Abstract
The objective of the study was to analyze the potential role of follicle stimulating hormone (FSH) in cytogenetic changes of in vivo and in vitro matured mouse oocytes and to determine whether the lower developmental potential of immature oocytes is due to a higher incidence of abnormalities in meiotic spindle organization and chromosome alignment as well as aneuploidy. In vivo matured oocytes were collected from naturally ovulated and superovulated (5.0 I U of recombinant follicle-stimulating hormone [rec-FSH] + recombinant human chorionic gonadotropin [rec-HCG]) mice. Immature oocytes were retrieved from naturally cycling mice and from mice primed with rec-FSH for 48 h. The immature oocytes were cultured 18 h for in vitro maturation (IVM). In vivo and in vitro matured oocytes were assessed for the meiotic spindle organization and chromosome alignment as well as aneuploidy. There was no significant difference of meiotic spindle organization, chromosomal alignment and aneuploidy between in vivo and in vitro matured oocytes derived from naturally cycling and stimulated mice. Therefore, the lower developmental potential of immature oocytes does not appear to be directly related to the incidence of abnormal meiotic spindle organization and chromosome alignment or to aneuploidy.
| Abbreviations | ||
| FSH | = | follicle stimulating hormone |
| rec-FSH | = | recombinant follicle-stimulating hormone |
| rec-HCG | = | recombinant human chorionic gonadotropin |
| IVM | = | in vitro maturation |
| IVF | = | in vitro fertilization |
| LH | = | luteinizing hormone |
| ICSI | = | intracytoplasmic sperm injection |
| PCOS | = | polycystic ovary syndrome |
| HCG | = | human chronic gonadotropin |
| mHTF-HEPES | = | modified human tubal fluid-HEPES buffered medium |
| COCs | = | cumulus-oocyte complexes |
| DPBS | = | Dulbecco's phosphate buffered saline |
| FITC | = | fluorescein isothiocynate |
| DAPI | = | 4,6-diamidino-2-phenylindole |
Introduction
The use of in vitro fertilization (IVF) has helped millions of infertile couples. Current protocols for IVF treatment involve the use of gonadotropin to stimulate the ovaries to produce a greater number of mature oocytes. When more mature oocytes are available for collection, more embryos can be generated, therefore, increasing the chance of pregnancy from each treatment cycle. However, due to the side effects of gonadotropin stimulation, more and more patients are becoming interested in unstimulated cycle IVF or minimal stimulation cycle IVF treatments as well in vitro maturation (IVM) of immature oocytes.
Although IVM has resulted in more than 500 healthy live births worldwide [Edwards [Citation2007]], it is still considered an experimental procedure [Piquette [Citation2006]; Lanzendorf [Citation2006]], as it results in a lower pregnancy rate than standard stimulated IVF treatment. Recently, it has been reported that IVM increases the risk of abnormal spindles and chromosome configurations of human oocytes compared with oocytes matured in vivo [Li et al. [Citation2006]]. The investigators postulated that this is one possible explanation for the reduced developmental potential of IVM oocytes compared with those matured in vivo.
The development of follicles from the antral to pre-ovulatory stage is normally considered gonadotropin dependent and is regulated by the cyclic changes of follicle-stimulating hormone (FSH) and luteinizing hormone (LH). The endocrine environment of the follicles in the later stage of folliculogenesis has a profound impact on oocyte quality. Although in vivo priming with FSH prior to immature oocyte retrieval followed by IVM may improve maturation and fertilization rates as well as embryonic development [Wynn et al. [Citation1998]; Mikkelsen and Lindenberg [Citation2001]], it was reported that developmentally lethal chromosomal abnormalities are common defects, affecting more than 25% of normal-looking mature human oocytes retrieved from stimulated ovaries [Delhanty and Handyside [Citation1995]; Wall et al. [Citation1996]]. While aneuploidy occurs at a high rate in mature human oocytes retrieved from stimulated ovaries [Gras et al. [Citation1992]; Hodges et al. [Citation2002]], it is not clear whether this is caused by FSH stimulation or not. Therefore, it is important to investigate the cause of abnormal meiotic spindle organization, chromosome alignment and aneuploidy.
In addition, we have reported that in vivo matured oocytes are more competent than those matured in vitro due to both a lesser degree of DNA damage and that in vivo priming with FSH prior to the retrieval of immature oocytes is beneficial to subsequent embryonic development [Wang et al. [Citation2006]]. Therefore, it is important to answer the questions that have been raised whether to lower development potential of immature oocytes is due to high incidence of abnormal meiotic spindle organization and alignment as well as aneuploidy.
