ABSTRACT
What is the impact of intentional weight loss and regain on serum androgens in women? We conducted an ancillary analysis of prospectively collected samples from a randomized controlled trial. The trial involved supervised 10% weight loss (8.5 kg on average) with diet and exercise over 4-6 months followed by supervised intentional regain of 50% of the lost weight (4.6 kg on average) over 4-6 months. Participants were randomized prior to the partial weight regain component to either continuation or cessation of endurance exercise. Analytic sample included 30 obese premenopausal women (mean age of 40 ± 5.9 years, mean baseline body mass index (BMI) of 32.9 ± 4.2 kg/m2) with metabolic syndrome. We evaluated sex hormone binding globulin (SHBG), total testosterone (T), free androgen index (FAI), and high molecular weight adiponectin (HMWAdp). Insulin, homeostasis model assessment (HOMA), and quantitative insulin sensitivity check index (QUICKI), and visceral adipose tissue (VAT) measured in the original trial were reanalyzed for the current analytic sample. Insulin, HOMA, and QUICKI improved with weight loss and were maintained despite weight regain. Log-transformed SHBG significantly increased from baseline to weight loss, and then significantly decreased with weight regain. LogFAI and logVAT decreased similarly and increased with weight loss followed by weight regain. No changes were found in logT and LogHMWAdp. There was no significant difference in any tested parameters by exercise between the groups. SHBG showed prominent sensitivity to body mass fluctuations, as reduction with controlled intentional weight regain showed an inverse relationship to VAT and occurred despite stable HMWAdp and sustained improvements with insulin resistance. FAI showed opposite changes to SHBG, while T did not change significantly with weight. Continued exercise during weight regain did not appear to impact these findings.
Introduction
Androgen excess is associated with increased cardiovascular risk including lipid abnormalities, hypertension, and insulin resistance in women [Bell et al. Citation2007; Sutton-Tyrrell et al. Citation2005]. In older women, elevated serum total testosterone is associated with multiple features of metabolic syndrome including abdominal adiposity [Patel et al. Citation2009]. Furthermore, higher quartiles of total testosterone in older women are associated with worsened insulin resistance and higher odds of metabolic syndrome and coronary heart disease [Patel et al. Citation2009]. Sex hormone binding globulin (SHBG) is a glycoprotein that binds to sex steroids. Only unbound androgens, such as free testosterone, are biologically active. Reduced serum SHBG is independently associated with worsened glucose homeostasis and development of type 2 diabetes in both men and women [Ding et al. Citation2009]. Low levels of SHBG have also been associated with hyperlipidemia, elevated C-reactive protein, and disordered lipid profile in women [Bell et al. Citation2007]. Adiponectin is a hormone with anti-inflammatory and anti-atherogenic properties that is exclusively derived from adipose tissue, and has been found in vitro to decrease hepatic SHBG production [Simo et al. Citation2014]. SHBG is used in the calculation of and is inversely related to free androgen index (FAI). Both high FAI and low SHBG are negatively associated with markers of metabolic syndrome independent of body mass index [Sutton-Tyrrell et al. Citation2005].
Nearly two-thirds of U.S. adults are overweight or obese with profound implications for cardiovascular health [Xu et al. Citation2013]. Although cardiovascular risk associated with adiposity can be mitigated by weight loss, the impact is rarely sustainable. Short term weight loss can be achieved with caloric restriction and lifestyle modifications, but approximately half of patients who achieve weight loss will regain up to 75% of the lost weight following 12-30 months of treatment completion [Donnelly et al. Citation2013]. It is unknown how weight regain impacts the androgenic profile, a critical marker of metabolic dysregulation. In order to bridge this knowledge gap, we undertook a secondary analysis of serum stored from premenopausal women who participated in a randomized trial of controlled weight loss and subsequent regain with or without exercise. All participants in the randomized trial upon which the current study was based agreed to undergo supervised weight loss using calorie restriction and endurance exercise as part of the trial. The participants then intentionally regained some of the lost weight. For the weight regain portion, participants all increased caloric intake but were randomized to either continue or cease exercise.
