amha-belay
- Billington CK, Ojo OO, Penn RB, Ito S. 2013. cAMP regulation of airway smooth muscle function. Pulm Pharmacol Ther. 26(1):112–120. doi:10.1016/j.pupt.2012.05.007.
- Bobescu E, Bălan A, Moga MA, Teodorescu A, Mitrică M, Dima L. 2020. Are there any beneficial effects of spirulina supplementation for metabolic syndrome components in postmenopausal women? Mar Drugs. 18(12):651. doi:10.3390/md18120651.
- Capelli B, Cysewski GR. 2010. Potential health benefits of spirulina microalgae* A review of the existing literature. Nutrafoods. 9(2):19–26. doi:10.1007/BF03223332.
- Costa JAV, Freitas BCB, Rosa GM, Moraes L, Morais MG, Mitchell BG. 2019. Operational and economic aspects of Spirulina-based biorefinery. Bioresour Technol. 292:121946. doi:10.1016/j.biortech.2019.121946.
- D’Agostino RB, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. 2008. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 117(6):743–753. doi:10.1161/CIRCULATIONAHA.107.699579.
- Demir BS, Tükel SS. 2010. Purification and characterization of lipase from Spirulina platensis. J Mol Catal B: Enzym. 64(3–4):123–128. doi:10.1016/j.molcatb.2009.09.011.
- Drozdz D, Alvarez-Pitti J, Wójcik M, Borghi C, Gabbianelli R, Mazur A, Herceg-Čavrak V, Lopez-Valcarcel BG, Brzeziński M, Lurbe E, et al. 2021. Obesity and cardiometabolic risk factors: from childhood to adulthood. Nutrients. 13(11):4176. doi:10.3390/nu13114176.
- Estrada JP, Bescós PB, Del Fresno AV. 2001. Antioxidant activity of different fractions of Spirulina platensis protean extract. Il Farmaco. 56(5–7):497–500.
- Fujimoto M, Tsuneyama K, Fujimoto T, Selmi C, Gershwin ME, Shimada Y. 2012. Spirulina improves non-alcoholic steatohepatitis, visceral fat macrophage aggregation, and serum leptin in a mouse model of metabolic syndrome. Dig Liver Dis. 44(9):767–774. doi:10.1016/j.dld.2012.02.002.
- Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. 2017. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 114(12):1752–1761. doi:10.1172/JCI21625.
- Graille M, Wild P, Sauvain JJ, Hemmendinger M, Guseva Canu I, Hopf NB. 2020. Urinary 8-isoprostane as a biomarker for oxidative stress. A systematic review and meta-analysis. Toxicol Lett. 328:19–27. doi:10.1016/j.toxlet.2020.04.006.
- Guo W, Zeng M, Zhu S, Li S, Qian Y, Wu H. 2022. Phycocyanin ameliorates mouse colitis via phycocyanobilin-dependent antioxidant and anti-inflammatory protection of the intestinal epithelial barrier. Food Funct. 13(6):3294–3307. doi:10.1039/d1fo02970c.
- Han L-K, Li D-X, Xiang L, Gong X-J, Kondo Y, Suzuki I, Okuda H. 2006. Isolation of pancreatic lipase activity-inhibitory component of Spirulina platensis and it reduce postprandial triacylglycerolemia. Yakugaku Zasshi. 126(1):43–49. doi:10.1248/yakushi.126.43.
- Hernández-Lepe MA, López-Díaz JA, Juárez-Oropeza MA, Hernández-Torres RP, Wall-Medrano A, Ramos-Jiménez A. 2018. Effect of Arthrospira (Spirulina) maxima supplementation and a systematic physical exercise program on the body composition and cardiorespiratory fitness of overweight or obese subjects: a double-blind, randomized, and crossover controlled trial. Mar Drugs. 16(10):364. doi:10.3390/md16100364.
