Abstract
Male infertility has traditionally been diagnosed by microscopic assessment of concentration, motility and morphology of sperm in the ejaculate. Most laboratories use sperm isolated by various methods such as density gradient centrifugation to enrich for subpopulations of sperm believed to have greater fertilization potential. These tests are essential to provide the fundamental information on which clinicians base their initial diagnosis. However, in the clinical setting, tests with superior prognostic value are needed. Tests showing much promise are those determining sperm DNA integrity, particularly the Comet, TUNEL, and Sperm Chromatin Structure assays.
Sperm nuclear DNA fragmentation has been positively correlated with lower fertilization rates in IVF, impaired implantation rates, an increased incidence of abortion and disease in offspring, including childhood cancer. The mitochondrial genome of sperm has also been shown to be a sensitive marker of sperm health. Although the usefulness of these tests is recognized, insufficient resources have been available to develop standardized tests and protocols that could lead to universally accepted clinical thresholds. Associated with the lack of useful prognostic tests is the lack of improvement in assisted conception success rates despite thirty years of worldwide use. International collaborations should be initiated to develop agreed protocols and establish clinical thresholds.
| Abbreviations: | ||
| CASA | = | computer assisted sperm analysis |
| ROS | = | reactive oxygen species |
| DHE | = | dihydroethidium |
| SCF | = | sperm chromatin fragmentation |
| SCSA | = | Sperm Chromatin Structural Structure Assay |
| TdT, TUNE | = | terminal deoxynucleotidyl transferase |
| DFI | = | DNA fragmentation index |
| HDS | = | higher than normal stainability |
| ICSI | = | intracytoplasmic sperm injection |
| ART | = | artificial reproductive technologies |
| FCM | = | flow cytometric assay. |
Necessity of initial semen analysis
The conventional diagnosis of male infertility has relied on microscopic assessment and biochemical assays to determine human semen quality through measures of concentration, motility and morphology of sperm. Semen analysis provides the fundamental information on which clinicians base their initial diagnosis so it is imperative that it is performed as accurately as possible. However, in the two decades since the WHO manuals [World Health Organization Citation[1987]; Citation[2006]] have been our core reference points, it has become apparent that a basic semen analysis is insufficient for the determination of the fertility status of individual men (reviewed by [Lewis [Citation2007]]). One of the reasons for the lack of power of conventional parameters and the disagreement between studies, is the inherent heterogeneity of human semen. It is one of the most variable of all biological fluids. Its conventional parameters vary significantly between countries, regions, individuals and even between consecutive samples from the same individual. Semen analyses are confined to visual observations of a continually variable biological product at one point in time; giving no information as to how or why deficiencies in that product occurred. Furthermore they provide no clue to site or time when dysfunction occurred so no therapy (if we had such an entity) can be considered. Ongoing research into teratozoospermic RNA may provide useful information as to the root cause and interval during the spermatogenic cycle that is disturbed [Platts et al. [Citation2007]], but this has yet to be validated.
To address this deficiency, a plethora of sperm function tests such as computer assisted sperm analysis (CASA), acrosome reaction, zona free hamster egg penetration and sperm zona binding tests (ESHRE Andrology Special Interest Group [1996]; reviewed by [Aitken [Citation2006]]) have been developed over the past two decades. However, these tests are not sufficiently robust, or reproducible by multi-users nor do they have useful thresholds that can be adopted in a clinical setting. This had led to new avenues of investigation.
Oxidative stress tests
One group of tests showing promise in identifying sperm dysfunction is the measure of reactive oxygen species (ROS). Numerous studies by Aitken and associates (reviewed by [Aitken [Citation2006]]), have shown that ROS are necessary for the normal functioning of sperm. Indeed, sperm have multiple plasma membrane redox systems involved in the physiological control of the cell. However, oxidative stress resulting from excess ROS, such as hydrogen peroxide, superoxide anion and hydroxyl radical present either as the result of increased production or reduced antioxidant protection [Lewis et al. [Citation1995]; Aitken et al. [Citation1998]] are thought to be a major cause of sperm dysfunction. Sperm are susceptible to ROS damage because they have a high content of polyunsaturated fatty acids and little ability to repair ROS-induced damage. Further studies have reported associations between oxidative stress and structural [Cummins et al. [Citation1994]; Twigg et al. [Citation1998]; Irvine et al. [Citation2000]] and functional damage (reviewed by [Aitken and Fisher [Citation1994]]). Potential and actual oxidative injury can be determined by chemi-luminescence tests that assess ROS generation [Donnelly et al. [Citation1999]]. Recently, dihydroethidium (DHE) has been introduced as a probe for detecting the generation of superoxide anion and to elucidate the specific instigator of oxidative stress [De Iuliis et al. [Citation2006]]. Lipid peroxidation tests can be employed to determine the irreversible damage induced [Agarwal et al. [Citation2006]] and antioxidant capacity tests are useful [Lewis et al. [Citation1995]] to measure the sperm and seminal plasma protection against ROS. Such tests are ongoing and becoming increasingly sensitive as they are optimized. However, since the lifespan of oxygen radicals is of the order of nano to microseconds, it is very difficult to accurately measure ROS levels in semen in a clinical setting. One promising approach to monitoring oxidative stress in semen is to measure isoprostanes, which are stable by-products of lipid peroxidation that can be readily measured by either gas chromatography or immunocytochemistry [Lysiak et al. [Citation2007]].
Causes of Sperm DNA Damage
The high incidence of DNA damage in sperm compared to somatic cells [Ollero et al. [Citation2001]] may be due to its vulnerability to damage at a number of different stages of its unique development. Sperm DNA damage may occur within the testes, epididymis or ejaculate. It may be the result of abortive apoptosis [Sakkas et al. [Citation1999]] since the cellular machinery that allows male germ cells to complete apoptosis is discarded during spermatogenesis. Differentiated germ cells cannot be removed by apoptosis, even though, as evidenced by Fas expression or even endonuclease activation [Gorczyca et al. [Citation1993]; McVicar et al. [Citation2004]], the process has begun. DNA damage may also be due to the incomplete rejoining of the nicks within the DNA produced during the remodeling of the sperm chromatin. The third stage at which damage may occur is during the migration of the sperm through the epididymis. This damage can be induced in mature sperm by ROS-producing immature sperm during co-migration through the epididymis or by ROS-producing epithelial epididymal cells shown to play an important role in the oxidation of thiol groups and the crosslinking of sperm chromatin [Moore [Citation1996]]. Although DNA repair does occur during spermatogenesis [Van Loon et al. [Citation1991]] this ceases late in spermiogenesis [Matsuda et al. [Citation1989]; Aitken [Citation2004]], making post testicular sperm particularly vulnerable to DNA damage. Recently, sperm DNA degradation through a sperm chromatin fragmentation (SCF)—induced pathway has been proposed [Shaman et al. [Citation2007]; Yamauchi et al. [Citation2007a]]. These authors have reported a mechanism in mouse sperm by, which the DNA is degraded to facilitate decondensation of the tight toroid. This is likely initiated by topoisomerase IIB and finally by sperm nuclease action. The ability of sperm to execute this mechanism increases as it passes through the epididymis [Yamauchi et al. [Citation2007a]]. Physiologically, this mechanism is associated with DNA replication in oocytes and is thought to be part of the process by which paternal DNA is prevented from influencing initial cleavage of the embryo [Yamauchi et al. [Citation2007b]]. It may help to thwart the introduction of damaged sperm DNA into the fertilized oocyte [Yamauchi et al. [Citation2007b]]. Thus, the increased load of DNA damage (reviewed by [Aitken et al. [Citation2007]]) may also be explained, at least in part, by an aberrant SCF-induced DNA degradation pathway in infertile sperm.
The vulnerability of epididymal sperm is supported by studies showing that DNA damage is lowest in testicular sperm and increases in caudal epididymal and ejaculated sperm [Steele et al. [Citation1999]; O'Connell et al. [Citation2002]; Lewis et al. Citation[2004]]. This is further supported in a recent study of Greco et al. [[Citation2005b]]. DNA damage from testicular and ejaculated sperm from 18 men was measured and the incidence of DNA fragmentation was markedly lower in testicular sperm than in the ejaculated sperm. In agreement, defective sperm show an increase in damage during passage through the epididymis [Suganuma et al. [Citation2005]]. We know that even the proximal epididymis will contain substantial proportions of senescent sperm [Steele et al. [Citation1999]] damaging sperm adjacent to them as they age and die [Moore [Citation1996]]. Again, an aberrant SCF- induced DNA degradation pathway is in accord with the observed increase of DNA damage as sperm mature from testes to ejaculate. Oxidative stress appears to be the major cause of sperm DNA damage [Ollero et al. [Citation2001]; Aitken et al. [Citation2003a]; b; Aitken and Sawyer [Citation2003]; Saleh et al. [Citation2003]], irrespective of the site of damage, as with numerous sperm dysfunctions discussed earlier (see Oxidative Stress Tests).