The published findings of this randomized trial showed that sustained exercise during weight regain appeared to maintain many cardiovascular benefits obtained from the original weight loss, with the exceptions of increased abdominal fat, triglycerides, and cholesterol toward baseline levels [Thomas et al. Citation2010]. It is known that SHBG fluctuates with weight [Harvie et al. Citation2011; Wildman et al. Citation2012; Guzick et al. Citation1994] but this has not been previously evaluated in the setting of supervised weight regain or by exercise.
We hypothesized that total testosterone (T) and free androgen index (FAI) would undergo changes opposite to SHBG with regain of weight. We postulated that T and FAI would increase to a lesser extent in women who continued to exercise whilst regaining weight compared to those who stopped exercising. We also hypothesized that high molecular weight adiponectin (HMWAdp) would change similarly to SHBG [Simo et al. Citation2014].
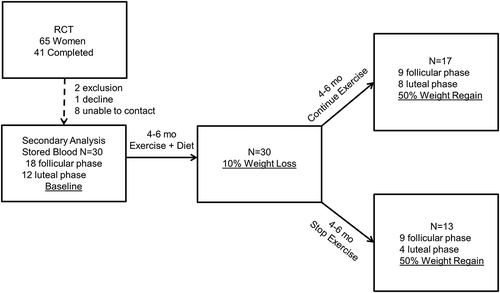
Results
The samples were collected between 2007 and 2008, stored at -80°C, and were analyzed for the current study in 2010. It is possible that some degradation could have occurred in that time though a widely cited study evaluating this issue was reassuring in this regard [Bolelli et al. Citation1995]. Serum samples from 30/41 women from the original study were available for further analysis (). The women were of mean age of 40 ± 5.9 years. Twenty-eight were non-Hispanic-Caucasian, one was of Asian Indian and one was of Hispanic descent. Eighteen were in the follicular phase, 12 were luteal (), and blood was sampled during the same phase at baseline, weight loss, and weight regain. By design (), during the weight loss phase, weight decreased 9.6% through dietary calorie reduction of approximately 600 kcal/day from baseline and expenditure of approximately 450 kcal/day achieved with aerobic walking/jogging exercise at 60% maximal oxygen consumption measured through expired gas [Thomas et al. Citation2010]. Following completion of the weight loss phase, participants intentionally regained 54% of the lost weight (). In those who were randomized to continue exercise at 60% maximal oxygen consumption, weight regain was accomplished by increased dietary intake of healthy food, while in the remaining participants weight regain occurred by a combination of cessation of exercise and increased dietary intake [Thomas et al. Citation2010]. Weight regain was confirmed by additional data indicating that percent fat by dual X-ray absorptiometry decreased and increased similarly to weight and body mass index (BMI) (). There was no difference in fasting glucose whereas insulin, homeostasis model assessment (HOMA) (marker of insulin resistance), and quantitative insulin sensitivity check index (QUICKI) (marker of insulin sensitivity) showed improvements using repeated measures ANOVA testing from baseline to weight loss that were maintained despite weight regain (). Due to skewed distributions, high molecular weight adiponectin (HMWAdp), total testosterone (T), sex hormone binding globulin (SHBG), and free androgen index (FAI) were natural-log-transformed. LogHMWAdp showed no changes () using linear mixed modeling.
Table 1. All measured study parameters for baseline, post weight loss, and post weight regain with or without exercise.
Figure 1. Study design flowchart from the original randomized controlled trial (RCT) and for the current secondary analysis.
The Wilcoxon Signed Rank test was used to test for differences across time points for logT and logFAI. Due to samples below T assay detection limit, imputation and linear mixed models were also used to analyze Log T and log FAI. LogT did not change from baseline with either weight loss or weight regain (). However, logFAI significantly decreased with weight loss, then increased with weight regain (). Notably, LogSHBG significantly increased from baseline to weight loss, and then significantly decreased with weight regain ( and Supplemental ), while log visceral adipose tissue (VAT) by computed tomography mirrored these fluctuations over the same time points (). There were trends of higher weight, BMI, insulin, and logFAI along with lower logSHBG in the nonexercisers but none of the exercise vs. nonexercise comparisons achieved statistical significance using Student’s t test and Wilcoxon Sign Rank test as appropriate ().
