Abstract
The human spermatozoal centrosome acts as a microtubule organizing center and is essential for male and female pronuclear migration and apposition. In this study, we assess centrosomal function of spermatozoa from infertile patients using heterologus intracytoplasmic sperm injection (ICSI) into bovine eggs. Spermatozoa from 15 infertile patients undergoing assisted reproductive technology (ART) treatment and 3 fertile donors were tested. Microtubules and DNA were imaged by immuocytochemistry and epifluorescence microscopy. Decondensed female chromosomes and sperm nuclei, pronuclear formation and sperm aster formation were examined. The average rate of sperm aster formation using spermatozoa from infertile individuals was lower (47.0%) than that with spermatozoa from fertile individuals (66.1%). We compared the sperm aster formation rates after ART with various clinical parameters, including semen characteristics, pronuclear formation rates, embryonic cleavage rates and pregnancy outcome. Clinical semen characteristics and the rate of pronuclear formation appeared independent of sperm centrosomal function. In contrast, the centrosomal function had a substantial effect on embryonic cleavage rate and pregnancy after ART. These results suggested that centrosomal function is essential for pregnancy and embryonic development. The method described using bovine eggs is suitable to assay human centrosome function and predict pregnancy after ART.
| Abbreviations | ||
| ART | = | assisted reproductive technology |
| ICSI | = | intracytoplasmic sperm injection |
| IVF | = | in vitro fertilization |
| MTOC | = | microtubule organizing center |
| SSS | = | synthesized serum substitute |
Introduction
Assisted reproductive technology (ART) has been advancing at a remarkable pace. For example, intracytoplasmic sperm injection (ICSI) has become common within the ART clinical setting. However, not all ICSI cases succeed in pregnancy. Therefore, clinicians should consider the process of fertilization.
The goal of fertilization is the fusion of the male and the female genome in the egg cytoplasm rather than just the entry of spermatozoon into the egg [Yanagimachi [Citation1994]]. Many events, such as egg activation, completion of meiotic maturation, second polar body extrusion and pronuclear formation, occur during the process of fertilization following sperm entry into the egg [Schatten et al. [Citation1998]]. However, these events in fertilization are not well understood. ICSI does not assist in initial sperm incorporation; it completely bypasses the sperm incorporation step of fertilization.
During the process of human fertilization, the sperm delivers the male genome, the egg activator [Kimura et al. [Citation1998]], the centrosome [Schatten [Citation1994]] and a population of RNAs [Krawetz [Citation2005]]. Microtubules form radially from the sperm-contributed zygotic centrosome, and this is called a ‘sperm aster’. The sperm aster is essential for the pronuclear movement that leads to the union of the male and female genomes [Schatten [Citation1994]; Navara et al. [Citation1994]; Simerly et al. [Citation1995]]. Sperm centrosomal functional assays have been reported with a heterologous ICSI system in vivo and with Xenopus egg extracts in vitro [Simerly et al. [Citation1999]]. Heterologous ICSI systems have been applied to numerous species including hamster [Hewitson et al. [Citation1997]], rabbit [Terada et al. [Citation2000]] and bovine [Nakamura et al. [Citation2001]]. Egg activation and sperm aster formation have been observed in heterologous ICSI of fertile human spermatozoa into both rabbit and bovine eggs [Terada et al. [Citation2000]; Nakamura et al. [Citation2001]]. Centrosomal function of spermatozoa from infertile human subjects following heterologus ICSI into rabbit eggs has also been described. The rate of human sperm aster formation within the rabbit egg correlated with embryonic cleavage rates of human clinical in vitro fertilization (IVF). However, the rate of sperm aster formation was approximately 30% for normal fertile spermatozoa [Terada et al. [Citation2004a]]. To achieve higher rates of aster formation as a tool to assess human sperm centrosomal function, we have examined the use of bovine eggs [Terada et al. [Citation2004b]]. The rate of aster formation following heterologus ICSI with fertile donor spermatozoa into bovine eggs is 60%. This is significantly higher than the rate of 30% in rabbit eggs. Using bovine eggs, we examined several cases of infertility associated with teratozoospermia and poor sperm centrosomal function. The rates of sperm aster formation were only 15.8% in a case of globozoospermia [Nakamura et al. [Citation2002]] and 16.0% in a case of dysplasia of the fibrous sheath [Rawe et al. [Citation2002]; Nakamura et al. [Citation2005]].