Types and tests of Sperm DNA damage
There is little doubt, that the tests showing most promise in the diagnosis and treatment of male infertility are those measuring sperm nuclear DNA integrity. In recent years, the rapid advance of molecular biology has led to the development of numerous techniques directed towards assessing various aspects of DNA and chromatin quality. Of these, the COMET, terminal deoxynucleotidyl transferase (TdT, TUNEL) and Sperm Chromatin Structure assays (reviewed by [Evenson et al. [Citation2002]; Agarwal and Said Citation[2004]; Lewis and Aitken [Citation2005]]) have been shown to be the most informative. Each of these tests determines a different aspect of DNA damage.
Before discussing each assay, we must understand that damage to sperm DNA can be assessed in a number of ways. Real or actual damage is generally recognized as the damage indicated by assays, which do not subject the sperm to conditions that will induce damage. These include the TUNEL [Henkel et al. [Citation2004]; Greco et al. [Citation2005a]] and COMET using neutral conditions [Morris et al. [Citation2002]; McVicar et al. [Citation2004]] and in situ nick translation [Irvine et al. [Citation2000]] of the native structure. The next set of assays, (COMET using alkaline conditions, Sperm Chromatin Structural Structure Assay; SCSA) also measure damage. However the sperm are exposed to denaturing conditions. This could be classified as ‘potential’ since strand breaks are measured only when induced by adverse conditions. This type of damage could also be described as potential in that without laboratory intervention it has not yet formed a break. The third type of assay is that measuring the earlier stages of (potential) DNA damage; those sites that exhibit DNA base modifications such as 8-OH-guanosine and 8-OH -2′-deoxyguanosine. The extent to which each of these contributes to infertility is unknown.
Those assays measuring double strand breaks are thought to be more useful as a clinical tool, since it is known that the oocyte is unable to effect repair [Genesca et al. [Citation1992]; Ahmadi and Ng [Citation1999a]; Alvarez [Citation2005a]]. Others [Aitken and De Luliis [Citation2007]] suggest that total DNA damage, as opposed to strand breaks only, is a more comprehensive approach. For example, the COMET, under alkaline conditions, measures single and double strand breaks as well as alkali labile sites, which, at high pH are susceptible to breakage, and conversion into single strand breaks. Thus the alkaline COMET assay can provide a comprehensive measure of DNA damage.
Over the past decade, the COMET assay has become one of the standard methods for assessing DNA damage, with applications in genotoxicity testing, human biomonitoring, molecular epidemiology and eco-toxicolology, as well as fundamental research in DNA damage in somatic and germ cells. It is sensitive, versatile, fast and economical and as it only requires 100 cells for analysis it has been particularly useful for studies when sperm are limited e.g., testicular sperm [Lewis et al. [Citation2004]].
The SCSA can measure both single and double strand breaks [Evenson et al. [Citation2007]]. Primarily single-stranded DNA using a DNA fragmentation index (DFI) is reported. It can also be used to measure ‘immature sperm populations’ which have higher than normal stainability (HDS). It can be used in conjunction with light microscopy or flow cytometry so that very large numbers of sperm can be assayed rapidly. It also benefits from all users adhering to a standardized protocol that minimizes inter-laboratory variation.
The TUNEL assay uses TdT that preferentially labels the blunt 3′-OH ends of double-stranded DNA breaks, but also measures the single strand 3′-OH [Sergerie et al. [Citation2005]]. It is relatively quick and easy to perform and does not induce additional damage. However, the absence of cell lysis may impede its effectiveness due to sperm DNA compaction and limit access of the enzyme to all 3′-OH ends. This may account for the range of suggested thresholds [Tesarik et al. [Citation2006]] for assisted conception success from 12, 15, 18%, [Duran et al. [Citation2002]; Benchaib et al. [Citation2003]; Greco et al. [Citation2005b]; Benchaib et al. [Citation2007]] compared to the 30% standard proposed by Evenson et al. [[Citation2007]]. A few studies compare alternative assays, SCSA and Comet [Aravindan et al. [Citation1997]], COMET and TUNEL [Donnelly et al. [Citation2000]] and SCSA and TUNEL [Zini et al. [Citation2001]; Perera et al. [Citation2002]; Erenpreiss et al. [Citation2004]] and suggest surprisingly close correlations in DNA damage despite the differences in protocol and in the parameters supposedly measured by each assay.
Measurement of DNA Adducts
The COMET, TUNEL and SCSA assays may only be measuring ‘the tip of the iceberg’ in terms of actual underlying DNA damage [Evenson and Jost [Citation2000]; Alvarez [Citation2005b]]. Methods of measuring ‘potential’ DNA damage in terms of precursors to actual strand breaks, combined with fragmentation assays, may give a more complete picture of the extent of the DNA damage [Shen and Ong [Citation2000]] and also reveal the mechanisms by which it is occurring.
Oxidative damage takes many forms, including loss of bases, deoxyribose damage and base modification or ‘adduct’ formation. In addition to causing strand breaks [Alvarez [Citation2005b]], these adducts can cause errors in DNA replication, transcription and translation and thus have mutagenic potential [Cheng et al. [Citation1992]; Halliwell [Citation2000]], contributing to the aetiology of a number of human diseases [Cooke et al. [Citation2003]; Baker and Aitken [Citation2005]]. There are numerous oxidative DNA adducts [Croteau and Bohr [Citation1997]], however modification of the purine guanosine (7, 8-dihydro-8-oxo-2′deoxoguanosine; 8-OHdG) is the most prevalent and easily studied [Aust and Eveleigh [Citation1999]].
Levels of 8-OHdG were first evaluated in sperm by Fraga et al. [Citation[1991]]. A series of studies have reported higher levels of this marker of sperm oxidative DNA damage in infertile men [Kodama et al. [Citation1997]; Shen et al. [Citation1999]]. The levels of 8-OHdG have even been found to be predictive of male fecundity [Loft et al. [Citation2003]], with the chance of pregnancy being negatively correlated with 8-OHdG. Even though semen profiles did not differ significantly, higher levels of 8-OHdG in sperm from diabetic men have been observed, [Agbaje et al. [Citation2007]]. An inverse relationship between sperm concentration and 8-OHdG has also been reported [Kodama et al. [Citation1997]; Shen et al. [Citation1999]; Loft et al. [Citation2003]; Xu et al. [Citation2003]] while sperm motility or morphology have not revealed a similar correlation [Kodama et al. [Citation1997]; Shen et al. [Citation1999]; Loft et al. [Citation2003]; Xu et al. [Citation2003]]. This is consistent with the notion that oxidative damaged sperm DNA may still be capable of reaching and fertilizing the oocyte and therefore contributing to mutations during embryonic development [Fraga et al. [Citation1991]].
Sperm exhibit much greater levels of oxidative DNA damage as measured by 8-OHdG compared to other cell types [Kodama et al. [Citation1997]]. This supports the view that ROS are a major source of damage in sperm and sperm are more easily damaged than somatic cells. Perhaps this or other forms of DNA testing may provide a more sensitive means to diagnose disease by uncovering molecular anomalies when cellular flaws are not apparent. If these tests are to become useful as diagnostic tools, we must establish clinical thresholds. A clinical threshold for the COMET assay has not been established although inverse correlations between sperm DNA damage and embryo quality [Morris et al. [Citation2002]] and pregnancy rates following ICSI with testicular and ejaculated sperm [Lewis et al. [Citation2004]] have been reported. Over the last 5 years investigators have turned their attention to the TUNEL assay reporting a range of thresholds. As well as the lower thresholds of 12–18%, higher thresholds of 24% for ICSI success [Henkel et al. [Citation2003]], 36% for IVF success [Henkel et al. [Citation2004]], and 30% for ICSI success [Hazout et al. [Citation2006]] have been reported. Another ART outcome predicted by sperm DNA fragmentation is spontaneous abortion. This has been shown to increase four-fold when DNA damage is >5% [Benchaib et al. [Citation2007]]. The wide range of thresholds for the TUNEL assay may be related to different protocols used. Recently, an interesting although small study from Greco et al. [[Citation2005b]], showed that ICSI outcomes were higher when testicular sperm were used from 18 men who had previously had unsuccessful ICSI cycles with their highly damaged ejaculated sperm. This needs to be reconciled with the relatively large study of 303 couples where sperm DNA fragmentation measured by TUNEL was not correlated with pregnancy rates after IVF or ICSI [Huang et al. [Citation2005]].