Discussion
In our secondary analysis of stored blood collected from a cohort of premenopausal women with metabolic syndrome, log sex hormone binding globulin (SHBG) increased with supervised weight loss, then decreased with controlled intentional weight regain (50% of lost weight), while opposite changes were seen with log free androgen index (FAI). Log total T and log high molecular weight adiponectin (HMWAdp) did not exhibit significant changes. Endurance exercise during the weight regain component did not significantly affect these findings.
Although the changes in SHBG with weight loss and gain were consistent with previous investigations [Harvie et al. Citation2011; Wildman et al. Citation2012; Guzick et al. Citation1994] they were unexpected in that no changes with weight were observed for HMWAdp. In the longitudinal Study of Women’s Health Across the Nation (SWAN), HWAdp negatively correlated with BMI and fat mass, and HWAdp levels were highest (6.3 µg/ml) with low T levels <25.8 ng/dL even after adjustment for SHBG [Wildman et al. Citation2013]. Intra-abdominal adipose tissue in our sample decreased and increased as expected with weight [Azrad et al. Citation2012], and we observed a similar inverse relationship of visceral adipose tissue (VAT) to SHBG to that found in SWAN, which noted that the lowest VAT (78.7 cm2) was found in subjects with the highest SHBG (90.9 nmol/L) levels [Kavanagh et al. Citation2013]. Of note, HWAdp in our study was 6-fold lower than those found in SWAN, and so it is possible that the effects of weight change and exercise would require a larger sample size to detect a difference.
Therefore, given the reduction of SHBG with weight regain, we did not expect the sustained improvements in insulin, HOMA, and QUICKI. Insulin has an inhibitory effect on SHBG in-vitro [Plymate et al. Citation1988] and low SHBG could potentially be implicated in development of diabetes mellitus [Ding et al. Citation2009; Simo et al. Citation2015], though others have also commented on the lack of correlation of insulin and SHBG [Azrad et al. Citation2012]. The original randomized controlled trial (RCT) found similar findings with insulin resistance markers only in the continuing exercise group [Thomas et al. Citation2010]. Our findings contrast with previous investigations which found worsening of HOMA and QUICKI with unsupervised weight regain [Beavers et al. Citation2013; Beavers et al. Citation2015]. These studies involved older populations who most likely did not maintain exercise during weight regain, though our differing findings also may relate to our insufficient power and trend of differing weights in the exercising vs. non exercising groups. More sensitive measurements of insulin sensitivity such as hyperinsulinemic euglycemic clamp would likely have provided more clarity but were not performed in the RCT. Based on results from the landmark Diabetes Prevention Program Trial, it seems reasonable to assume that the approximately 9 kg (10%) weight loss and 5 kg weight regain in our study would be sufficient to cause changes in insulin/glucose homoeostasis [Orchard et al. Citation2005; Delahanty et al. Citation2014; Knowler et al. Citation2002]. SHBG is a sex-steroid transport protein produced in the liver, and its regulation is affected by liver fat and liver adipogenesis [Kavanagh et al. Citation2013]. Medical conditions such as nonalcoholic steatohepatitis or thyroid disease could thus influence SHBG levels. Our study participants reported no other health issues beyond obesity and metabolic syndrome [Thomas et al. Citation2010]. Undiagnosed polycystic ovary syndrome may have potentially affected the findings but this was unlikely given low serum T. We did exclude participants using hormonal contraceptives as those could raise SHBG. It is possible that analysis by exercise was affected by menstrual cycle phase, since SHBG rises in the luteal phase [Thys-Jacobs et al. Citation2008] and more women in the exercise group were in the luteal phase. However, we would then expect to see significant differences by exercise group, which was not the case. Furthermore, since each participant was sampled in the same phase of the cycle for each time point (baseline, weight loss, and weight regain), changes with SHBG across each time point are not expected to be affected by menstrual phase. In support of this contention, published data demonstrated that serum T remains stable across the menstrual cycle [Elliott et al. Citation2003].