To address whether the heterologous ICSI system is useful to assay human centrosome function and predict the reproductive success we have assessed the centrosomal function of spermatozoa from ‘infertile’ individuals using heterologous ICSI into bovine eggs. The results of this study are described in this paper.
Results
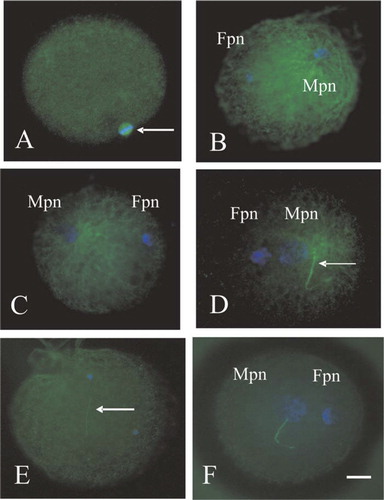
The microtubules present in unfertilized metaphase II-arrested bovine egg are organized as an anastral barrel-shaped meiotic spindle (). The microtubule organization of the human sperm centrosome as well as chromatin configuration in bovine eggs following ICSI was observed at 6 h post ICSI (). The decondensation of the female chromosomes and sperm nuclei, pronuclear formation and the sperm aster were observed (). The configuration of a normal sperm aster extends radially to fill the cytoplasm. In contrast, small sperm asters () and a failure to activate the eggs () were observed in most the ICSI-zygotes in the present study. The male and female nuclei were decondensed, but the sperm aster was absent, indicating that zygotic centrosome function was impaired (). As summarized in , the rate of activation, 2PN formation, and sperm aster formation (in bovine eggs at 6 h post-ICSI) was examined. The average rate of sperm aster formation with fertile spermatozoa was 66.1% (). In contrast, the average rate of activation, 2PN formation and sperm aster formation were 80.8%, 76.0% and 47.0%, respectively (), when spermatozoa from infertile individuals were used.
Figure 1 Microtubule (green) and chromatin (blue) configurations in the bovine eggs (A) and in the bovine eggs after heterologus ICSI with human sperm (B–F). Fpn, Female pronucleus; Mpn, Male pronucleus. Bar=10 μm. A: Unfertilized bovine egg that is arrested in Metaphase II displays a second meiotic spindle (arrow). B, C: The male and female pronuclei have decondensed. Microtubules are formed radially from sperm centrosome and are not present around the female pronucleus. D: The short sperm aster is observed, separated from male nucleus (arrow: sperm tail). E: The injected sperm nucleus remains intact and sperm tail (arrow) is observed away from sperm head. The female pronucleus, microtubule organization and the sperm aster are not visible. F: Male and female pronuclei with absent sperm asters were observed.
Heterologous ICSI with Sperm from Infertile Individuals.
Heterologous ICSI with Donor Sperm from Fertile Individuals.
The rates of sperm aster formation are summarized in . The rate of sperm aster formation from oligozoospermic and non-oligozoospermic patients was 35.4% and 52.8%, respectively, from asthenozoospermic and non-asthenozoospermic patients, 45.4% and 48.3%, respectively, and from infertile patients with low and high rates of pronuclear formation following ART, 42.9% and 51.6%, respectively. There was no statistically significant correlation among these parameters. The rate of sperm aster formation from infertile patients which provided high and low cleavage rates following ART were 57.0% and 32.2%, respectively. Whereas, the rate of sperm aster formation in those individuals that achieved pregnancy after clinical ART was 67.3%. Thus the rate of sperm aster formation was significantly different between embryos that cleaved versus those that did not, correlating with a successful clinical ART pregnancy.
