ABSTRACT
This study aimed to investigate the protective effects of Cordyceps militaris (C. militaris) against reproductive damage induced by bisphenol A (BPA). Rats were administrated 200 mg/kg BPA for 4 weeks and treated with C. militaris (200, 400, and 800 mg/kg body weight/day). By the end of the fourth week, the level of oxidative damage, sperm parameters, hormone levels, and histopathological changes were examined. In the group that only received BPA, there was a significant decrease in body weight compared with the normal control (NC) group. C. militaris significantly alleviated the BPA-induced reproductive damage by increasing testicular superoxide dismutase (SOD), glutathione peroxidase (GSH-PX), and glutathione (GSH); as well as by reducing serum malondialdehyde (MDA). C. militaris not only obviously enhanced the levels of serum LH and T, but it also improved the sperm count and motility compared to the BPA-treated group. These results suggest that C. militaris could be used as a potential natural substance for preventing BPA induced reproductive damage.
Abbreviations BPA: bisphenol A; SOD: superoxide dismutase; GSH: glutathione; GSH-PX: glutathione peroxidase; MDA: malondialdehyde; ROS: reactive oxygen species; T: testosterone; LH: luteinizing hormone; FSH: follicle-stimulating hormone; UPLC: ultra performance liquid chromatography; RIA: radioimmunoassay; qRT-PCR: quantitative real time PCR; NC: normal control group; BPA: 200 mg/kg BPA administered group; H: 800 mg/kg C. militaris extract administered group; LB, MB, and HB: 200 mg/kg BPA + 200 mg/kg, 400 mg/kg, and 800 mg/kg C. militaris administered group, respectively; VeB: 200 mg/kg BPA + 300 mg/kg Vitamin E administered group; Star: steroidogenic acute regulatory protein; 3β-HSD: 3beta-hydroxyl-delta-5-steroid dehydrogenase; CYP11A1: cytochrome P 450 family 11 subfamily A member 1; CYP17A1: cytochrome P 450 family 17 subfamily A member 1
Introduction
Bisphenol A (BPA; 2, 2-bis (4-hydroxyphenyl) propane; CAS: 80-05-7), a mass-produced artificial compound, has been widely used as plasticizers [Huang et al. Citation2012]. According to a survey in 2013, a total of 7 million tons of BPA was produced, from which 73% was used for polycarbonate, followed by epoxy resins and other plastic products in a wide range of products in our daily use, including infant feeding bottles, water bottles, food storage containers, plastic bags, thermal paper, and dental sealant medical devices [Hormann et al. Citation2014; Kloukos et al. Citation2013; WHO Citation2010b]. BPA can be easily released from those materials into water, food, or environment by various means like under heat, acid, and base substances, which can increase the risk of human exposure to BPA via various routes [Welshons et al. Citation2006].
BPA has numerous toxic effects on multiple organ systems including brain, liver, lung, kidney, nervous system, and reproductive system [El-Missiry et al. Citation2014; Manfo et al. Citation2014; Michałowicz Citation2014; Spanier et al. Citation2014]. It has been confirmed that BPA can influence the regular hormonal secretions by acting as an estrogen agonist and as an androgen receptor antagonist in-vivo [Wolstenholme et al. Citation2011]. BPA can also induce free radical generation with oxidative stress, thereby reducing epididymal or testicular sperm counts, and increasing DNA damage and cell apoptosis. This has been considered an important factor in testicular structural damage and dysfunction [Anjum et al. Citation2011; Korkmaz et al. Citation2010; Li et al. Citation2009; Meeker et al. Citation2010].
Cordyceps militaris (L.) Link (C. militaris) is a medicinal fungus of traditional Chinese medicine used as a folk tonic food. C. militaris and Cordyceps sinensis (C. Sinensis) belong to the same fungus family with similar chemical composition, nutritional ingredients, and medicinal functions. As natural, C. Sinensis is scarce and expensive, C. militaris has been considered as an ideal substitute for C. Sinensis [Fan and Lin Citation2013]. C. militaris contains many active components, such as cordycepin, polysaccharide, cordycepic acid, amino acids, trace elements, and other chemical compositions [Fan and Lin Citation2013; Ni et al. Citation2007]. Traditional Chinese medicine believes that C. militaris can be used to treat impotence, seminal emission, and infertility, and invigorate kidney and lungs [Ch Citation2000; Zhu et al. Citation1998]. Pharmacological studies have confirmed that C. militaris possesses a wide range of biological activities including anti-bacterial, anti-tumor, anti-oxidation, and immune-modulatory [Liu et al. Citation1994; Ng and Wang Citation2005; Reis et al. Citation2013]. It can also improve reproductive function and repair reproductive dysfunction induced by cyclophosphamide in mice [Jin and Chen Citation2008].