We are limited in our interpretation of the T and FAI findings. Our a priori analysis suggested that the study was adequately powered to detect changes in T, but our study sample nevertheless was small with 30 women included out of the 41 who had completed the RCT. Other investigators have found no changes of T levels across quartiles of SHBG [Kavanagh et al. Citation2013]. Additionally, SHBG and FAI are more strongly correlated to BMI-adjusted serum markers of cardiovascular disease than T [Sutton-Tyrrell et al. Citation2005]. The chemiluminescent assays for our study were performed in 2010, and were similar to that recently used in SWAN [Polotsky et al. Citation2014; Wildman et al. Citation2013]. We acknowledge a limitation of immunoassays to be less accurate at the low levels of testosterone in women. This has been addressed in the assays used in SWAN [Polotsky et al. Citation2012] and could have decreased the number of samples below the detection limit. Future direct measurement of T may be helpful to determine whether changes in FAI were attributable to SHBG alone, and additional measurement of dehydroepiandrosterone sulfate would be appropriate as higher levels have been correlated with less severe metabolic syndrome [Polotsky et al. Citation2012]. Our results in a mostly white population may have limited generalizability, although SWAN found that SHBG and FAI correlations were very robust across ethnic groups [Sutton-Tyrrell et al. Citation2005].
To summarize, SHBG showed prominent sensitivity to body mass fluctuations, as reduction with controlled intentional weight regain showed an inverse relationship to VAT and occurred despite stable HMWAdp and sustained improvements with insulin resistance. FAI showed opposite changes to SHBG, while T did not change significantly with weight. Continued endurance exercise during weight regain did not appear to impact these findings. However, interpretation of T and exercise comparisons are limited by power.
Materials and methods
This study involved assays of stored blood samples collected from a completed RCT [Thomas et al. Citation2010] of the impact of exercise on metabolic and cardiovascular health. The RCT was conducted in 2007-2008 and was in accordance with the Declaration of Helsinki and approved by the Health Sciences Institutional Review Board at the University of Missouri. Informed consent for this secondary analysis conducted in 2010 was also obtained from the included participants.
In the original RCT the outcome of interest was whether continuing endurance exercise after weight loss would maintain metabolic benefits during weight regain. Participants were previously sedentary overweight to obese men and women age 21-52 with metabolic syndrome based on National Cholesterol Education Program III criteria [Third Report of the National Cholesterol Education Program Citation2002; Thomas et al. Citation2010]. All indicated that they were otherwise healthy in their responses on a health questionnaire. Subjects lost 10% weight with supervised calorie restriction of approximately 600 kcal/day and aerobic exercise over 4-6 months. This was followed by a prior designed regain of 50% of the lost weight by increasing caloric intake while randomized to either continued aerobic exercise or cessation of exercise over 4-6 months (). The 10% weight loss goal was perceived to be modest enough that the amount of subsequent weight regained would be within acceptable ethical boundaries, but still significant enough to produce beneficial changes in metabolic parameters for overweight to class II obese subjects. In order to further maintain ethical standards, the intervention was 50% regain rather than 100%. The exercise regimen consisted of supervised walking/jogging 5 times a week which increased over 4 weeks to >200 min a week for 4-6 months. Exercise was quantified as 60% maximal oxygen consumption (directly measured with expired gas with treadmill use) for >200 min per week, approximately 450 kcal/day, for all participants. Participants randomized to exercise during weight regain continued the supervised exercise at 60% maximal oxygen consumption for >200 min per week while the nonexercisers were also closely supervised; the randomization process of the original RCT for exercise vs. nonexercise during weight regain is described previously [Thomas et al. Citation2010]. Each subject in the no exercise group was required to report to the testing laboratory a minimum of 1-2 d per week for weigh-in, diet diary analysis, and for counseling.
Participants were non-smokers and were not taking any potentially confounding medications that could influence weight loss or metabolic syndrome variables [Thomas et al. Citation2010]. For the secondary analysis of the stored blood samples from the RCT, we also excluded subjects using hormonal contraceptives and those who had undergone hysterectomy or oophorectomy. Forty-one of the original 65 women completed the RCT. Thirty met inclusion criteria and agreed to the secondary analysis of their stored blood samples ().