- Hindy G, Engström G, Larsson SC, Traylor M, Markus HS, Melander O, Orho-Melander M. 2018. Role of blood lipids in the development of ischemic stroke and its subtypes: a Mendelian randomization study. Stroke. 49(4):820–827. doi:10.1161/STROKEAHA.117.019653.
- Holmes MV, Asselbergs FW, Palmer TM, Drenos F, Lanktree MB, Nelson CP, Dale CE, Padmanabhan S, Finan C, Swerdlow DI, et al. 2015. Mendelian randomization of blood lipids for coronary heart disease. Eur Heart J. 36(9):539–550. doi:10.1093/eurheartj/eht571.
- Hornych A, Oravec S, Girault F, Forette B, Horrobin D. 2002. The effect of gamma-linolenic acid on plasma and membrane lipids and renal prostaglandin synthesis in older subjects. Bratisl Lek Listy. 103(3):101–107.
- Horrobin D. 1992. Nutritional and medical importance of gamma-linolenic acid. Prog Lipid Res. 31(2):163–194. doi:10.1016/0163-7827(92)90008-7.
- Ionov VA, Basova MM. 2003. Use of blue-green micro-seaweed Spirulina platensis for the correction of lipid and hemostatic disturbances in patients with ischemic heart disease. Vopr Pitan. 72(6):28–31. https://www.scopus.com/inward/record.uri?eid=2-s2.0-2942679819&partnerID=40&md5=984f5c6983e5a80afe5a38354aad1b38.
- Iyer Uma M, Sophia A, Uliyar V. 1999. Glycemic and lipemic responses of selected spirulina-supplemented rice-based recipes in normal subjects. Age Years. 22:1–8.
- Karizi SR, Armanmehr F, Azadi HG, Zahroodi HS, Ghalibaf AM, Bazzaz BSF, Abbaspour M, Boskabadi J, Eslami S, Taherzadeh Z. 2023. A randomized, double-blind placebo-controlled add-on trial to assess the efficacy, safety, and anti-atherogenic effect of spirulina platensis in patients with inadequately controlled type 2 diabetes mellitus. Phytother Res. 37(4):1435–1448. doi:10.1002/ptr.7674.
- Karkos PD, Leong SC, Karkos CD, Sivaji N, Assimakopoulos DA. 2011. Spirulina in clinical practice: evidence-based human applications. Evid Based Complement Alternat Med. 2011:531053–531054. doi:10.1093/ecam/nen058.
- Kennedy DO. 2016. B vitamins and the brain: mechanisms, dose and efficacy—a review. Nutrients. 8(2):68. doi:10.3390/nu8020068.
- Koite NDLN, Sanogo NgI, Lépine O, Bard J-M, Ouguerram K. 2022. Antioxidant efficacy of a spirulina liquid extract on oxidative stress status and metabolic disturbances in subjects with metabolic syndrome. Mar Drugs. 20(7):441. doi:10.3390/md20070441.
- Kulshreshtha A, Zacharia AJ, Jarouliya U, Bhadauriya P, Prasad GBKS, Bisen PS. 2008. Spirulina in health care management. Curr Pharm Biotechnol. 9(5):400–405. doi:10.2174/138920108785915111.
- Le T-M, Knulst AC, Röckmann H. 2014. Anaphylaxis to Spirulina confirmed by skin prick test with ingredients of Spirulina tablets. Food Chem Toxicol. 74:309–310. doi:10.1016/j.fct.2014.10.024.
- Lee EH, Park J-E, Choi Y-J, Huh K-B, Kim W-Y. 2008. A randomized study to establish the effects of spirulina in type 2 diabetes mellitus patients. Nutr Res Pract. 2(4):295–300. doi:10.4162/nrp.2008.2.4.295.
- Liu R, Qin S, Li W. 2022. Phycocyanin: Anti-inflammatory effect and mechanism. Biomed Pharmacother. 153:113362. doi:10.1016/j.biopha.2022.113362.
- Lu YM, Xiang WZ, Wen YH. 2011. Spirulina (Arthrospira) industry in Inner Mongolia of China: current status and prospects. J Appl Phycol. 23(2):265–269. doi:10.1007/s10811-010-9552-4.