The objective of this study was to extend our previous work to analyze the potential role of FSH in cytogenetic changes of in vivo and in vitro matured oocytes and to determine whether the lower developmental potential of immature oocytes is due to a higher incidence of abnormal meiotic spindle organization and chromosome alignment as well as aneuploidy using the mouse model.
Results
As shown in , the percentage of oocytes with meiotic spindle plate () under PolScope was not significantly different between in vivo and in vitro matured oocytes derived from naturally cycling and stimulated mice (91.1%, 90.2%, 89.1% and 90.7%, respectively). There was no difference in the length of spindle plates of in vivo matured oocytes between naturally ovulated oocytes (4.7±1.1 μm) and superovulated oocytes (4.8±1.4 μm). Also, the length of the spindle plates was not significantly different between in vivo (4.7±1.1 μm) and in vitro (5.0±1.1 μm) matured oocytes derived from naturally cycling mice. Similarly, there was no difference in the length of the meiotic spindle between immature oocytes derived from naturally cycling mice (5.0±1.1 μm) and from rec-FSH primed mice (5.1±0.8 μm). In addition, there was no difference in the length of the meiotic spindle between in vivo (4.8±1.4 μm) and in vitro (5.1±0.8 μm) matured oocytes derived from stimulated cycles. As shown in , there was no difference in the percentage of abnormal meiotic spindle as shown in , and chromosome alignment among the four groups (1.8%, 3.8%, 5.4% and 3.8%, respectively). Also, the percentage of aneuploidy as determined by chromosome analysis compared to normal chromosomes shown in was not significantly different among the four groups (; 6.0%, 6.2%, 9.8% and 9.6%, respectively).
Comparison of the Length of Meiotic Spindle Plate of In Vivo and In Vitro Matured Oocytes Derived from Naturally Cycling or Gonadotropin Stimulated Mice (5 Replicates)*
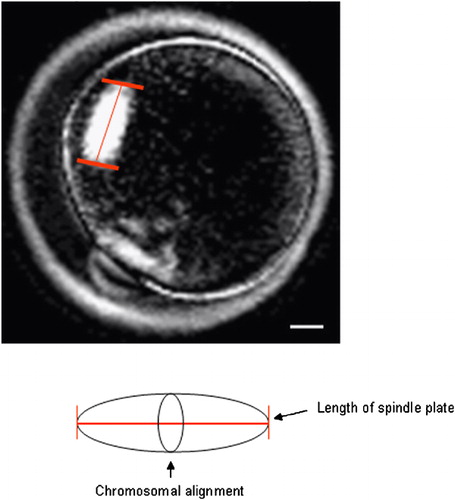
Figure 1 Measurement for the length of spindle plate of mouse oocyte using the PolScope imaging system. The length of spindle plate was measured by crossing the chromosomal alignment (arrow). Scale bar indicates 10 μm.
Comparison of Meiotic Spindle Organization and Chromosomal Alignment of In Vivo and In Vitro Matured Oocytes Derived from Naturally Cycling or Gonadotropin Stimulated mice (10 Replicates)*
Figure 2 Morphology of meiotic spindle organization and chromosome alignment in normal and abnormal mouse oocytes. (A) Oocyte with normal meiotic spindle organization (green); (a) The same oocyte with normal chromosome alignment (blue); (B) Oocyte with abnormal meiotic spindle organization (green); (b) The same oocyte with abnormal chromosome alignment (blue). Scale bar indicates 20 μm.