The predictive value of DNA fragmentation tests in IVF, when using testicular sperm, which, for the most part, have low levels of DNA damage, cannot be compared to their predictive value when using ejaculated sperm with high DNA fragmentation levels. In a recent prospective study, the indication of TESA-ICSI in couples with repeated IVF failure without an apparent cause and with high DNA fragmentation levels in semen, resulted in an overall pregnancy rate of 55% (n=40) [Garcia et al. unpublished results]. Therefore, the negative predictive value of the sperm DNA fragmentation tests in IVF cycles, when ejaculated sperm with high sperm DNA fragmentation values were used, cannot necessarily be compared to the predictive value of these tests when using testicular sperm with low DNA fragmentation. This can be reconciled, at least in part, based on the following two factors: (i) the number of oocytes usually available in IVF is relatively high and (ii) in a given cohort of oocytes some may have a high sperm DNA repair capacity. In addition, embryo polymerases may also repair DNA fragmentation introduced through the paternal genome. Thus, when using ejaculated sperm, there is the possibility that a spermatozoon with intact DNA may fertilize a viable mature oocyte or that a spermatozoon with fragmented DNA may fertilize an oocyte with high DNA repair capacity. This is in contrast to the use of testicular sperm where DNA fragmentation values are usually low and, therefore, the positive predictive value of these tests in couples with idiopathic infertility would be expected to be high.
The third widely used assay for sperm DNA fragmentation, is the SCSA developed in 1980 [Evenson [Citation1980]]. The SCSA is a flow cytometric assay (FCM) based on the principle that abnormal sperm chromatin is more susceptible to DNA denaturation in-situ following exposure to heat or acid. The degree of denaturation is measured by the metachromatic shift from green fluorescence (acridine orange, AO associated with double stranded DNA) to red fluorescence (AO associated with single stranded DNA). Outcomes are measured as the percentage of spermatozoa with non-detectable (formerly the main population) and detectable (moderate and high populations) of DNA fragmentation indices (now called % DFI, formerly COMPα1). The SCSA is less specific than Comet or TUNEL in that it detects changes in protamine content and disulphide cross linkage as well as DNA fragmentation. However its advantage is that clinical thresholds have been reported both for spontaneous pregnancies and for each type of assisted conception [Evenson et al. 2007]. In the ‘Georgetown’ study [Evenson et al. [Citation1999]], couples conceived without intervention were 6.5 times more likely to become pregnant if they had DFI <30%. In the only other in vivo study to date, Spano et al. [2000] reported that in a group of 144 couples, they were 10 times more likely to achieve a pregnancy if their DFI was <40%. The utility of SCSA as a predictive test is supported by the inverse relationships between DFI and outcomes of IUI, IVF and ISCI reported in large studies [Benchaib et al. [Citation2003]; Bungum et al. [Citation2004]; Gandini et al. [Citation2004]; Virro et al. [Citation2004]]. An association between spontaneous abortions and DFI >30% has also been suggested [Zini et al. [Citation2005]]. However, there are a number of studies disputing these findings. These include the study of Bungum et al. [[Citation2004]], reporting with a small group of 13 couples that a DFI >27% did not affect pregnancy by IVF or ICSI. More recently, Bungum et al. [Citation[2007]] reported that in a study of 510 ART cycles no DFI cut off values could be set for in vivo or in vitro fertility using native semen [Bungum et al. [Citation2007]] or prepared sperm [Bungum et al. 2008]. Similarly, Benchaib et al. [[Citation2003]], showed no differences in IVF pregnancies between couples with high or low DFI.
Factors that influence the predictive value of DNA damage tests
As with all predictive tests, there are limitations to what one can expect from sperm DNA tests, given the additional variables present within each couple. For example, the age of the female partner and the quality of her oocytes will have a major impact on her ability to repair the sperm DNA damage presented at fertilization. Although DNA damage is observed at a cellular level, it is currently impossible to detect which sequences of the genome are affected. Whether the damage is occurring in the vital exons or in the much more common non-coding DNAs remains to be explored. This could account for some of the unexpected reports [Bungum et al. [Citation2004]; Gandini et al. [Citation2004]; Payne et al. [Citation2005]], of high pregnancy rates despite the male partner having severely damaged sperm DNA. The predictive value of DNA fragmentation tests depends on several factors associated with the DNA damage itself. These include:
1. Single- vs. double-stranded DNA fragmentation. In general, single-stranded DNA damage is of better prognosis than double-stranded since it is easier to repair, either by the oocyte and/or the embryo.
2. Percentage of spermatozoa with DNA damage. The higher the percentage of spermatozoa affected, the lower the probability that a spermatozoon with intact DNA will fertilize the oocyte.
3. Degree of DNA fragmentation per spermatozoon. The higher the degree of DNA fragmentation, the lower the probability that the oocyte or the embryo could repair the damage.
4. Whether there is primary and/or secondary DNA damage. For example, in cases of apoptosis or DNA nicks produced during the process of spermatogenesis and spermiogenesis, respectively, this type of damage is generally not associated with secondary damage. In sharp contrast, hydroxyl radical-induced damage or damage produced following exposure to ionizing radiation is associated to secondary damage as for example exhibited in 8-OHdG. Primary damage in this case consists, for the most part, in double-stranded DNA fragmentation.
5. Type of DNA fragmentation test used. Tests that measure DNA damage under physiological conditions like COMET at neutral pH or TUNEL would in principle have a higher predictive value than tests that measure DNA damage or susceptibility to DNA denaturation under non physiological conditions such as acid or alkaline pH, like the alkaline COMET, the SCSA or the SCD tests.
6. Whether DNA damage affects coding sequences. More than 90% of DNA consists of noncoding sequences. Therefore, the probability that DNA damage affects a protein coding sequence is relatively low.
7. Ability of the oocyte to repair sperm DNA damage in ART. The ability of the oocyte to repair fragmented DNA of the fertilizing spermatozoon will vary depending on the extent of sperm DNA fragmentation and on the cytoplasmic and genomic quality of oocytes obtained in a given ovarian stimulation cycle. The repair capacity may even vary among oocytes of a given retrieval cohort.
8. Ability of the embryo to repair DNA damage. The ability of the embryo to repair DNA damage will depend on the extent of DNA damage introduced in the embryo's genome by the fertilizing spermatozoon and embryo quality. Unlike the case of the oocyte, this property could be tested in vitro following embryo biopsy of either 8-cell embryos or, preferably, trophoectoderm cells of blastocysts.
9. Number of metaphase II oocytes. In IVF cycles where the level of DNA fragmentation is relatively high, the probability that viable embryos with normal implantation potential are produced will increase with the number of metaphase II oocytes and with their DNA damage repair capacity. If, for example, six metaphase II oocytes were available (), the probability of obtaining a viable embryo would be higher than if only three oocytes were available (). In the first case, the probability that a spermatozoon with intact DNA will fertilize the oocyte is going to be higher than in the second case. Although two high-quality embryos could be obtained (case 1), this does not imply that the DNA fragmentation test lacked predictive value. In fact, pregnancy rate per retrieval would be lower, since the pregnancy potential of the remaining embryos would be lower, whether they are directly transferred or transferred after cryopreservation. Therefore, the number of metaphase II oocytes available is an important factor that is going to impact the predictive value of the DNA fragmentation assay. This same rational could be applied to IUI cycles, where the number of oocytes obtained is usually one or two. Pregnancy rates would be lower in cases of high DNA fragmentation and, therefore, the negative predictive value would be higher than in IVF. This is supported by the results reported by Duran et al. [[Citation2002]].
Figure 1 Hypothetical case of an IVF cycle where five oocytes were microinjected with spermatozoa from a semen sample with a DNA fragmentation value of 50%, measured by TUNEL and flow cytometry. The DNA repair capacity of the oocyte is indicated as a + symbol. The higher the oocyte repair capacity, the higher the number of + symbols and vice versa. The higher the level of sperm DNA fragmentation, the higher the intensity of green fluorescence in the sperm head. Of the five embryos obtained, 2 were of good quality (2×G1) and 3 of low quality (G2, G4 and G4). The transfer of two G1 embryos resulted in a term pregnancy. Embryo quality is shown on day 3 for mere didactic purposes since, in general, the effect of sperm DNA fragmentation on embryo quality is expressed at later stages of embryo development (late paternal effects).