Comparison of the Rates of Sperm Aster Formation.
Discussion
The human zygotic centrosome, arising from the sperm and oocyte pericentriolar material, acts as a microtubule organizing center (MTOC) during fertilization to assemble microtubules in the inseminated egg [Vorobjev and Chentsov [Citation1982]; Simerly et al. [Citation1995]] and regulate microtubule dynamics within the cytoplasm thereby controlling cellular configuration. During the cell division, the centrosome is replicated. The two daughter centrosomes provide the two spindle poles that function as the MTOC to regulate the formation of the mitotic spindle that is required for chromosomal parting.
The centrosome contains a pair of centriole and pericentriolar proteins. The oocytes lose centrioles during oogenesis in human [Simerly et al. [Citation1995]; Sathananthan et al. 1996], cow [Navara et al. [Citation1994]] and other non-rodent mammals [Schatten [Citation1994]], but they retain pericentriolar proteins. In spermatogenesis, spermatozoa lose some centrosomal proteins during spermiogenesis. The distal centriole, one of two microtuble cylinders oriented at a 90° angle to each other, degenerates [Palermo et al. [Citation1997]; Manandhar et al. [Citation2005]]. The proximal centriole remains in the sperm connecting piece and functions as the base for the future zygotic centrosome. During fertilization, the spermatozoon introduces a single centriole, which becomes a zygotic centrosome by duplicating and attracting maternally derived pericentriolar material. Therefore, the zygote centrosome consists of both paternal and maternal centrosomal components [Schatten [Citation1994]; Manandhar et al. [Citation2005]].
The pattern of the centrosome inheritance during fertilization differs among species. In rodents, often the animal model system of choice, the paternal centrosome degenerates completely during spermiogenesis [Manandhar et al. [Citation2005]] and the zygotic microtubles are organized from the functional maternally inherited, acentriolar MTOC. In a heterologous ICSI system using hamster eggs, microtubules emanating from the injected sperm centriole were not observed [Hewitson et al. [Citation1997]]. This strongly suggests that rodents do not serve as a suitable model for studying human sperm centrosomal function. In contrast, rabbits have biparental inheritance of centrosomes during fertilization [Terada et al. [Citation2000]]. The maternal cytoplasm can replace the function of the sperm-derived centrosome [Morita et al. [Citation2005]]. In heterologous ICSI employing rabbit eggs, sperm aster microtubules are observed. Thus, unlike the rodent model system, this system is compatible with studying human sperm centrosomal function.
However, in both bovine and human, inheritance is biparental; the spermatozoon contributes the centriole, while the oocyte contributes pericentiolar material. There is a variance in the proportion of the paternally derived centrosome that can present as variance in the sperm aster [Navara et al. [Citation1996]]. Nakamura et al. [[Citation2001]] reported that the rate of oocyte activation and sperm aster formation after heterologus ICSI of human spermatozoa in bovine eggs by piezo injection was 80% and 60%, respectively. This is higher than in the other species and heterologous ICSI using bovine eggs is suitable for studying human sperm centrosomal function.
Sperm aster formation and the configuration of chromatin in bovine eggs following ICSI of spermatozoa of 15 infertile patients was assessed (). The approximate distances between the male and female pronucleus varied (, F). The sperm aster expanded within the cytoplasm as a function of the time in culture. Firstly, the sperm aster just surrounded the male pronucleus. During this time the female pronucleus moved to the center of the egg, and microtubules elongated throughout the cytoplasm of the bovine eggs. Then, the female pronucleus became surrounded by the sperm aster. At 6 h post-ICSI, the phenotype, size and configuration of sperm aster were visible in eggs fertilized with sperm from all patients. The association between presence of a large sperm aster and morula/blastocyst formation has been previously reported in bovine in vitro fertilization [Navara et al. [Citation1996]]. Accordingly, sperm aster morphology reflects the developmental potential [Comizzoli et al. [Citation2006]]. For example, a large sperm aster with a defined focus of microtubles as well as elongations that extend from the paternal chromatin towards the maternal chromatin, a short sperm aster with a defined focus but without elongated microtubles, and an absent sperm aster without a defined focus of microtubles that does not extend toward the maternal chromatin have all been observed. In this study, the rate of sperm aster formation using spermatozoa from infertile patients was lower (47.0%) than that of the fertile donors (66.1%). Sperm centrosomal function was estimated from the sperm aster formation rate. These results suggested that sperm centrosomal dysfunction can result in infertility.