Weight, BMI in kg/m2, visceral adipose tissue (VAT) in cm2 by computed tomography, and percent body fat by dual x-ray absorptiometry were measured at baseline, post-weight-loss, and post-weight-regain in the original RCT [Thomas et al. Citation2010]. Insulin, glucose, homeostasis model assessment (HOMA), and quantitative insulin sensitivity check index (QUICKI) were determined during the original trial at baseline, post weight loss, and post weight regain. All of the aforementioned original RCT data points were reanalyzed for the secondary analysis [Thomas et al. Citation2010].
For the ancillary analysis, the frozen blood samples from the original RCT were assayed in 2010 for total T, sex hormone binding globulin (SHBG), and high molecular weight adiponectin (HMWAdp) at baseline, post weight loss, and post weight regain. Each draw was obtained in the same phase of the menstrual cycle for a given female participant ().
All samples from a single subject were analyzed in a single assay in duplicate or triplicate. T (intraassay coefficient of variance (CV) 8.7%, interassay CV 10.6%) and SHBG (intraassay CV 6.5%, interassay CV 8.7%) were determined using the Immulite 1000 solid phase chemiluminescent immunoassay (Siemens Medical Solutions Diagnostics, Flanders, NJ, USA). The lower limit of detection for T was 20 ng/dl. HMWAdp (intraassay CV 3.1%, interassay CV 2.6%) was analyzed with enzyme-linked immunosorbent assay (Alpco Diagnostics, Salem, NH, USA). Free androgen index (FAI) was calculated: 100 x (T ng/dl * 0.0347 nmol/l)/SHBG nmol/l. There was one unmeasurable sample for SHBG and two for HMWAdp. For T (and therefore free androgen index FAI), 12 samples were below the assay detection limit.
A priori power analysis was based on highly significant changes in SHBG, T, and FAI by 15-20% each after a 4 kg (4% from baseline) weight loss [Moran et al. Citation2007]. With a 10% weight loss, an estimated 20 participants would detect a 40% effect size at power=0.80, α=0.05 (Epi Info Version 6, Centers for Disease Control, 1993). Values below the T detection limit were addressed both by linear mixed model methods [Thiebaut and Jacqmin-Gadda Citation2004] and by imputing [Helsel Citation2005] T to 10 ng/dL and calculating the corresponding values for FAI. Due to skewed distributions of SHBG, T, FAI, VAT, and HMWAdp, log transformation was performed. The Wilcoxon Signed Rank test was used to test for differences across time points for logT and logFAI, linear mixed modeling was used for HMWAdp, and repeated measures analysis of variance (ANOVA) was used for the remaining variables with post-hoc Tukey HSD test when appropriate. Comparisons of exercise and non-exercise during the weight regain segment were conducted with Student t test and Wilcoxon Sign Rank test as appropriate. Two-tailed statistical analysis was performed with statistical significance set at an α-level of p < 0.05 (Stata, College Station, TX, USA).
Declaration of interest
The material contained in the manuscript is original, has not been published, has not been submitted or is not being submitted elsewhere. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article. This work was supported by a grant from the University of Missouri Research Council URC-10-044.
IAAN_1177619_Supplementary_files.zip
Download Zip (176.5 KB)Supplemental Material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Notes on contributors
Mira Aubuchon
Conceived and designed the experiments: MA,TRT; Performed the experiments: YL,TRT; Analyzed the data: MA, AJP, GFP,YL; Wrote the manuscript: MA, AJP.
Ying Liu
Conceived and designed the experiments: MA,TRT; Performed the experiments: YL,TRT; Analyzed the data: MA, AJP, GFP,YL; Wrote the manuscript: MA, AJP.
Gregory F. Petroski
Conceived and designed the experiments: MA,TRT; Performed the experiments: YL,TRT; Analyzed the data: MA, AJP, GFP,YL; Wrote the manuscript: MA, AJP.
Tom R. Thomas
Conceived and designed the experiments: MA,TRT; Performed the experiments: YL,TRT; Analyzed the data: MA, AJP, GFP,YL; Wrote the manuscript: MA, AJP.