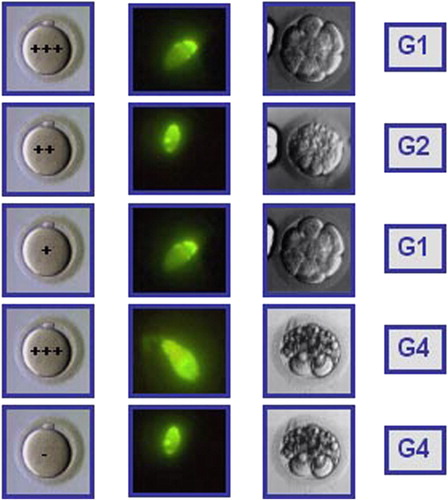
Figure 2 Hypothetical case of an IVF cycle where three oocytes were microinjected with spermatozoa from a semen sample with a DNA fragmentation value of 50%, measured by TUNEL and flow cytometry. All embryos were of low quality (G4, G2, and G4). All three embryos were transferred and no pregnancy ensued.
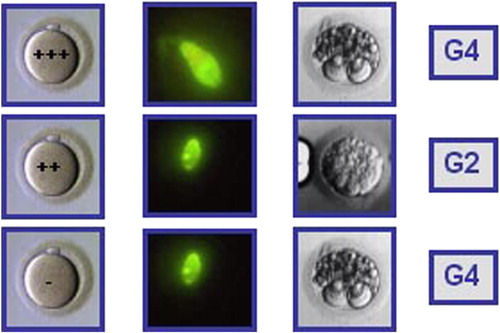
10. Sample processing. It is not unusual that during sperm processing iatrogenic DNA damage may be induced. This could be related to centrifugation of semen samples with a high concentration of ROS-producing immature sperm and/or leukocytes, or to prolonged incubation of sperm samples with a high oxidative stress potential after semen processing. In conclusion, as a first approximation, the predictive value of a DNA fragmentation test, will be the sum of multiple factors: Ptest=P(1)+P(2)+P(3)……+P(n).
For these reasons, we hold that the quest for single-value clinical thresholds is inappropriate. The goal should be to establish discrete ranges rather than single-value cut-offs. For example, the analysis of the same sample using the same DNA fragmentation assay, i.e., TUNEL, performed in different laboratories produces significantly different values. Given the potential inter-laboratory variability, a range between 15 and 25% should be recommended. Additionally, reference laboratories may have an important role in such testing.
It is thus inappropriate to attempt to enforce any threshold above which patients will be informed that they will be unsuccessful and therefore should not be treated. Nevertheless, assisted reproduction is expensive financially and emotionally, highly invasive and the long-term consequences remain unknown. Therefore, couples justifiably want to know the likelihood of success before embarking on a treatment cycle. These patients have a right to the fullest information that we can provide.
In a position paper from the Practice Committee of the American Society for Reproductive Medicine, it was accepted that “[fragmented] sperm DNA is more common in infertile men and may contribute to poor reproductive performance” [ASRM Citation[2006, p. S37]]. However, the authors commented that, as yet “there was no proven role for routine DNA integrity testing in the evaluation of infertility” since “current methods do not reliably predict treatment outcomes” [ASRM 2006, p. S35]. The ASRM Practice Committee [[Citation2006]] concluded that current data is only sufficient to suggest rather than confirm an association between sperm DNA testing and ART outcomes. This is indeed a matter of pressing concern to all. The breakout group at the 1st international Conference on Male Mediated Developmental Toxicity [Perreault et al. [Citation2003]] first highlighted this issue and recommended the development of consensus documents standardizing protocols and the participation of international multi center groups to provide a program with adequate power to give definitive results. Unfortunately lack of funding has impeded progress. Nevertheless, this strategy must be implemented in order to either confirm or refute the prognostic usefulness of sperm DNA testing.
Recently, there have been numerous reviews highlighting sperm DNA tests as holding promise [Holt [Citation2005]; De Iuliis et al. [Citation2006]; Makhlouf and Niederger [Citation2006]; Evenson et al. [Citation2007]; Lewis [Citation2007]; Ozmen et al. [Citation2007]]. Given the recent acknowledgment that semen analysis itself is of limited clinical utility [Guzick et al. [Citation2001]; Jequier [Citation2005]; Lewis [Citation2007]], it is now even more important to identify those tests with improved diagnostic power.
The Potential Effects of ICSI on Subsequent Children's Health
Progress in developing useful prognostic tests has been impeded by the short-term success of intracytoplasmic sperm injection (ICSI). This therapeutic technique allows us to bypass the natural hurdles to fertilization so that even unfit sperm succeed. The consequence of this has been a further reduction in the diagnostic significance of parameters such as sperm concentration, motility and morphology but without the inclusion of more appropriate selection tests. ICSI allows previously untreatable patients the chance to become genetic fathers, by circumventing most of the biological processes involved in natural conception. However, the attainment of this therapeutic advance has been achieved at what cost to the offspring? From the inception of ICSI, there were concerns that ICSI babies would show an increase in sex chromosome abnormalities based on the higher incidence of cytogenetic abnormalities in men with oligozoospermia and obstructive azoospermia, who are typical of the patient population treated with this procedure [Hewitson et al. [Citation1999]]. In 1998, the ESHRE ICSI Task Force of ICSI child and family outcomes group over a three year period confirmed the findings of smaller studies; reporting a small increased risk (2%) of chromosomal abnormalities in ICSI babies [Tarlatzis and Bill [Citation1998]]. An International Collaborative 5-year study [2004] reported no differences in cognitive, neurological or socio-economic development although Wennerholm et al. [[Citation2000]] highlighted an increase in urogenital problems in males. Further, in 2004 a meta-analysis [Rimm et al. [Citation2004]] reported major malformations rates ranging between 1.1–9.7% in ICSI compared with 0–6.9% in spontaneously conceived infants. However they stressed that some of the studies were not adequately controlled, including infertile patients who had then conceived without treatment and suggested that this increased the rate of malformations in the controls. In marked contrast, Hansen et al. [2005] undertook a systematic review of data relating to birth defects in infants following ICSI and or IVF compared with spontaneously conceived infants and concluded that there was a dramatic 30–40% increase of birth defects associated with ART. This latest paper has led to renewed interest and active debate. Further, it will not be until the end of these childrens’ lives that we will know the effects of ICSI on long term health of children thus conceived. With this view, it is particularly important to monitor the genetic integrity of the gametes used for ART, especially ICSI [Singh et al. [Citation2003]; Aitken [Citation2004]].
Sperm DNA damage and fertilization
Since sperm with damaged DNA can still achieve fertilization [Ahmadi and Ng Citation[1999a; b]] with subsequent early embryonic development [Braude et al. [Citation1988]; Tesarik et al. [Citation2004]] it is conceivable that sperm with damaged DNA may retain the potential to produce a viable embryo. The oocyte is limited in its ability to repair damaged sperm DNA [Matsuda et al. [Citation1989]; Genesca et al. [Citation1992]]. If inadequately repaired, such damage can be indicative of a predisposition to mutations in the developing embryo with the potential to induce disease in the offspring [Aitken and Krausz [Citation2001]]. It is acknowledged that a greater number of inherited diseases have their origin in the paternal as opposed to the maternal germ line [Crow [Citation2000]]. Additionally, cancers arising from germ cell mutations show a much greater paternal contribution [Vogel and Rathenberg [Citation1975]]. This fact is further illustrated in the finding of higher rates of cancers (leukemias and lymphomas) in men who smoke [Ji et al. [Citation1997]] and thus have increased levels of oxidatively damaged sperm DNA [Fraga et al. [Citation1996]].
The relationships between sperm DNA damage and fertilization have been reviewed by Aitken and Sawyer [[Citation2003]]. This group has shown that at low levels of oxidative stress, DNA damage was induced yet the fertilizing potential of the spermatozoa was actually enhanced, reflecting the importance of cellular redox status in driving the tyrosine phosphorylation events associated with sperm capacitation [Aitken et al. [Citation2003b]; Aitken and Sawyer [Citation2003]]. These results are clinically significant since supporting the study of Braude et al. [[Citation1988]] that since the oocyte controls fertilization and early cleavage stages, sperm with damaged DNA still have fertilizing potential. The damage can become part of the next generation's genome.
Additional studies have shown that in vitro fertilization is negatively correlated with DNA damage [Aitken [Citation2004]] as opposed to ICSI. With the latter, successful fertilization is not disrupted by high levels of DNA damage in the injected spermatozoa, regardless of whether ejaculated or testicular spermatozoa are used in the course of therapy [Sun et al. [Citation1997]; Hammadeh et al. [Citation1998]; Lopes et al. [Citation1998]; Twigg et al. [Citation1998]; Esterhuizen et al. [Citation2000]; Host et al. [Citation2000]; Aitken [Citation2004]; Lewis et al. [Citation2004]]. ICSI does not depend on the functional competence of the spermatozoa in terms of the ability of these cells to capacitate, acrosome react, penetrate the zona pellucida or fuse with the vitelline membrane of the oocyte.