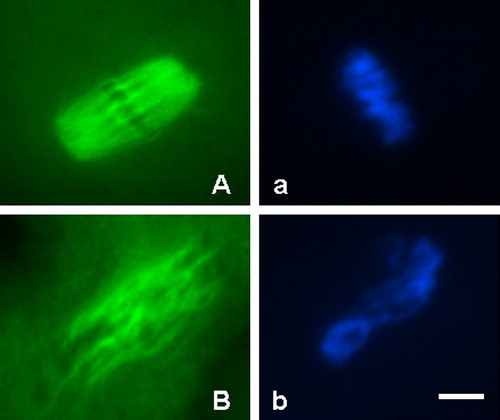
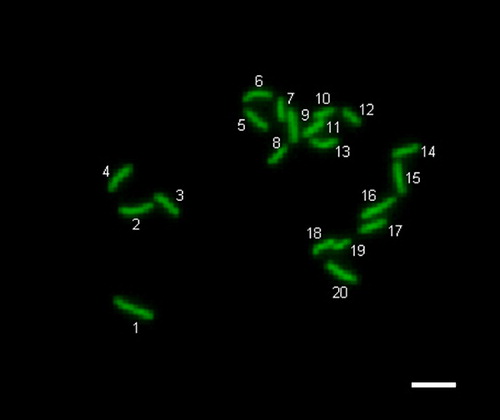
Figure 3 Normal (haploidy) chromosomes in parthenogenetically activated mouse oocyte. The oocyte with 20 chromosomes. Scale bar indicates 20 μm.
Ploidy Analysis of In Vivo and In Vitro Matured Oocytes Derived from Naturally Cycling or Gonadotropin Stimulated Mice (6 Replicates)*
Discussion
The results of the present study demonstrate that there is no significant difference in abnormal meiotic spindle organization and chromosomal alignment as well as aneuploidy between in vivo and in vitro matured oocytes derived from naturally cycling and stimulated mice. The integrity of the meiotic spindles and chromosomes may be of critical importance to the oocytes’ capacity to be fertilized, develop into a normal embryo and ultimately produce a healthy live birth. The development of PolScope has allowed the noninvasive observation of the spindle [Liu et al. [Citation2000]]. In most cases, this technology is applied to intracytoplasmic sperm injection (ICSI) or nuclear transfer in order to localize the first polar body as an indication of spindle position without damaging the spindle. It has been reported that oocytes with invisible meiotic spindles have a lower fertilization rate and poorer developmental potential, suggesting that spindle position and displacement may be used to predict the quality of oocytes [Wang et al. [Citation2001a]; Citation[b]]. Moon et al. [[Citation2003]] reported that IVM of mouse oocytes had significantly lower fertilization and blastocyst formation rates compared with in vivo matured oocytes, indicating that these lower rates may be associated with the significant differences in meiotic spindle arrangement between in vitro and in vivo matured oocytes measured by PolScope. However, the results obtained by the present study indicate that there is no direct correlation in terms of the appearance of meiotic spindles and the length of spindle plate between in vivo and in vitro matured oocytes derived from naturally cycling and stimulated mice ().
It has been reported that the IVM procedure can have deleterious effects on the organization of the meiotic spindle and chromosome alignment of oocytes derived from women with polycystic ovary syndrome (PCOS). This has been suggested as one possible explanation for the reduced developmental potential of IVM oocytes compared with those matured in vivo [Li et al. [Citation2006]]. Aneuploidy is a major cause of pregnancy loss and increases with age. In the mouse, the different rates of oocyte aneuploidy reported depend upon the methods of examination used and the different environments involved in oocyte meiotic maturation [Eichenlaub-Ritter et al. [Citation1986]; Albertini [Citation1992]; Sun et al. [Citation2001]; Sanfins et al. [Citation2003]]. Sanfins et al. [[Citation2003]] reported that differences in cytoskeletal organization between in vitro and in vivo matured mouse oocytes were detected using a fluorescence microscope, highlighting a fundamental distinction in the spatial and temporal regulation of microtubule dynamics between in vitro and in vivo matured oocytes. However, the results obtained by the present study indicate that there is no difference in terms of meiotic spindle organization and chromosomal alignment between in vivo and in vitro matured oocytes derived from naturally cycling and stimulated mice ().
It appears that ovarian stimulation with gonadotropins enhances oocyte availability but compromises oocyte developmental competence. Recently, it has been indicated that ovarian stimulation may influence oocyte developmental competence and aneuploidy formation [Hodges et al. [Citation2002]; Combelle and Albertini [Citation2003]]. Furthermore, it has been suggested that exposure to high concentrations of FSH during IVM can accelerate nuclear maturation but can also induce chromosomal abnormalities [Robert et al. [Citation2005]]. Although we have reported that in vivo priming with FSH prior to immature oocyte retrieval is beneficial to subsequent embryonic development [Wang et al. [Citation2006]], the nature of the defects caused by exogenous ovarian stimulation on oocyte quality is still unclear.