- Lupatini AL, Colla LM, Canan C, Colla E. 2017. Potential application of microalga Spirulina platensis as a protein source. J Sci Food Agric. 97(3):724–732. doi:10.1002/jsfa.7987.
- Maddiboyina B, Vanamamalai HK, Roy H, Gandhi S, Kavisri M, Moovendhan M. (2023). Food and drug industry applications of microalgae Spirulina platensis: a review. J Basic Microbiol, 63(6), 573–583. doi:10.1002/jobm.202200704.
- Mani U, Desai S, Iyer U. 2000. Studies on the long-term effect of spirulina supplementation on serum lipid profile and glycated proteins in NIDDM patients. J Nutraceuticals Funct Med Foods. 2(3):25–32. doi:10.1300/J133v02n03_03.
- Marles RJ, Barrett ML, Barnes J, Chavez ML, Gardiner P, Ko R, Mahady GB, Low Dog T, Sarma ND, Giancaspro GI, et al. 2011. United States pharmacopeia safety evaluation of spirulina. Crit Rev Food Sci Nutr. 51(7):593–604. doi:10.1080/10408391003721719.
- Martínez-Sámano J, Torres-Montes de Oca A, Luqueño-Bocardo OI, Torres-Durán PV, Juárez-Oropeza MA. 2018. Spirulina maxima decreases endothelial damage and oxidative stress indicators in patients with systemic arterial hypertension: Results from exploratory controlled clinical trial. Mar Drugs. 16(12):496. doi:10.3390/md16120496.
- Masuda K, Chitundu M. 2019. Multiple micronutrient supplementation using spirulina platensis during the first 1000 days is positively associated with development in children under five years: a follow up of a randomized trial in Zambia. Nutrients. 11(4):730. doi:10.3390/nu11040730.
- Matsuura E, Hughes GR, Khamashta MA. 2008. Oxidation of LDL and its clinical implication. Autoimmun Rev. 7(7):558–566. doi:10.1016/j.autrev.2008.04.018.
- Mazokopakis EE, Papadomanolaki MG, Fousteris AA, Kotsiris DA, Lampadakis IM, Ganotakis ES. 2014. The hepatoprotective and hypolipidemic effects of Spirulina (Arthrospira platensis) supplementation in a Cretan population with non-alcoholic fatty liver disease: a prospective pilot study. Ann Gastroenterol. 27(4):387–394.
- Mazokopakis EE, Starakis IK, Papadomanolaki MG, Mavroeidi NG, Ganotakis ES. 2014. The hypolipidaemic effects of Spirulina (Arthrospira platensis) supplementation in a Cretan population: a prospective study. J Sci Food Agric. 94(3):432–437. doi:10.1002/jsfa.6261.
- Mezhal F, Oulhaj A, Abdulle A, AlJunaibi A, Alnaeemi A, Ahmad A, Leinberger-Jabari A, Al Dhaheri AS, AlZaabi E, Al-Maskari F, et al. 2023. High prevalence of cardiometabolic risk factors amongst young adults in the United Arab Emirates: the UAE healthy future study. BMC Cardiovasc Disord. 23(1):137. doi:10.1186/s12872-023-03165-3.
- Miczke A, Szulińska M, Hansdorfer-Korzon R, Kręgielska-Narożna M, Suliburska J, Walkowiak J, Bogdański P. 2016. Effects of spirulina consumption on body weight, blood pressure, and endothelial function in overweight hypertensive Caucasians: a double-blind, placebo-controlled, randomized trial. Eur Rev Med Pharmacol Sci. 20(1):150–156.
- Mohiti S, Zarezadeh M, Naeini F, Tutunchi H, Ostadrahimi A, Ghoreishi Z, Ebrahimi Mamaghani M. 2021. Spirulina supplementation and oxidative stress and pro-inflammatory biomarkers: A systematic review and meta-analysis of controlled clinical trials. Clin Exp Pharmacol Physiol. 48(8):1059–1069. doi:10.1111/1440-1681.13510.