Alex J. Polotsky
Conceived and designed the experiments: MA,TRT; Performed the experiments: YL,TRT; Analyzed the data: MA, AJP, GFP,YL; Wrote the manuscript: MA, AJP.
References
- Azrad, M., Gower, B.A., Hunter, G.R. and Nagy, T.R. (2012) Intra-abdominal adipose tissue is independently associated with sex-hormone binding globulin in premenopausal women. Obesity (Silver Spring) 20:1012-1015.
- Beavers, D.P., Beavers, K.M., Lyles, M.F. and Nicklas, B.J. (2013) Cardiometabolic risk after weight loss and subsequent weight regain in overweight and obese postmenopausal women. J Gerontol A Biol Sci Med Sci 68:691-698.
- Beavers, K.M., Case, L.D., Blackwell, C.S., Katula, J.A., Goff, D.C., Jr. and Vitolins, M.Z. (2015) Effects of weight regain following intentional weight loss on glucoregulatory function in overweight and obese adults with pre-diabetes. Obes Res Clin Pract 9:266-273.
- Bell, R.J., Davison, S.L., Papalia, M.-A., McKenzie, D.P. and Davis, S.R. (2007) Endogenous androgen levels and cardiovascular risk profile in women across the adult life span. Menopause 14:630–638 610.1097/gme.1090b1013e31802b31806cb31801.
- Bolelli, G., Muti, P., Micheli, A., Sciajno, R., Franceschetti, F., Krogh, V., et al. (1995) Validity for epidemiological studies of long-term cryoconservation of steroid and protein hormones in serum and plasma. Cancer Epidemiol Biomarkers Prev 4:509-513.
- Delahanty, L.M., Pan, Q., Jablonski, K.A., Aroda, V.R., Watson, K.E., Bray, G.A., et al. (2014) Effects of weight loss, weight cycling, and weight loss maintenance on diabetes incidence and change in cardiometabolic traits in the Diabetes Prevention Program. Diabetes Care 37:2738-2745.
- Ding, E.L., Song, Y., Manson, J.E., Hunter, D.J., Lee, C.C., Rifai, N., et al. (2009) Sex hormone-binding globulin and risk of type 2 diabetes in women and men. N Engl J Med 361:1152-1163.
- Donnelly, J.E., Washburn, R.A., Sullivan, D.K., Honas, J.J., Mayo, M.S., Goetz, J., et al. (2013) The Midwest Exercise Trial for the Prevention of Weight Regain: MET POWeR. Contemp Clin Trials 36:470–478.
- Elliott, K.J., Cable, N.T., Reilly, T. and Diver, M.J. (2003) Effect of menstrual cycle phase on the concentration of bioavailable 17-beta oestradiol and testosterone and muscle strength. Clin Sci (Lond) 105:663-669.
- Guzick, D.S., Wing, R., Smith, D., Berga, S.L. and Winters, S.J. (1994) Endocrine consequences of weight loss in obese, hyperandrogenic, anovulatory women. Fertil Steril 61:598-604.
- Harvie, M.N., Pegington, M., Mattson, M.P., Frystyk, J., Dillon, B., Evans, G., et al. (2011) The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. Int J Obes (Lond) 35:714-727.
- Helsel, D.R. (2005) Nondetects and data analysis: statistics for censored environmental data. Statistics in practice. Wiley-Interscience, Hoboken, NJ, USA.
- Kavanagh, K., Espeland, M.A., Sutton-Tyrrell, K., Barinas-Mitchell, E., Khoudary, S.R. and Wildman, R.P. (2013) Liver fat and SHBG affect insulin resistance in midlife women: the Study of Women’s Health Across the Nation (SWAN). Obesity (Silver Spring) 21:1031-1038.
- Knowler, W.C., Barrett-Connor, E., Fowler, S.E., Hamman, R.F., Lachin, J.M., Walker, E.A., et al. (2002) Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 346:393-403.
- Moran, L.J., Noakes, M., Clifton, P.M., Wittert, G.A., Belobrajdic, D.P. and Norman, R.J. (2007) C-Reactive Protein before and after Weight Loss in Overweight Women with and without Polycystic Ovary Syndrome. J Clin Endocrinol Metab 92:2944-2951.