In the mouse model, the ovarian stimulation regimen involves a single injection of FSH to induce the development of multiple follicles followed by administration of human chronic gonadotropin (HCG) with luteinizing hormone (LH) surge to trigger oocyte maturation and ovulation. It is important to note that although the principle is similar, ovarian stimulation regimens used in human infertility treatment for IVF are quite different from the current mouse stimulation protocol. Human ovarian stimulation regimens involve longer and multiple doses of FSH injection. Interestingly, Battaglia et al. [[Citation1996]] found that the proportion of oocytes with abnormal meiotic spindles is significantly higher in naturally cycling women of increased maternal age than in younger women, suggesting that abnormal spindles may contribute to the high prevalence of aneuploidy in embryos from this population. Therefore, the cytoplasmic factors of oocytes may be dependent on maternal age and genetic background and may not only be related to stimulation regimens.
Aneuploidy occurs at high rates (up to 52.1%) in mature human oocytes collected from gonadotropin stimulated cycles [Gras et al. [Citation1992]; Delhanty and Handyside [Citation1995]; Wall et al. [Citation1996]; Racowsky et al. [Citation1997]; Hodges et al. [Citation2002]]. Interestingly it has been reported that only 18% of in vitro matured human oocytes have gross meiotic aberrations [Kuliev et al. [Citation2003]]. Very high frequencies of chromosomal aneuploidy have been observed in human oocytes with cytoplasmic defects, suggesting that these genetic abnormalities may develop during gonadotropin stimulation as a result of degenerative cytoplasmic alterations [Racowsky and Kaufman [Citation1992]]. Indeed, it has been shown that increasing maternal age in naturally cycling women is associated with high frequencies of spindle defects and chromosomal segregation disorders [Van Blerkom and Henry [Citation1992]; Battaglia et al. [Citation1996]]. The results from the present study indicate that chromosomal aneuploidy rates are not increased by either gonadotropin ovarian stimulation or by IVM (). This implies that chromosomal aneuploidy may not be the main factor for the low embryonic developmental competence of in vitro matured oocytes.
In conclusion, the abnormalities of meiotic spindle organization and chromosomal alignment as well as aneuploidy are not significantly different between in vitro and in vitro matured oocytes derived from naturally cycling and stimulated mice. These results suggest that the lower embryonic development of in vitro matured oocytes may not be related directly to the abnormal meiotic spindle organization and chromosomal alignment as well as aneuploidy.
Materials and methods
Animals
The mice used in this study were cluster of differentiation 1 (CD1), female: 8–10 weeks-old and male: 10–12 weeks-old. They were housed in a temperature- and light-controlled room and had free access to food and water under a photoperiod of 12 hours-light and 12 hours-dark. The experimental protocols and animal handling procedures were reviewed and approved by the Animal Ethics Committee of McGill University.
Mature and Immature Oocytes from Ovaries Stimulated by Gonadotropins
Female mice were injected intraperitoneally with 5.0 IU of recombinant follicle-stimulating hormone (rec-FSH; Serono Canada). For mature oocyte collection, the female mice were further injected intraperitoneally with 5 IU of recombinant human chorionic gonadotropin (rec-hCG; Serono Canada) after 48 h of rec-FSH injection. Post 14 hours of rec-hCG injection, the mice were sacrificed and the oviducts were dissected and placed into a Petri-dish containing modified human tubal fluid-HEPES buffered medium (mHTF-HEPES) [Quinn et al. [Citation1995]] supplemented with 1.0 mg/ml bovine serum albumin (BSA; Sigma, St. Louis, MO, USA). Cumulus-oocyte complexes (COCs) were released by tearing the ampullae of the oviducts, COCs were denuded from cumulus cells by mechanically pipetting with a fine diameter pipette in mHTF-HEPES supplemented with 85 unit/ml hyaluronidase for assessment of maturity, and then mature oocytes were used for experiments. For immature oocyte collection, the mice were sacrificed after 48 hours of rec-FSH injection and the ovaries were dissected and placed in mHTF-HEPES supplemented with 1.0 mg/ml BSA. The visible follicles were punctured under a stereomicroscope using a 25-gauge needle. The compacting COCs were collected for IVM culture.