Centrosomal dysfunction as a factor contributing to infertility, is not considered part of the standard semen analysis. There was no correlation between sperm centrosomal function from infertile patients and the pronuclear formation rates with clinical ART. However, sperm centrosomal function in infertile patients was significantly higher in this study, yielding a rather high cleavage rate as opposed to the low cleavage rate observed by clinical ART in other studies [Terada et al. [Citation2004a]]. Nevertheless, centrosomal function in this study was associated with pregnancy outcome. Using bovine eggs provides a suitable assay of human centrosomal function and predicts the likely pregnancy outcome for clinical ART.
Materials and methods
All samples and procedures were performed under the approval of the internal review board of Tohoku University School of Medicine.
Sperm Samples
Sperm samples from 15 patients participating in the clinical ART program were examined. Patient characteristics and the results of human clinical ART are summarized in . Characteristics include indications, semen analysis, fertilization rates, cleavage rates and the number of successful pregnancies. Sperm samples from 3 normal fertile donors were examined as a control.
Patient Characteristics and Outcome of Clinical ART.
IVF Procedure
Controlled ovarian hyperstimulation was performed using a GnRH analogue (Hoechst, Tokyo, Japan), FSH (Fertinome-P, Serono, Tokyo, Japan), hMG (Humegon, Organon, Osaka, Japan), and hCG (Mochida, Tokyo, Japan). Egg collection was performed under guidance by transvaginal ultrasonography.
Semen was liquefied for 30 min and washed once in human tubal fluid medium (HTF; Irvine Scientific, Santa Ana, CA) supplemented with synthesized serum substitute (SSS; Irvine Scientific). After a 30 min swim-up, motile spermatozoa were collected. Each egg was inseminated with 1.0×105 spermatozoa.
Male and female pronuclear formation was confirmed in successfully fertilized eggs 16 h after insemination. After 2 days in culture, transvaginal embryo transfer was performed using a maximum of 3 eggs. A urine pregnancy test was performed two weeks after transfer.
In Vitro Maturation of Bovine Eggs
Bovine ovaries were obtained from a local abattoir. Eggs were recovered by aspiration from 2–8 mm follicles and were matured at 22°C for 4 h in Medium 199 (M199; Gibco, Grand Island, NY) supplemented with 10% (v/v) fetal calf serum (FCS; Gibco), 0.12 IU/ml of FSH (Antrin, Denka Pharmaceutical, Kanagawa, Japan), and 50 ng/ml of recombinant human epidermal growth factor (Upstate, Lake placid, NY) at 38.5°C with 5% CO2. Cumulus cells were removed by a brief incubation with 1 mg/ml of collagenase (Sigma, St. Louis, MO, USA) and 2 mg/ml of hyaluronidase (Sigma) in M2 culture medium (Sigma). Eggs that had paused at the second meiotic metaphase were selected for ICSI.
Human Sperm ICSI Using a Piezo-Micromanipulator
Surplus spermatozoa from 15 infertile ART patients were obtained with informed consent and frozen in a TEST-yolk buffer (Irvine Scientific, Santa Ana, CA). The sperm samples were thawed at room temperature. Then, samples were washed with modified HTF Medium (Irvine Scientific, Santa Ana, CA), supplemented with 10% SSS, pelleted by centrifugation at 500×g for 5 min and then M2 culture medium containing 10% (w/v) polyvinylpyrrolidone (PVP; Sigma, St. Louis, MO) added. Only motile and normal shaped spermatozoa were used. After being immobilized with a Piezo-micromanipulator (MB-U; Prim Tech, Tsuchiura, Japan), a spermatozoon was loaded in the pipette. The zona pellucida was penetrated using piezo-pulses. The section of the zona pellucida contained within the pipette was discharged, and immobilized spermatozoon was pushed to the tip of the injection pipette. The pipette was inserted into the ooplasm as the oolemma was punctured by applying one piezo-pulse. The spermatozoon on the tip of the pipette was injected into the ooplasm simultaneously with a minimum amount of sperm suspension medium. After injection, eggs were cultured in M199 under mineral oil, and supplemented with 10% FCS at 38.5°C with 5% CO2. Following the 6 h incubation the eggs were fixed and stained.