- Naeini F, Zarezadeh M, Mohiti S, Tutunchi H, Ebrahimi Mamaghani M, Ostadrahimi A. 2021. Spirulina supplementation as an adjuvant therapy in enhancement of antioxidant capacity: A systematic review and meta-analysis of controlled clinical trials. Int J Clin Pract. 75(10):e14618. doi:10.1111/ijcp.14618.
- Nagaoka S, Shimizu K, Kaneko H, Shibayama F, Morikawa K, Kanamaru Y, Otsuka A, Hirahashi T, Kato T. 2005. A novel protein C-phycocyanin plays a crucial role in the hypocholesterolemic action of Spirulina platensis concentrate in rats. J Nutr. 135(10):2425–2430. doi:10.1093/jn/135.10.2425.
- Panday A, Sahoo MK, Osorio D, Batra S. 2015. NADPH oxidases: an overview from structure to innate immunity-associated pathologies. Cell Mol Immunol. 12(1):5–23. doi:10.1038/cmi.2014.89.
- Pande A, Sarkar R, Krishnamoorthi K. 1981. Toxicity of copper sulphate to the alga Spirulina platensis, & the ciliate Tetrahymena pyriformis. Indian J Exp Biol. New Delhi. 19(5):500–502.
- Parikh P, Mani U, Iyer U. 2001. Role of Spirulina in the control of glycemia and lipidemia in type 2 diabetes mellitus. J Med Food. 4(4):193–199. doi:10.1089/10966200152744463.
- Park HS, Park JY, Yu R. 2005. Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF-α and IL-6. Diabetes Res Clin Pract. 69(1):29–35. doi:10.1016/j.diabres.2004.11.007.
- Patel RV, Mistry BM, Shinde SK, Syed R, Singh V, Shin HS. 2018. Therapeutic potential of quercetin as a cardiovascular agent. Eur J Med Chem. 155:889–904. doi:10.1016/j.ejmech.2018.06.053.
- Piché M-E, Tchernof A, Després J-P. 2020. Obesity phenotypes, diabetes, and cardiovascular diseases. Circ Res. 126(11):1477–1500. doi:10.1161/CIRCRESAHA.120.316101.
- Powell-Wiley TM, Poirier P, Burke LE, Després J-P, Gordon-Larsen P, Lavie CJ, Lear SA, Ndumele CE, Neeland IJ, Sanders P, et al. 2021. Obesity and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 143(21):e984–e1010. doi:10.1161/CIR.0000000000000973.
- Quagliariello V, Basilicata MG, Pepe G, De Anseris R, Di Mauro A, Scognamiglio G, Palma G, Vestuto V, Buccolo S, Luciano A, et al. 2022. Combination of Spirulina platensis, ganoderma lucidum and moringa oleifera improves cardiac functions and reduces pro-inflammatory biomarkers in preclinical models of short-term doxorubicin-mediated cardiotoxicity: new frontiers in cardioncology? J Cardiovasc Dev Dis. 9(12):423. doi:10.3390/jcdd9120423.
- Quagliariello V, Berretta M, Buccolo S, Iovine M, Paccone A, Cavalcanti E, Taibi R, Montopoli M, Botti G, Maurea N. 2021. Polydatin reduces cardiotoxicity and enhances the anticancer effects of sunitinib by decreasing pro-oxidative stress, pro-inflammatory cytokines, and NLRP3 inflammasome expression. Front Oncol. 11:680758. doi:10.3389/fonc.2021.680758.
- Rahnama I, Arabi SM, Chambari M, Bahrami LS, Hadi V, Mirghazanfari SM, Rizzo M, Hadi S, Sahebkar A. 2023. The effect of Spirulina supplementation on lipid profile: GRADE-assessed systematic review and dose-response meta-analysis of data from randomized controlled trials. Pharmacol Res. 193:106802. doi:10.1016/j.phrs.2023.106802.