Mature and Immature Oocytes from Natural Cycling Ovaries
For mature oocyte collection, the natural cycling female mice were mated with a vasectomized male. Briefly, the female and the male mice were caged together on the evening before the day of oocyte collection. After checking vagina-mating plug the next morning, the female mice were sacrificed and the oviducts dissected into a Petri dish. By tearing the ampullae, the COCs were released and collected. COCs were denuded from cumulus cells by mechanically pipetting with a fine diameter pipette in mHTF-HEPES supplemented with 85 unit/ml hyaluronidase for assessment of maturity, and then mature oocytes were used for experiments. For immature oocyte collection, the natural cycling mice (without any stimulation with gonadotropins) were sacrificed and the ovaries were dissected and placed into a Petri dish containing mHTF-HEPES. As in the above, the visible follicles were punctured under a stereomicroscope using a 25-gauge needle. The compacting COCs were collected for IVM culture.
In Vitro Maturation (IVM) of Immature Oocytes
After collection, the immature COCs were rapidly washed three times in mHTF-HEPES containing 1.0 mg/ml BSA and then placed in culture for maturation. Oocyte maturation involved placing the COCs in Oocyte Maturation Medium (Cooper Surgical/SAGE, USA) supplemented with 10% fetal bovine serum (FBS), and 75 mIU/ml rec-FSH and rec-LH (Serono, Canada). Briefly, following washing, 30–40 selected COCs were cultured for 17–18 hours in an Organ Tissue Culture Dish (60×15 mm; Falcon) containing 1.0 ml of Oocyte Maturation Medium in an incubator at 37.0 °C with an atmosphere of 5% CO2 and 95% air and high humidity. Following maturation in culture, COCs were denuded from cumulus cells by mechanically pipetting with a fine diameter pipette in mHTF-HEPES supplemented with 85 unit/ml hyaluronidase. Mature oocytes were identified by the presence of a first polar body extrusion under a stereomicroscope, and then prepared for experiments.
Analysis of Spindle View with PolScope
Oocytes were transferred to 20 μl of mHTF-HEPES medium [Quinn et al. [Citation1995]] supplemented with 1.0 mg/ml bovine serum albumin (BSA; Sigma) covered with warm paraffin oil in a Bioptechs δTC3 Culture Dish System (Bioptechs Inc., Butler, PA, USA). The system consists of a temperature controller, a stage adapter and a δTC3 Culture Dish, which has a specially coated clear glass bottom that is 0.15 mm thick. The dish was maintained at 37 °C during examination. Mature oocytes were examined under an invert microscope (Olympus X70) equipped with LC Pol-Scope optics and controller (CRI, Cambridge, MA, USA), combined with a computerized image analysis system. All oocytes were imaged for analysis of spindle plate length () [Liu et al. [Citation2000]; Wang et al. [Citation2001a]; Citation[b]; Moon et al. [Citation2003]; 2005].
Assessment of Spindle and Chromosomes with Immunocytochemistry
Fixation and immunofluorescent staining of tubulin and chromatin were adapted from a previously published method [Liu and Keefe [Citation2002]]. The oocytes were fixed in 4% paraformaldehyde (Sigma) for five minutes. After fixation, the oocytes were washed in Dulbecco's phosphate buffered saline (DPBS) and transferred to 0.01% Triton X-100 in DPBS for 40 min at room temperature. The oocytes were then washed extensively and blocked at 4 °C in wash medium (DPBS supplemented with 0.02% NaN3, 0.2% non-fat dry milk, 2% goat serum, 2% bovine serum albumin and 0.1 mol/l glycine) for 45 min at room temperature. After rinsing in DPBS, the oocytes were incubated with β-tubulin monoclonal antibody diluted 1:500 in DPBS at 4 °C overnight. Subsequent to washing twice in DPBS, the microtubulins were stained with fluorescein isothiocynate (FITC)—conjugated anti-mouse IgG (Molecular Probes, OR, USA) diluted 1:320 in DPBS for 60 min in the dark at room temperature. After DPBS washing three times, the samples were mounted onto a slide under a coverslip in the Vectashield mounting medium (Vector Laboratories, Burlingame, CA, USA), containing 4,6-diamidino-2-phenylindole (DAPI).
The localization of tubulin and chromatin revealed by FITC and DAPI fluorescence was observed under×400 magnification using a fluorescence miscroscope (Leica DMIL) with a Hamamatsu digital camera imaging system (C4742-80). Optical filters specific for the wavelengths of 450–590 nm and 330–380 nm were used to detect the green signal of FITC and the blue signal of DAPI. Morphologically normal meiotic spindles and chromosome alignments were barrel-shaped with microtubules traversing between both poles, and metaphase chromosomes aligned in a compact group along the metaphase plate. Otherwise, the meiotic spindles and chromosome configuration were considered abnormal ().