Immunocytochemical Detection of Microtubules and DNA
The zonae pellucidae were removed and oocytes placed in M2 culture medium supplemented with 0.75% Protease (Sigma). After allowing the eggs to recover for 30 min, the zona-free eggs were extracted for 15 min with buffer M (25% [v/v] glycerol, 50 mM KCl, 0.5 mM MgCl2, 0.1 mM EDTA, 1 mM EGTA, 50 mM imidazole hydrochloride, and 1 mM 2-mercaptoethanol, pH 6.8 containing 5% (v/v) methanol and 1% (v/v) Triton X-100) and were fixed in cold methanol for 10 min [Simerly and Schatten [Citation1993]]. Fixed eggs were then permeabilized for approximately 12 h in 0.1 mM PBS (Sigma) containing 0.1% (v/v) Triton X-100.
Microtubules were labeled with a mixture of monoclonal antibodies against β-tubulin (clone 2-28-33; diluted 1:100; Sigma, St. Louis, MO) and acetylated α-tubulin (clone 6-11-B1; diluted 1:100; Sigma, St. Louis, MO). Primary antibodies were detected with a secondary antibody conjugated to fluorescein-isothiocyanate (FITC; diluted 1:40; Zymed, San Francisco, CA). The DNA was stained with 10 μg/ml of Hoechst 33342 (Dojindo, Kumamoto, Japan). Coverslips were mounted in antifade medium (Vectashield; Vector Labs, Burlingame, CA) to retard photobleaching, and were examined using epifluorescence microscopy (Optiphot-2; Nikon, Tokyo, Japan).
Images were digitally captured, archived on magnetic optical disks and processed using Adobe Photoshop software (Adobe Systems Inc, Mountain View, CA). Images were used to compare the rate of sperm aster formation with the various clinical characteristics of ART using the Mann–Whitney-test. A P≤0.05 was considered to be statistically significant.
Acknowledgments
Human sperm samples were used in accordance with Tohoku University's Internal Review Board. We thank Drs. Yuki Morito-Shima and Soichi Nakamura, Tohoku University, for their collaboration.
References
- Comizzoli P., Wildt D. E., Pukazhenthi B. S. Poor centrosomal function of cat testicular spermatozo impairs embryo development in vitro after intracytoplasmic sperm injection. Biol Reprod 2006; 75: 252–260
- Hewitson L., Haavisto A., Simerly C., Jones J., Schatten G. Microtubule organization and chromatin configurations in hamster oocytes during fertilization and parthenogenetic activation, and after insemination with human sperm. Biol Reprod 1997; 57: 967–975
- Kimura Y., Yanagimachi R., Kuretake S., Bortkiewics H., Perry A. C., Yanagimachi H. Analyses of mouse oocyte activation suggests the involvement of sperm perinuclear material. Biol Reprod 1998; 58: 1407–1415
- Krawetz S. A. Paternal Contribution: new insights and future challenges. Nature Reviews Genetics 2005; 6: 633–642
- Manandhar G., Schatten H., Sutovsky P. Centrosome reduction during gametogenesis and its significance. Biol Reprod 2005; 72: 2–13
- Morita J., Terada Y., Hosoi Y., Fujinami N., Sugimoto M., Nakamura S., Murakami T., Yaegashi N., Okamura K. Microtubule organization during rabbit fertilization by intracytoplasmic sperm injection with and without sperm centrosome. Reprod Med Biol 2005; 4: 169–177
- Nakamura S., Terada Y., Horiuchi T., Emuta C., Murakami T., Yaegashi N., Okamura K. Human sperm aster formation and pronuclear decondensation in bovine eggs following intracytoplasmic sperm injection using a Piezo-driven pipette. Biol Reprod 2001; 65: 1359–1363