- Ramamoorthy A, Premakumari S. 1996. Effect of supplementation of Spirulina on hypercholesterolemic patients. J Food Sci Technol-Mysore. 33:124–127.
- Rostami HAA, Marjani A, Mojerloo M, Rahimi B, Marjani M. 2022. Effect of spirulina on lipid Profile, glucose and malondialdehyde levels in type 2 diabetic patients. Braz J Pharm Sci. 58:106802. doi:10.1590/s2175-97902022e191140.
- Sarubin-Fragakis A. 2007. The health professional’s guide to popular dietary supplements. Chicago: American Dietetic Association.
- Serban M-C, Sahebkar A, Dragan S, Stoichescu-Hogea G, Ursoniu S, Andrica F, Banach M. 2016. A systematic review and meta-analysis of the impact of Spirulina supplementation on plasma lipid concentrations. Clin Nutr. 35(4):842–851. doi:10.1016/j.clnu.2015.09.007.
- Shah A, Mehta N, Reilly MP. 2008. Adipose inflammation, insulin resistance, and cardiovascular disease. JPEN J Parenter Enteral Nutr. 32(6):638–644. doi:10.1177/0148607108325251.
- Shah TZ, Ali AB, Jafri SA, Qazi M. 2013. Effect of nicotinic acid (vitamin B3 or niacin) on the lipid profile of diabetic and non–diabetic rats. Pak J Med Sci. 29(5):1259–1264. doi:10.12669/pjms.295.4193.
- Shariat A, Farhangi MA, Zeinalian R. 2019. Spirulina platensis supplementation, macrophage inhibitory cytokine-1 (MIC-1), oxidative stress markers and anthropometric features in obese individuals: a randomized controlled trial. J Herbal Med. 17-18:100264. doi:10.1016/j.hermed.2019.100264.
- Sharma NK, Tiwari SP, Tripathi K, Rai AK. 2011. Sustainability and cyanobacteria (blue-green algae): facts and challenges. J Appl Phycol. 23(6):1059–1081. doi:10.1007/s10811-010-9626-3.
- Skrypnik K, Suliburska J, Skrypnik D, Pilarski Ł, Reguła J, Bogdański P. 2017. The genetic basis of obesity complications. Acta Sci Pol Technol Aliment. 16(1):83–91. doi:10.17306/J.AFS.2017.0442.
- Strasky Z, Zemankova L, Nemeckova I, Rathouska J, Wong RJ, Muchova L, Subhanova I, Vanikova J, Vanova K, Vitek L, et al. 2013. Spirulina platensis and phycocyanobilin activate atheroprotective heme oxygenase-1: a possible implication for atherogenesis. Food Funct. 4(11):1586–1594. doi:10.1039/c3fo60230c.
- Szulinska M, Gibas-Dorna M, Miller-Kasprzak E, Suliburska J, Miczke A, Walczak-Gałezewska M, Stelmach-Mardas M, Walkowiak J, Bogdanski P. 2017. Spirulina maxima improves insulin sensitivity, lipid profile, and total antioxidant status in obese patients with well-treated hypertension: a randomized double-blind placebo-controlled study. Eur Rev Med Pharmacol Sci. 21(10):2473–2481.
- Talior I, Tennenbaum T, Kuroki T, Eldar-Finkelman H. 2005. PKC-δ-dependent activation of oxidative stress in adipocytes of obese and insulin-resistant mice: role for NADPH oxidase. Am J Physiol Endocrinol Metab. 288(2):E405–E411. doi:10.1152/ajpendo.00378.2004.
- Terry MJ, Maines M, Lagarias J. 1993. Inactivation of phytochrome-and phycobiliprotein-chromophore precursors by rat liver biliverdin reductase. J Biol Chem. 268(35):26099–26106. doi:10.1016/S0021-9258(19)74286-0.
- Tomaselli L. 1997. Morphology, ultrastructure and taxonomy of Arthrospira (Spirulina) maxima and Arthrospira (Spirulina) platensis. In: Avigad Vonshak, editor. Spirulina platensis (Arthrospira): physiology, cell-biology and biotechnology. London: CRC Press. p. 1–16.