Chromosome Counts with Oocyte Spreading
Mature oocytes were parthenogenetically activated by exposure to 10 mM strontium chloride (Sigma) in calcium-free potassium simplex optimized medium (KSOM) [Lawitts and Biggers [Citation1993]] for 1 hour. The oocytes were then cultured in 20 μl of modified KSOM (mKSOM) containing 1.0 mg/ml BSA microdrop under mineral oil at 37 °C in a high humidified and 5.0% CO2 incubator. One h post-activation, chromosome spreading was performed according to the air-dry method described by Tarkowski [[Citation1966]]. Briefly, the oocytes were placed on a glass slide, exposed to hypotonic 1% trisodium citrate solution for 5 min and allowed to dry at room temperature. The chromosome spreads were stained with DAPI and counted under fluorescent microscope. Ploidy screening was classified as being normal when 20 chromosomes (haploidy) were present in the activated oocyte. If there were less or more than 20 chromosomes (), the oocyte was classified as being abnormal (aneuploidy).
Statistical Analysis
The data were analyzed for agreement with Kappa statistics using StatsDirect (version 1.9.14 for Windows; StatsDirect Ltd, Cheshire, UK). The results of the length of meiotic spindle plate were presented as mean±SD and were analyzed by the one-way analysis of variance (ANOVA). Differences among the groups were analyzed by using the χ2-test and were considered statistically significant if P < 0.05.
Acknowledgments
This work was partially supported by the Program on Oocyte Health funded under the Healthy Gametes and Great Embryos Strategic Initiative of Canadian Institutes of Health Research (CIHR), Institute of Human Development, Child and Youth Health (IHDCYH), grant number HCG62293.
References
- Albertini D. F. Cytoplasmic microtubular dynamics and chromatin organization during mammalian oogenesis and oocyte maturation. Mutat Res 1992; 296: 57–68
- Battaglia D. E., Goodwin P., Klein N. A., Soules M. R. Influence of maternal age on meiotic spindle in oocytes from naturally cycling women. Hum Reprod 1996; 11: 2217–2222
- Combelle C. M., Albertini D. F. Assessment of oocyte quality following repeated gonadotropin stimulation in the mouse. Biol Reprod 2003; 68: 812–821
- Delhanty J., Handyside A. The origin of genetic defects in the human and their detection in the preimplantation embryo. Hum Reprod Update 1995; 1: 210–215
- Edwards R. G. In-vitro maturation comes of age. In-vitro Maturation of Human Oocytes: Basic science to clinical application, S. L. Tan, R. C. Chian, W. M. Buckett. Informa Healthcare Press Ltd, New York 2007; XV–XX
- Eichenlaub-Ritter U., Chandley A. C., Gosden R. G. Alteration to the microtubular cytoskeleton and increased disorder of chromosome alignment in spontaneously ovulated mouse oocytes aged in vivo: an immunofluorescence study. Chromosoma 1986; 94: 337–345
- Gras L., McBain J., Trounson A., Kola I. The incidence of chromosomal aneuploidy in stimulated and unstimulated (natural) uninseminated human oocytes. Hum Reprod 1992; 7: 1396–1401
- Kuliev A., Cieslak J., Ilkevitch Y., Verlinsky Y. Chromosomal abnormalities in a series of 6733 human oocytes in Preimplantation diagnosis for age-related aneuploidies. Reprod BioMed Online 2003; 6: 54–59
- Hodges C. A., Ilagan A., Jennings D., Keri R., Nilson J., Hunt P. A. Experimental evidence that changes in oocyte growth influence meiotic chromosome segregation. Hum Reprod 2002; 17: 1171–1180
- Lanzendorf S. E. Developmental potential of in vitro- and in vivo-matured human oocytes collected from stimulated and unstimulated ovaries. Fertil Steril 2006; 85: 836–837
- Lawitts J. A., Biggers J. D. Culture of preimplantation embryos. Methods Enzymol 1993; 225: 153–164
- Li Y., Feng H. L., Cao Y. J., Zheng G. L., Yang Y., Mullen S., Critser J. K., Chen Z. J. Confocal microscopic analysis of the spindle and chromosome configurations of human oocytes matured in vitro. Fertil Steril 2006; 85: 827–832