- Nakamura S., Terada Y., Horiuchi T., Emuta C., Murakami T., Yaegashi N., Okamura K. Analysis of the human sperm centrosomal function and the oocyte activation ability in a case of globozoospermia, by ICSI into bovine oocytes. Hum Reprod 2002; 17: 2930–2934
- Nakamura S., Terada Y., Rawe V. Y., Uehara S., Morito Y., Yoshimoto T., Tachibana M., Murakami T., Yaegashi N., Okamura K. A trial to restore defective human sperm centrosomal function. Hum Reprod 2005; 14: 1–5
- Navara C. S., First N. L., Shatten G. Microtubule organization in the cow during fertilization, polyspermy, parthenogenesis, and nuclear transfer. Dev Biol 1994; 162: 29–40
- Navara C. S., First N. L., Shatten G. Phenotypic variations among paternal centrosomes expressed within the Zygote as disparate microtubule lengths and sperm aster organization. PNAS 1996; 93: 5384–5388
- Palermo G. D., Colombero L. T., Rosenwakes Z. The human sperm centrosome is responsible for normal syngamy and early development. Rev Reprod 1997; 2: 19–27
- Rawe V., Terada Y., Nakamura S., Chillik C. F., Brugo Olmedo S., Chemes H. E. A pathology of the sperm centriole responsible for defective sperm aster formation, sygamy and cleavage. Hum Reprod 2002; 17: 2344–2349
- Sathananthan A. H., Ratnam S. S., Ng S. C., Tarin J. J., Ganaroli L., Trounson A. The sperm centriole: Its inheritance, replication and perpetuation in early human embryos. Hum Reprod 1994; 11: 34
- Schatten G. The centrosome and its mode of inheritance. Dev Biol 1994; 165: 299–335
- Schatten G., Hewitson L., Simerly C., Sutovsky P., Huszer G. Cell and molecular challenges of ICSI. J Law Med Ethic 1998; 26: 29–27
- Simerly C., Schatten G. Techniques for localization of specific molecules in oocytes and embryos. Methods Enzymol 1993; 225: 516–553
- Simerly C., Wu G. J., Zoran S., Ord T., Rawlins R., Jones J., Navara C., Geritty S., Rinehart J., Binor Z. The paternal inheritance of centrosome, the cells microtubule organizing center, in humans, and the implications for infertility. Nat Med 1995; 1: 47–52
- Simerly C., Zoran S. S., Payne C., Sutovsky P., Navara C. S., Salisbury J. L., Schatten G. Biparental inheritance of gamma-tubulin during human fertilization. Mol Biol Cell 1999; 10: 2955–2969
- Terada Y., Simerly C. R., Hewitson L., Schatten G. Sperm aster formation and pronuclear decondensation during rabbit fertilization and development of a functional assay for human sperm. Biol Reprod 2000; 62: 557–563
- Terada Y., Nakamura S., Simerly C., Hewitson L., Murakami T., Yaegashi N., Okamura K., Schatten G. Centrosomal function assessment in human sperm using heterologous ICSI with rabbit eggs. Mol Reprod Dev 2004a; 67: 360–365
- Terada Y., Nakamura S., Morita J., Tachibana M., Morito Y., Murakami T., Ito K., Yaegashi N., Okamura K. Use of mammalian eggs for assessment of human sperm function. Am J Reprod Immunol 2004b; 51: 290–293
- Vorobjev I. A., Chentsov Yu. S. Centrioles in the cell cycle. J Cell Biol 1982; 98: 938–949
- Yanagimachi R. Fertilization. The physiology of reproduction, 2nd ed., E. Knobil, J. Neil. Raven Press, New York, USA 1994; 189–317