Although the maintenance, and indeed survival, of somatic cells depends on the integrity of the genome, sperm appear to function without significant nuclear control. Even in IVF, where sperm competence is a prerequisite for fertilization, there is conflicting evidence about the relationship between sperm DNA fragmentation and fertilization rates. The presence of damaged DNA regardless of the degree of damage remains to be clarified. For example, it was reported to have no effect on fertilization in one study [Ahmadi and Ng 1999] but was shown to have a significant inverse relationship in other studies [Sun et al. [Citation1997]; Host et al. [Citation2000]] and to contribute to a failure of fertilization even in ISCI [Lopes et al. [Citation1998]].
Sperm DNA damage and pre-implantation development
While fertilization may be independent of sperm DNA integrity, the post-fertilization development of the embryo can be impaired by such damage. After the third cleavage, the paternal genome exerts its influence [Braude et al. [Citation1988]; Tesarik et al. [Citation2004]] and evidence of DNA damage is observed in stunted development. Preimplantation development has been negatively correlated with DNA damage as assessed by Sakkas et al. [[Citation1998]], the COMET assay [Morris et al. [Citation2002]] TUNEL [Sun et al. [Citation1997]; Ahmadi and Ng 1999] and SCSA [Saleh et al. [Citation2003]; Erenpreiss et al. [Citation2006]]. In addition, assessment of nuclear packaging [Filatov et al. 1999] has shown that abnormalities in chromatin structure are associated with reduced rates of embryo cleavage, again stressing the association between chromatin integrity and embryo development.
Sperm DNA damage and pregnancy
Artificial insemination cycles where sperm have high levels of DNA fragmentation negatively correlate with pregnancy [Duran et al. [Citation2002]]. An inverse relationship has also been reported between pregnancy rates with ICSI and the level of DNA fragmentation in testicular [Lewis et al. [Citation2004]] and ejaculated sperm [Benchaib et al. [Citation2003]; Bungum et al. [Citation2004]; Lewis et al. [Citation2004]; Virro et al. [Citation2004]; Erenpreiss et al. [Citation2006]]. Moreover, in a large group of Danish first pregnancy planners, fertile couples took longer to conceive by natural conception, the time to pregnancy increasing as a function of the proportion of sperm with abnormal chromatin according to the SCSA assay [Spano et al. [Citation2000]]. Loft et al. [[Citation2003]] have also reported that the likelihood of pregnancy occurring in a single menstrual cycle was inversely associated with DNA adducts suggesting a major cause of this DNA damage may be oxidative stress.
Sperm DNA damage and pregnancy loss
The rate of spontaneous abortion after ART cycles is markedly higher than in the general population [Simpson et al. 2001], reducing the already low (25%) ART success rates and increasing the psychological distress of infertile patients. Approximately 50–70% of spontaneous abortions in natural conceptions can be attributed to aneuploidies, mostly trisomies [Philipp and Kalousek [Citation2002]] with the remaining 30–50% remaining unexplained and possibly due to unknown genetic and epigenetic factors [Shi and Haaf [Citation2002]]. Unexplained recurrent pregnancy loss has been associated with a variety of DNA anomalies including sperm DNA fragmentation [Carrell et al. [Citation2003]; Saleh et al. [Citation2003]]. Furthermore, an increased mutation frequency caused by testicular toxicants (reviewed by [Hales and Robaire [Citation2001]]) has been shown to cause a marked increase in postimplantation embryonic loss in animal models. The abortion rate was higher in apparently fertile couples where the partner's sperm had poor chromatin quality [Evenson et al. [Citation1999]]. In addition, higher rates of pregnancy loss have been documented in ICSI as compared to IVF cycles [Bar-Hava et al. [Citation1997]]. As these couples presented with male infertility and sperm incapable of fertilizing an oocyte naturally, there may well be a link between their negative outcome and sperm DNA damage.
Sperm DNA damage and the health of future generations
While sperm with damaged DNA may have a reduced capacity for fertilization and implantation, they are still capable of achieving both biological outcomes with ART intervention [Twigg et al. [Citation1998]; Gandini et al. [Citation2004]]. The effect of sperm DNA damage on the health of future generations is not yet known. Epidemiological data has shown an association between paternal occupations involving exposure to metals, solvents and pesticides and an increase in birth defects and childhood diseases [Olshan and Mattison [Citation1994]]. Animal studies have also given clear indications that sperm damaged by paternal exposure to cancer therapeutic agents transmit heritable translocations, mutations and malformations such as hydrocephaly and micrognathia to the next generation [Trasler et al. [Citation1986]; Robaire and Hales 1999; Trasler and Doerksen [Citation1999]]. Cigarette smoke is a particularly noxious mutagen [Smith et al. [Citation2000]] that can induce DNA damage in the sperm [Arabi and Moshtaghi [Citation2005]] and thereby increase the chances of childhood cancer in the offspring [Ji et al. [Citation1997]; Sorahan et al. [Citation1997]]. One epidemiological study [Sorahan et al. [Citation1997]], has reported that 14% of all such cancers are linked to paternal smoking. Recently, an increased incidence of schizophrenia, achondroplasia and Apert's syndrome has also been reported in children of older men with high levels of sperm DNA damage [Aitken and De Illius [Citation2007]]. There is little doubt, that sperm DNA damage has a negative impact at each check point of fertility.
Urgency of Appropriate Test implementation
The advent of ICSI has provided a means of treatment for infertile couples with severe male factor infertility previously considered untreatable by conventional artificial reproductive technologies (ART). Since success rates with ICSI approximate that of IVF, there has been little incentive for the development of sperm selection tests to date. However, in general terms, ART is in its ‘infancy’, with comparatively little long-term data on the health and well being of subsequent generations. With this in mind, increasing interest in the functional and genomic integrity of male gametes used in ART is inevitable.
Despite the rapid advances in ART techniques, success rates have not kept apace. In Europe they are similar [Andersen 2002] to those achieved a decade ago [Harari et al. [Citation1995]; Van den Bergh et al. [Citation2005]; Van Steirteghem et al. [Citation1993]]. As a matter of urgency the clinical and scientific community must revisit the way in which male fertility potential is assessed and develop sperm structure and function tests with established thresholds of clinical relevance and prognostic value.
Acknowledgements
The authors would like to thank Mrs Margaret Kennedy, Biomedical Scientist, Andrology Laboratory, Royal Jubilee Maternity Service, Belfast and the staff of the Regional Centre for Endocrinology and Diabetes, Royal Victoria Hospital, Belfast, for their contributions to this work. The authors thank their sponsors The Wellcome Trust, The Fertility Research Trust, and Northern Ireland Research and Development Office, Northern Ireland.
References
- Agarwal, A. and Said, T. M. (2004) Sperm Chromatin Assessment. Textbook of ART, PLC, London, UK. 7:93–106.