- Liu L., Oldenbourg R., Trimarchi J. R., Keefe D. L. A reliable, non-invasive technique for spindle imaging and enucleation of mammalian oocytes. Nat Biotechnol 2000; 18: 223–225
- Liu L., Keefe D. L. Ageing-associated aberration in meiosis of oocytes from senescence-accelerated mice. Hum Reprod 2002; 17: 2678–2685
- Mikkelsen A. L., Lindenberg S. Benefit of FSH priming of women with PCOS to the in vitro maturation procedure and the outcome: a randomized prospective study. Reproduction 2001; 122: 587–592
- Moon J. H., Hyun C. S., Lee S. W., Son W. Y., Yoon S. H., Lim J. H. Visualization of the metaphase II meiotic spindle in living human oocytes using Polscope enables the prediction of embryonic developmental competence after ICSI. Hum Reprod 2003; 18: 817–820
- Moon J. H., Jee B. C., Ku S. Y., Suh C. S., Kim S. H., Choi Y. M., Kim J. G., Moon S. Y. Spindle positions and their distributions in in vivo and in vitro matured mouse oocytes. Hum Reprod 2005; 20: 2207–2210
- Piquette G. N. The in vitro maturation (IVM) of human oocytes for in vitro fertilization (IVF): is it time yet to switch to IVM-IVF?. Fertil Steril 2006; 85: 833–835
- Quinn P., Moinipanah R., Steinberg J. M., Weatherbee P. Successful human in-vitro fertilization using a modified human tubal fluid medium lacking glucose and phosphate ions. Fertil Steril 1995; 63: 922–924
- Racowsky C., Prather A. L., Johnson M. K. Prematurely condensed chromosomes and meiotic abnormalities in unfertilized human oocytes after ovarian stimulation with and without gonadotropin-releasing hormone agonist. Fertil Steril 1997; 67: 932–938
- Racowsky C., Kaufman M. L. Nuclear degeneration and meiotic aberration observed in human oocytes matured in vitro: analysis by light microscopy. Fertil Steril 1992; 58: 750–755
- Robert R., Iatropoulou A., Ciantar D., Stark J., Becker D. L., Franks S., Hardy K. Follicle-stimulating hormone affects metaphase I chromosome alignment and increases aneuploidy in mouse oocytes matured in vitro. Biol Reprod 2005; 72: 107–118
- Sanfins A., Lee G. Y., Plancha C. E., Overstrom E. W., Albertini D. F. Distinction in meiotic spindle structure and assembly during in vitro and in vivo maturation of mouse oocytes. Biol Reprod 2003; 69: 2059–2067
- Sun F., Yin H., Eichenlaub-Ritter U. Differential chromosome behaviour in mammalian oocytes exposed to the tranquilizer diazepam in vitro. Mutagenesis 2001; 16: 407–417
- Tarkowski A. K. An air-drying method for chromosome preparation from mouse eggs. Cytogenet 1966; 5: 394–400
- Van Blerkom J., Henry G. Oocyte dysmorphism and aneuploidy in meiotically-mature human oocytes after ovarian stimulation. Hum Reprod 1992; 7: 379–390
- Wang W. H., Meng L., Hackett R. J., Odenbourg R., Keefe K. L. The spindle observation and its relationship with fertilization after ICSI in living human oocytes. Fertil Steril 2001a; 75: 348–353
- Wang W. H., Meng L., Hackett R. J., Keefe D. L. Development ability of human oocytes with or without birefringent spindles imaged by Polscope before insemination. Hum Reprod 2001b; 16: 1464–1468
- Wall M. B., Marks K., Smith T. A., Gearon C. M., Muggleton-Harris A. L. Cytogenetic and fluorescent in-situ hybridization chromosomal studies on in-vitro fertilized and intracytoplasmic sperm injected ‘failed-fertilized’ human oocytes. Hum Reprod 1996; 11: 2230–2238
- Wang Y., Ock S. A., Chian R. C. Effect of gonadotrophin stimulation on mouse oocytes quality and subsequent embryonic development in vitro. Reprod BioMed Online 2006; 12: 304–314
- Wynn P., Picton H. M., Krapez J. A., Rutherford A. J., Balen A. H., Gosden R. G. Pretreatment with follicle stimulating hormone promotes the numbers of human oocytes reaching metaphase II by in-vitro maturation. Hum Reprod 1998; 13: 3132–3138