- Ugalde U, Castrillo J. 2002. Single cell proteins from fungi and yeasts. In: George G. Khachatourians, Dilip K. Arora, editors. Applied Mycology and Biotechnology. Vol. 2. Elsevier; p. 123–149.
- Upasani CD, Balaraman R. 2003. Protective effect of Spirulina on lead induced deleterious changes in the lipid peroxidation and endogenous antioxidants in rats. Phytother Res. 17(4):330–334. doi:10.1002/ptr.1135.
- Wang Y, Wang S, Harvat T, Kinzer K, Zhang L, Feng F, Qi M, Oberholzer J. 2015. Diazoxide, a K(ATP) channel opener, prevents ischemia-reperfusion injury in rodent pancreatic islets. Cell Transplant. 24(1):25–36. doi:10.3727/096368913x673441.
- Wells ML, Potin P, Craigie JS, Raven JA, Merchant SS, Helliwell KE, Smith AG, Camire ME, Brawley SH. 2017. Algae as nutritional and functional food sources: revisiting our understanding. J Appl Phycol. 29(2):949–982. doi:10.1007/s10811-016-0974-5.
- Westerbacka J, Lammi K, Häkkinen A-M, Rissanen A, Salminen I, Aro A, Yki-Järvinen H. 2005. Dietary fat content modifies liver fat in overweight nondiabetic subjects. J Clin Endocrinol Metab. 90(5):2804–2809. doi:10.1210/jc.2004-1983.
- Westphal SA, Gannon MC, Nuttall FQ. 1990. Metabolic response to glucose ingested with various amounts of protein. Am J Clin Nutr. 52(2):267–272. doi:10.1093/ajcn/52.2.267.
- World Health Organization. 2022. Noncommunicable diseases (NCDs) - fact sheet. https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases.
- Wu JM, Hsieh TC. 2011. Resveratrol: a cardioprotective substance. Ann N Y Acad Sci. 1215(1):16–21. doi:10.1111/j.1749-6632.2010.05854.x.
- Yang Y, Du L, Hosokawa M, Miyashita K. 2020. Spirulina lipids alleviate oxidative stress and inflammation in mice fed a high-fat and high-sucrose diet. Mar Drugs. 18(3):148. doi:10.3390/md18030148.
- Yousefi R, Mottaghi A, Saidpour A. 2018. Spirulina platensis effectively ameliorates anthropometric measurements and obesity-related metabolic disorders in obese or overweight healthy individuals: a randomized controlled trial. Complement Ther Med. 40:106–112. doi:10.1016/j.ctim.2018.08.003.
- Zarezadeh M, Faghfouri AH, Radkhah N, Foroumandi E, Khorshidi M, Rasouli A, Zarei M, Mohammadzadeh Honarvar N, Hazhir Karzar N, Ebrahimi Mamaghani M. 2021. Spirulina supplementation and anthropometric indices: a systematic review and meta-analysis of controlled clinical trials. Phytother Res. 35(2):577–586. doi:10.1002/ptr.6834.
- Zeinalian R, Farhangi MA, Shariat A, Saghafi-Asl M. 2017. The effects of Spirulina Platensis on anthropometric indices, appetite, lipid profile and serum vascular endothelial growth factor (VEGF) in obese individuals: a randomized double blinded placebo controlled trial. BMC Complement Altern Med. 17(1):225. doi:10.1186/s12906-017-1670-y.
- Zheng J, Inoguchi T, Sasaki S, Maeda Y, McCarty MF, Fujii M, Ikeda N, Kobayashi K, Sonoda N, Takayanagi R. 2013. Phycocyanin and phycocyanobilin from Spirulina platensis protect against diabetic nephropathy by inhibiting oxidative stress. Am J Physiol Regul Integr Comp Physiol. 304(2):R110–R120. doi:10.1152/ajpregu.00648.2011.