- Orchard, T.J., Temprosa, M., Goldberg, R., Haffner, S., Ratner, R., Marcovina, S., et al. (2005) The effect of metformin and intensive lifestyle intervention on the metabolic syndrome: the Diabetes Prevention Program randomized trial. Ann Intern Med 142:611-619.
- Patel, S.M., Ratcliffe, S.J., Reilly, M.P., Weinstein, R., Bhasin, S., Blackman, M.R., et al. (2009) Higher serum testosterone concentration in older women is associated with insulin resistance, metabolic syndrome, and cardiovascular disease. J Clin Endocrinol Metab 94:4776-4784.
- Plymate, S.R., Matej, L.A., Jones, R.E. and Friedl, K.E. (1988) Inhibition of sex hormone-binding globulin production in the human hepatoma (Hep G2) cell line by insulin and prolactin. J Clin Endocrinol Metab 67:460-464.
- Polotsky, A.J., Allshouse, A., Crawford, S.L., Harlow, S.D., Khalil, N., Santoro, N., et al. (2012) Relative contributions of oligomenorrhea and hyperandrogenemia to the risk of metabolic syndrome in midlife women. J Clin Endocrinol Metab 97:E868–877.
- Polotsky, A.J., Allshouse, A.A., Crawford, S.L., Harlow, S.D., Khalil, N., Kazlauskaite, R., et al. (2014) Hyperandrogenic oligomenorrhea and metabolic risks across menopausal transition. J Clin Endocrinol Metab 99:2120-2127.
- Simo, R., Saez-Lopez, C., Barbosa-Desongles, A., Hernandez, C. and Selva, D.M. (2015) Novel insights in SHBG regulation and clinical implications. Trends Endocrinol Metab 26:376-383.
- Simo, R., Saez-Lopez, C., Lecube, A., Hernandez, C., Fort, J.M. and Selva, D.M. (2014) Adiponectin upregulates SHBG production: molecular mechanisms and potential implications. Endocrinology 155:2820-2830.
- Sutton-Tyrrell, K., Wildman, R.P., Matthews, K.A., Chae, C., Lasley, B.L., Brockwell, S., et al. (2005) Sex Hormone-Binding Globulin and the Free Androgen Index Are Related to Cardiovascular Risk Factors in Multiethnic Premenopausal and Perimenopausal Women Enrolled in the Study of Women Across the Nation (SWAN). Circulation 111:1242-1249.
- Thiebaut, R. and Jacqmin-Gadda, H. (2004) Mixed models for longitudinal left-censored repeated measures. Comput Methods Programs Biomed 74:255-260.
- Third Report of the National Cholesterol Education Program (2002) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, E., and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Circulation 106:3143-3421.
- Thomas, T.R., Warner, S.O., Dellsperger, K.C., Hinton, P.S., Whaley-Connell, A.T., Rector, R.S., et al. (2010) Exercise and the metabolic syndrome with weight regain. J Appl Physiol 109:3-10.
- Thys-Jacobs, S., McMahon, D. and Bilezikian, J.P. (2008) Differences in free estradiol and sex hormone-binding globulin in women with and without premenstrual dysphoric disorder. J Clin Endocrinol Metab 93:96-102.
- Wildman, R.P., Tepper, P.G., Crawford, S., Finkelstein, J.S., Sutton-Tyrrell, K., Thurston, R.C., et al. (2012) Do changes in sex steroid hormones precede or follow increases in body weight during the menopause transition? Results from the Study of Women’s Health Across the Nation. J Clin Endocrinol Metab 97:E1695–1704.
- Wildman, R.P., Wang, D., Fernandez, I., Mancuso, P., Santoro, N., Scherer, P.E., et al. (2013) Associations of testosterone and sex hormone binding globulin with adipose tissue hormones in midlife women. Obesity (Silver Spring) 21:629-636.
- Xu, F., Town, M., Balluz, L.S., Bartoli, W.P., Murphy, W., Chowdhury, P.P., et al. (2013) Surveillance for certain health behaviors among States and selected local areas - United States, 2010. MMWR Surveill Summ 62:1-247.