- Agarwal A., Said T. M., Bedaiwy M. A., Banerjee J., Alvarez J. G. Oxidative stress in an assisted reproductive techniques setting. Fertil Steril 2006; 86: 503–512
- Agbaje I. M., McVicar C. M., Schock B. C., McClure N., Atkinson A. B., Rogers D. A., Lewis S. E. M. Increased levels of the Oxidative DNA adduct 7, 8-dihydro-8-oxo-2′-deoxoguanosine in the germ-line of men with type-1 diabetes. Reprod Biomed 2007; 16: 401–409
- Ahmadi A., Ng S. C. Developmental capacity of damaged spermatozoa. Hum Reprod 1999a; 14: 2279–2285
- Ahmadi A., Ng S. C. Fertilizing ability of DNA-damaged spermatozoa. Journal of Experimental Zoology 1999b; 284: 696–704
- Aitken J., Fisher H. Reactive oxygen species generation and human spermatozoa: the balance of benefit and risk. Bioessays 1994; 16: 259–267
- Aitken R. J. Founders’ Lecture. Human spermatozoa: fruits of creation, seeds of doubt. Reprod Fertil Dev 2004; 16: 655–664
- Aitken R. J. Sperm function tests and fertility. Int J Androl 2006; 29: 69–75, discussion 105–108
- Aitken R. J., Baker M. A., Sawyer D. Oxidative stress in the male germ line and its role in the aetiology of male infertility and genetic disease. Reprod Biomed Online 2003a; 7: 65–70
- Aitken R. J., Ryan A. L., Curry B. J., Baker M. A. Multiple forms of redox activity in populations of human spermatozoa. Mol Hum Reprod 2003b; 9: 645–661
- Aitken R. J., De Luliis G. N. Value of DNA integrity assays for fertility evaluation. Soc Reprod Fertil Suppl 2007; 65: 81–92
- Aitken R. J., Gordon E., Harkiss D., Twigg J. P., Milne P., Jennings Z., Irvine D. S. Relative impact of oxidative stress on the functional competence and genomic integrity of human spermatozoa. Biol Reprod 1998; 59: 1037–1046
- Aitken R. J., Krausz C. Oxidative stress, DNA damage and the Y chromosome. Reproduction 2001; 122: 497–506
- Aitken R. J., Sawyer D. The human spermatozoon--not waving but drowning. Adv Exp Med Biol 2003; 518: 85–98
- Alvarez J. G. ‘Efficient treatment of infertility due to sperm DNA damage by ICSI with testicular sperm’. Hum Reprod 2005a; 20: 2031–2032, author reply 2032–2033
- Alvarez J. G. The predictive value of sperm chromatin structure assay. Hum Reprod 2005b; 20: 2365–2367
- Andersen A. N., Erb K. Register data on assisted reproductive technology (ART) in Europe. Including a detailed description of ART in Denmark. International Journal of Andrology 2006; 29: 12–16
- Arabi M., Moshtaghi H. Influence of cigarette smoking on spermatozoa via seminal plasma. Andrologia 2005; 37: 119–124
- Aravindan G. R., Bjordahl J., Jost L. K., Evenson D. P. Susceptibility of human sperm to in situ DNA denaturation is strongly correlated with DNA strand breaks identified by single-cell electrophoresis. Exp Cell Res 1997; 236: 231–237
- ASRM. The clinical utility of sperm DNA integrity testing. Fertil Steril 2006; 86: S35–S37
- Aust A. E., Eveleigh J. F. Mechanisms of DNA oxidation. Proc Soc Exp Biol Med 1999; 222: 246–252
- Baker M. A., Aitken R. J. Reactive oxygen species in spermatozoa: methods for monitoring and significance for the origins of genetic disease and infertility. Reprod Biol Endocrinol 2005; 3: 67
- Bar-Hava I., Ashkenazi J., Shelef M., Schwartz A., Brengauz M., Feldberg D., Orvieto R., Ben-Rafael Z. Morphology and clinical outcomes of embryos after in vitro fertilization are superior to those after intracytoplasmic sperm injection. Fertil Steril 1997; 68: 653–657
- Benchaib M., Braun V., Lornage J., Hadj S., Salle B., Lejeune H., Guerin J. F. Sperm DNA fragmentation decreases the pregnancy rate in an assisted reproductive technique. Hum Reprod 2003; 18: 1023–1028
- Benchaib M., Lornage J., Mazoyer C., Lejeune H., Salle B., Francois Guerin J. Sperm deoxyribonucleic acid fragmentation as a prognostic indicator of assisted reproductive technology outcome. Fertil Steril 2007; 87: 93–100
- Braude P., Bolton V., Moore S. Human gene expression first occurs between the four- and eight-cell stages of preimplantation development. Nature 1988; 332: 459–461
- Bungum M., Humaidan P., Axmon A., Spano M., Bungum L., Erenpreiss J., Giwercman A. Sperm DNA integrity assessment in prediction of assisted reproduction technology outcome. Hum Reprod 2007; 22: 174–179
- Bungum M., Humaidan P., Spano M., Jepson K., Bungum L., Giwercman A. The predictive value of sperm chromatin structure assay (SCSA) parameters for the outcome of intrauterine insemination, IVF and ICSI. Hum Reprod 2004; 19: 1401–1408
- Bungum M., Spanò M., Humaidan P., Eleuteri P., Rescia M., Giwercman A. Sperm chromatin structure assay parameters measured after density gradient centrifugation are not predictive for the outcome of ART. Hum Reprod 2008; 1: 4–10
- Carrell D. T., Liu L., Peterson C. M., Jones K. P., Hatasaka H. H., Erickson L., Campbell B. Sperm DNA fragmentation is increased in couples with unexplained recurrent pregnancy loss. Arch Androl 2003; 49: 49–55
- Cheng K. C., Cahill D. S., Kasai H., Nishimura S., Loeb L. A. 8-Hydroxyguanine, an abundant form of oxidative DNA damage, causes G—T and A—C substitutions. J Biol Chem 1992; 267: 166–172
- Cooke M. S., Evans M. D., Dizdaroglu M., Lunec J. Oxidative DNA damage: mechanisms, mutation, and disease. Faseb J 2003; 17: 1195–1214
- Croteau D. L., Bohr V. A. Repair of oxidative damage to nuclear and mitochondrial DNA in mammalian cells. J Biol Chem 1997; 272: 25409–25412
- Crow J. F. The origins, patterns and implications of human spontaneous mutation. Nat Rev Genet 2000; 1: 40–47
- Cummins J. M., Jequier A. M., Kan R. Molecular biology of human male infertility: links with aging, mitochondrial genetics, and oxidative stress?. Mol Reprod Dev 1994; 37: 345–362
- De Iuliis G. N., Wingate J. K., Koppers A. J., McLaughlin E. A., Aitken R. J. Definitive evidence for the nonmitochondrial production of superoxide anion by human spermatozoa. J Clin Endocrinol Metab 2006; 91: 1968–1975
- Donnelly E. T., McClure N., Lewis S. E. The effect of ascorbate and alpha-tocopherol supplementation in vitro on DNA integrity and hydrogen peroxide-induced DNA damage in human spermatozoa. Mutagenesis 1999; 14: 505–512
- Donnelly E. T., O'Connell M., McClure N., Lewis S. E. Differences in nuclear DNA fragmentation and mitochondrial integrity of semen and prepared human spermatozoa. Hum Reprod 2000; 15: 1552–1561
- Duran E. H., Morshedi M., Taylor S., Oehninger S. Sperm DNA quality predicts intrauterine insemination outcome: a prospective cohort study. Hum Reprod 2002; 17: 3122–3128
- Erenpreiss J., Bungum M., Spano M., Elzanaty S., Orbidans J., Giwercman A. Intra-individual variation in sperm chromatin structure assay parameters in men from infertile couples: clinical implications. Hum Reprod 2006; 21: 2061–2064
- Erenpreiss J., Jepson K., Giwercman A., Tsarev I., Erenpreisa J., Spano M. Toluidine blue cytometry test for sperm DNA conformation: comparison with the flow cytometric sperm chromatin structure and TUNEL assays. Hum Reprod 2004; 19: 2277–2282
- ESHRE Andrology Special Interest Group. Andrology: Consensus workshop on advanced diagnostic andrology techniques. Hum Reprod 1996; 11(7)1463–1479
- Esterhuizen A. D., Franken D. R., Lourens J. G., Prinsloo E., van Rooyen L. H. Sperm chromatin packaging as an indicator of in-vitro fertilization rates. Hum Reprod 2000; 15: 657–661
- Evenson D. Relation of mamalian sperm chromatin heterogeneity to fertility. Science 1980; 210: 1131–1133
- Evenson D., Jost L. Sperm chromatin structure assay is useful for fertility assessment. Methods Cell Sci 2000; 22: 169–189
- Evenson, D., Kasperson, K., Wixon, R. Analysis of sperm DNA fragmentation using flow cytometry and other techniques. Nottingham University Press. 2007
- Evenson D. P., Jost L. K., Marshall D., Zinaman M. J., Clegg E., Purvis K., de Angelis P., Claussen O. P. Utility of the sperm chromatin structure assay as a diagnostic and prognostic tool in the human fertility clinic. Hum Reprod 1999; 14: 1039–1049
- Evenson D. P., Larson K. L., Jost L. K. Sperm chromatin structure assay: its clinical use for detecting sperm DNA fragmentation in male infertility and comparisons with other techniques. J Androl 2002; 23: 25–43
- Fraga C. G., Motchnik P. A., Shigenaga M. K., Helbock H. J., Jacob R. A., Ames B. N. Ascorbic acid protects against endogenous oxidative DNA damage in human sperm. Proc Natl Acad Sci USA 1991; 88: 11003–11006
- Fraga C. G., Motchnik P. A., Wyrobek A. J., Rempel D. M., Ames B. N. Smoking and low antioxidant levels increase oxidative damage to sperm DNA. Mutat Res 1996; 351: 199–203
- Gandini L., Lombardo F., Paoli D., Caruso F., Eleuteri P., Leter G., Ciriminna R., Culasso F., Dondero F., Lenzi A., et al. Full-term pregnancies achieved with ICSI despite high levels of sperm chromatin damage. Hum Reprod 2004; 19: 1409–1417
- Genesca A., Caballin M. R., Miro R., Benet J., Germa J. R., Egozcue J. Repair of human sperm chromosome aberrations in the hamster egg. Hum Genet 1992; 89: 181–186
- Gorczyca W., Gong J., Darzynkiewicz Z. Detection of DNA strand breaks in individual apoptotic cells by the in situ terminal deoxynucleotidyl transferase and nick translation assays. Cancer Res 1993; 53: 1945–1951
- Greco E., Romano S., Iacobelli M., Ferrero S., Baroni E., Minasi M. G., Ubaldi F., Rienzi L., Tesarik J. ICSI in cases of sperm DNA damage: beneficial effect of oral antioxidant treatment. Hum Reprod 2005a; 20: 2590–2594
- Greco E., Scarselli F., Iacobelli M., Rienzi L., Ubaldi F., Ferrero S., Franco G., Anniballo N., Mendoza C., Tesarik J. Efficient treatment of infertility due to sperm DNA damage by ICSI with testicular spermatozoa. Hum Reprod 2005b; 20: 226–230
- Guzick D. S., Overstreet J. W., Factor-Litvak P., Brazil C. K., Nakajima S. T., Coutifaris C., Carson S. A., Cisneros P., Steinkampf M. P., Hill J. A., et al. Sperm morphology, motility, and concentration in fertile and infertile men. N Engl J Med 2001; 345: 1388–1393
- Hales B. F., Robaire B. Paternal exposure to drugs and environmental chemicals: effects on progeny outcome. J Androl 2001; 22: 927–936
- Halliwell B. Why and how should we measure oxidative DNA damage in nutritional studies? How far have we come?. Am J Clin Nutr 2000; 72: 1082–1087
- Hammadeh M. E., Stieber M., Haidl G., Schmidt W. Association between sperm cell chromatin condensation, morphology based on strict criteria, and fertilization, cleavage and pregnancy rates in an IVF program. Andrologia 1998; 30: 29–35
- Hansen M., Bower C., Milne E., de Klerk N., Kurinczuk J. J. Assisted reproductive technologies and the risk of birth defects—a systematic review. Hum Reprod 2005; 20: 328–338
- Harari O., Bourne H., McDonald M., Richings N., Speirs A. L., Johnston W. I., Baker H. W. Intracytoplasmic sperm injection: a major advance in the management of severe male subfertility. Fertil Steril 1995; 64: 360–368
- Hazout A., Dumont-Hassan M., Junca A. M., Cohen Bacrie P., Tesarik J. High-magnification ICSI overcomes paternal effect resistant to conventional ICSI. Reprod Biomed Online 2006; 12: 19–25
- Henkel R., Hajimohammad M., Stalf T., Hoogendijk C., Mehnert C., Menkveld R., Gips H., Schill W. B., Kruger T. F. Influence of deoxyribonucleic acid damage on fertilization and pregnancy. Fertil Steril 2004; 81: 965–972
- Henkel R., Kierspel E., Hajimohammad M., Stalf T., Hoogendijk C., Mehnert C., Menkveld R., Schill W. B., Kruger T. F. DNA fragmentation of spermatozoa and assisted reproduction technology. Reprod Biomed Online 2003; 7: 477–484
- Hewitson L., Dominko T., Takahashi D., Martinovich C., Ramalho-Santos J., Sutovsky P., Fanton J., Jacob D., Monteith D., Neuringer M., et al. Unique checkpoints during the first cell cycle of fertilization after intracytoplasmic sperm injection in rhesus monkeys. Nat Med 1999; 5: 431–433
- Holt W. V. Is quality assurance in semen analysis still really necessary? A spermatologist's viewpoint. Hum Reprod 2005; 20: 2983–2986
- Host E., Lindenberg S., Smidt-Jensen S. The role of DNA strand breaks in human spermatozoa used for IVF and ICSI. Acta Obstet Gynecol Scand 2000; 79: 559–563
- Huang C. C., Lin D. P., Tsao H. M., Cheng T. C., Liu C. H., Lee M. S. Sperm DNA fragmentation negatively correlates with velocity and fertilization rates but might not affect pregnancy rates. Fertil Steril 2005; 84: 130–140
- Irvine D. S., Twigg J. P., Gordon E. L., Fulton N., Milne P. A., Aitken R. J. DNA integrity in human spermatozoa: relationships with semen quality. J Androl 2000; 21: 33–44
- Jequier A. M. Is quality assurance in semen analysis still really necessary? A clinician's viewpoint. Hum Reprod 2005; 20: 2039–2042
- Ji B. T., Shu X. O., Linet M. S., Zheng W., Wacholder S., Gao Y. T., Ying D. M., Jin F. Paternal cigarette smoking and the risk of childhood cancer among offspring of nonsmoking mothers. J Natl Cancer Inst 1997; 89: 238–244
- Kodama H., Yamaguchi R., Fukuda J., Kasai H., Tanaka T. Increased oxidative deoxyribonucleic acid damage in the spermatozoa of infertile male patients. Fertil Steril 1997; 68: 519–524
- Lewis S. Is sperm evaluation useful in predicting human fertility?. Reproduction 2007; 134: 1–11
- Lewis S. E., Aitken R. J. DNA damage to spermatozoa has impacts on fertilization and pregnancy. Cell Tissue Res 2005; 322: 33–41
- Lewis S. E., Boyle P. M., McKinney K. A., Young I. S., Thompson W. Total antioxidant capacity of seminal plasma is different in fertile and infertile men. Fertil Steril 1995; 64: 868–870
- Lewis S. E., O'Connell M., Stevenson M., Thompson-Cree L., McClure N. An algorithm to predict pregnancy in assisted reproduction. Hum Reprod 2004; 19: 1385–1394
- Loft S., Kold-Jensen T., Hjollund N. H., Giwercman A., Gyllemborg J., Ernst E., Olsen J., Scheike T., Poulsen H. E., Bonde J. P. Oxidative DNA damage in human sperm influences time to pregnancy. Hum Reprod 2003; 18: 1265–1272
- Lopes S., Sun J. G., Jurisicova A., Meriano J., Casper R. F. Sperm deoxyribonucleic acid fragmentation is increased in poor quality semen samples and correlates with failed fertilization in intracytoplasmic sperm injection. Fertil Steril 1998; 69: 528–532
- Lysiak J. J., Zheng S., Woodson R., Turner T. T. Caspase-9-dependent pathway to murine germ cell apoptosis: mediation by oxidative stress, BAX, and caspase 2. Cell Tissue Res 2007; 328: 411–419
- Makhlouf A. A., Niederberger C. DNA integrity tests in clinical practice: it is not a simple matter of black and white (or red and green). J Androl 2006; 27: 316–323
- Matsuda Y., Tobari I., Maemori M., Seki N. Mechanism of chromosome aberration induction in the mouse egg fertilized with sperm recovered from postmeiotic germ cells treated with methyl methanesulfonate. Mutat Res 1989; 214: 165–180
- McVicar C. M., McClure N., Williamson K., Dalzell L. H., Lewis S. E. Incidence of Fas positivity and deoxyribonucleic acid double-stranded breaks in human ejaculated sperm. Fertil Steril 2004; 81(Suppl 1)767–774
- Moore H. The influence of the epididymis on human and animal sperm maturation and storage. Hum Reprod 1996; 11(Suppl)103–110
- Morris I. D., Ilott S., Dixon L., Brison D. R. The spectrum of DNA damage in human sperm assessed by single cell gel electrophoresis (Comet assay) and its relationship to fertilization and embryo development. Hum Reprod 2002; 17: 990–998
- O'Connell M., McClure N., Lewis S. E. Mitochondrial DNA deletions and nuclear DNA fragmentation in testicular and epididymal human sperm. Hum Reprod 2002; 17: 1565–1570
- Ollero M., Gil-Guzman E., Lopez M. C., Sharma R. K., Agarwal A., Larson K., Evenson D., Thomas A. J., Jr., Alvarez J. G. Characterization of subsets of human spermatozoa at different stages of maturation: implications in the diagnosis and treatment of male infertility. Hum Reprod 2001; 16: 1912–1921
- Olshan, A., Mattison, D. Male mediated developmental toxicity, Plenum Press, New York 1994
- Ozmen B., Koutlaki N., Youssry M., Diedrich K., Al-Hasani S. DNA damage of human spermatozoa in assisted reproduction: origins, diagnosis, impacts and safety. Reprod Biomed Online 2007; 14: 384–395
- Payne J. F., Raburn D. J., Couchman G. M., Price T. M., Jamison M. G., Walmer D. K. Redefining the relationship between sperm deoxyribonucleic acid fragmentation as measured by the sperm chromatin structure assay and outcomes of assisted reproductive techniques. Fertil Steril 2005; 84: 356–364
- Perera D., Pizzey A., Campbell A., Katz M., Porter J., Petrou M., Irvine D. S., Chatterjee R. Sperm DNA damage in potentially fertile homozygous beta-thalassaemia patients with iron overload. Hum Reprod 2002; 17: 1820–1825
- Perreault S. D., Aitken R. J., Baker H. W., Evenson D. P., Huszar G., Irvine D. S., Morris I. D., Morris R. A., Robbins W. A., Sakkas D., et al. Integrating new tests of sperm genetic integrity into semen analysis: breakout group discussion. Adv Exp Med Biol 2003; 518: 253–268
- Philipp T., Kalousek D. K. Generalized abnormal embryonic development in missed abortion: embryoscopic and cytogenetic findings. Am J Med Genet 2002; 111: 43–47
- Platts A. E., Dix D. J., Chemes H. E., Thompson K. E., Goodrich R., Rockett J. C., Rawe V. Y., Quintana S., Diamond M. P., Strader L. F., Krawetz S. A. Success and failure in human spermatogenesis as revealed by teratozoospermic RNAs. Hum Mol Genet 2007; 16: 763–773
- Ponjaert-Kristoffersen I., Tjus T., Nekkebroek J., et al. Psychological follow-up study of 5-year-old ICSI children. Hum Reprod 2004; 19: 2791–2797
- Rimm A. A., Katayama A. C., Diaz M., Katayama K. P. A meta-analysis of controlled studies comparing major malformation rates in IVF and ICSI infants with naturally conceived children. J Assist Reprod Genet 2004; 21: 437–443
- Sakkas D., Mariethoz E., St John J. C. Abnormal sperm parameters in humans are indicative of an abortive apoptotic mechanism linked to the Fas-mediated pathway. Exp Cell Res 1999; 251: 350–355
- Sakkas D., Urner F., Bizzaro D., Manicardi G., Bianchi P. G., Shoukir Y., Campana A. Sperm nuclear DNA damage and altered chromatin structure: effect on fertilization and embryo development. Hum Reprod 1998; 13(Suppl 4)11–19
- Saleh R. A., Agarwal A., Nada E. A., El-Tonsy M. H., Sharma R. K., Meyer A., Nelson D. R., Thomas A. J. Negative effects of increased sperm DNA damage in relation to seminal oxidative stress in men with idiopathic and male factor infertility. Fertil Steril 2003; 79(Suppl 3)1597–1605
- Sergerie M., Laforest G., Bujan L., Bissonnette F., Bleau G. Sperm DNA fragmentation: threshold value in male fertility. Hum Reprod 2005; 20: 3446–3451
- Shaman J. A., Yamauchi Y., Ward W. S. Sperm DNA fragmentation: awakening the sleeping genome. Biochem Soc Trans 2007; 35: 626–628
- Shen H., Ong C. Detection of oxidative DNA damage in human sperm and its association with sperm function and male infertility. Free Radic Biol Med 2000; 28: 529–536
- Shen H. M., Chia S. E., Ong C. N. Evaluation of oxidative DNA damage in human sperm and its association with male infertility. J Androl 1999; 20: 718–723
- Shi W., Haaf T. Aberrant methylation patterns at the two-cell stage as an indicator of early developmental failure. Mol Reprod Dev 2002; 63: 329–334
- Singh N. P., Muller C. H., Berger R. E. Effects of age on DNA double-strand breaks and apoptosis in human sperm. Fertil Steril 2003; 80: 1420–1430
- Smith C. J., Perfetti T. A., Mullens M. A., Rodgman A., Doolittle D. J. “IARC group 2B Carcinogens” reported in cigarette mainstream smoke. Food Chem Toxicol 2000; 38: 825–848
- Sorahan T., Prior P., Lancashire R. J., Faux S. P., Hulten M. A., Peck I. M., Stewart A. M. Childhood cancer and parental use of tobacco: deaths from 1971 to 1976. Br J Cancer 1997; 76: 1525–1531
- Spano M., Bonde J. P., Hjollund H. I., Kolstad H. A., Cordelli E., Leter G. Sperm chromatin damage impairs human fertility. The Danish First Pregnancy Planner Study Team. Fertil Steril 2000; 73: 43–50
- Steele E. K., McClure N., Maxwell R. J., Lewis S. E. A comparison of DNA damage in testicular and proximal epididymal spermatozoa in obstructive azoospermia. Mol Hum Reprod 1999; 5: 831–835
- Suganuma R., Yanagimachi R., Meistrich M. L. Decline in fertility of mouse sperm with abnormal chromatin during epididymal passage as revealed by ICSI. Hum Reprod 2005; 20: 3101–3108
- Sun J. G., Jurisicova A., Casper R. F. Detection of deoxyribonucleic acid fragmentation in human sperm: correlation with fertilization in vitro. Biol Reprod 1997; 56: 602–607
- Tarlatzis B. C., Bili H. Survey on intracytoplasmic sperm injection: report from the ESHRE ICSI Task Force. European Society of Human Reproduction and Embryology. Hum Reprod 1998; 13(Suppl 1)165–177
- Tesarik J., Greco E., Mendoza C. Late, but not early, paternal effect on human embryo development is related to sperm DNA fragmentation. Hum Reprod 2004; 19: 611–615
- Tesarik J., Mendoza-Tesarik R., Mendoza C. Sperm nuclear DNA damage: update on the mechanism, diagnosis and treatment. Reprod Biomed Online 2006; 12: 715–721
- Trasler J. M., Doerksen T. Teratogen update: paternal exposures-reproductive risks. Teratology 1999; 60: 161–172
- Trasler J. M., Hales B. F., Robaire B. Chronic low dose cyclophosphamide treatment of adult male rats: effect on fertility, pregnancy outcome and progeny. Biol Reprod 1986; 34: 275–283
- Twigg J. P., Irvine D. S., Aitken R. J. Oxidative damage to DNA in human spermatozoa does not preclude pronucleus formation at intracytoplasmic sperm injection. Hum Reprod 1998; 13: 1864–1871
- Van den Bergh M., Hohl M. K., De Geyter C., Stalberg A. M., Limoni C. Ten years of Swiss National IVF Register FIVNAT-CH. Are we making progress?. Reprod Biomed Online 2005; 11: 632–640
- Van Loon A. A., Den Boer P. J., Van der Schans G. P., Mackenbach P., Grootegoed J. A., Baan R. A., Lohman P. H. Immunochemical detection of DNA damage induction and repair at different cellular stages of spermatogenesis of the hamster after in vitro or in vivo exposure to ionizing radiation. Exp Cell Res 1991; 193: 303–309
- Van Steirteghem A. C., Liu J., Joris H., Nagy Z., Janssenswillen C., Tournaye H., Derde M. P., Van Assche E., Devroey P. Higher success rate by intracytoplasmic sperm injection than by subzonal insemination. Report of a second series of 300 consecutive treatment cycles. Hum Reprod 1993; 8: 1055–1060
- Virro M. R., Larson-Cook K. L., Evenson D. P. Sperm chromatin structure assay (SCSA) parameters are related to fertilization, blastocyst development, and ongoing pregnancy in in vitro fertilization and intracytoplasmic sperm injection cycles. Fertil Steril 2004; 81: 1289–1295
- Vogel F., Rathenberg R. Spontaneous mutation in man. Adv Hum Genet 1975; 5: 223–318
- Wennerholm U. B., Bergh C., Hamberger L., Lundin K., Nilsson L., Wikland M., Kallen B. Incidence of congenital malformations in children born after ICSI. Hum Reprod 2000; 15: 944–948
- World Health Organization (WHO) (1987) WHO Laboratory Manual for the examination of human semen and sperm-cervical mucus interaction. 2nd ed. Cambridge, UK: Cambridge Univeristy Press
- WHO (2006) WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction, Cambridge University Press
- Xu D. X., Shen H. M., Zhu Q. X., Chua L., Wang Q. N., Chia S. E., Ong C. N. The associations among semen quality, oxidative DNA damage in human spermatozoa and concentrations of cadmium, lead and selenium in seminal plasma. Mutat Res 2003; 534: 155–163
- Yamauchi Y., Shaman J. A., Boaz S. M., Ward W. S. Paternal pronuclear DNA degradation is functionally linked to DNA replication in mouse oocytes. Biol Reprod 2007a; 77: 407–415
- Yamauchi Y., Shaman J. A., Ward W. S. Topoisomerase II-mediated breaks in spermatozoa cause the specific degradation of paternal DNA in fertilized oocytes. Biol Reprod 2007b; 76: 666–672
- Zini A., Bielecki R., Phang D., Zenzes M. T. Correlations between two markers of sperm DNA integrity, DNA denaturation and DNA fragmentation, in fertile and infertile men. Fertil Steril 2001; 75: 674–677
- Zini A., Meriano J., Kader K., Jarvi K., Laskin C. A., Cadesky K. Potential adverse effect of sperm DNA damage on embryo quality after ICSI. Hum Reprod 2005; 20: 3476–3480