In order to evaluate the possible protective effect of C. militaris against BPA-induced reproductive toxicity, this investigation was focused on oxidative damage, hormone synthesis, and spermatogenesis using a mouse model.
Results
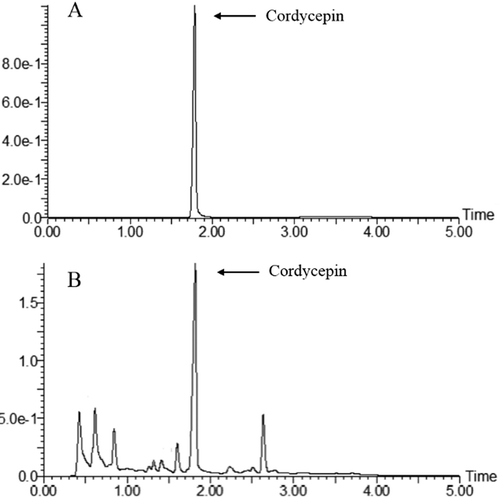
Total polysaccharide and cordycepin of C. militaris extracts
The values of a standard sample showed a linear relation y = 4.7787x + 0.1319 (R2=0.9896) and y = 277.44x + 328.12 (R2=0.9996) for glucose and cordycepin, respectively. The total polysaccharide and cordycepin contents in the extracts were 368.00 ± 28.56 mg/g and 9.41 ± 0.09 mg/g, respectively ().
Body weight and organ index
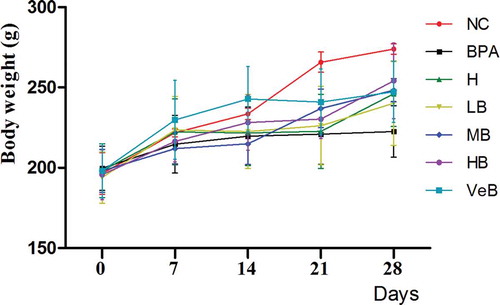
When compared to the normal control group (NC), the body weight decreased after 28 days of BPA exposure. C. militaris resisted the negative effect of BPA on body weight in a dosage dependent manner compared to the BPA group (). However, there were no significant changes in the organ indexes observed among all groups ().
Table 1. Reproductive organ indices.
Figure 2. Effects of Cordyceps militaris (C. militaris) and/or bisphenol A (BPA) on the changes of body weight. NC: normal control group; BPA represents the group treated with 200 mg/kg BPA; H (high dose of C. militaris group) represents 800 mg/kg C. militaris extract administered group; LB (low concentration of C. militaris combined with BPA group), MB (middle concentration of C. militaris combined with BPA group), and HB (high concentration of C. militaris combined with BPA group) represent the rats co-administrated 200 mg/kg BPA with 200, 400, and 800 mg/kg C. militaris, respectively; VeB (Vitamin E combined BPA group) represents 200 mg/kg BPA + 300 mg/kg Vitamin E administered group.
Biochemical, hormone analysis, and semen parameters
Malondialdehyde (MDA) is an important product of lipid peroxidation, and can be used to evaluate lipid peroxidation. It was measured by the thiobarbituric acid (TBA) method in each group. Our results showed that BPA significantly increased concentrations of MDA, however C. militaris could block the increase of MDA in BPA-treated mice in a dosage dependent manner (), suggesting that C. militaris could inhibit lipid peroxidation.
Table 2. Effect of Cordyceps militaris (C. militaris) against bisphenol A (BPA) on lipid peroxidation, antioxidant biochemical parameters, serum T, LH, and FSH level, mRNA levels of Star, CYP11A1, 3β-HSD, CYP17A1, and semen parameters.
Superoxide dismutase (SOD), glutathione peroxidase (GSH-PX), and glutathione (GSH) play important roles as antioxidants in vivo, and their imbalance could cause oxidative stress. The activity of SOD and GSH-PX were determined by using commercial kits, and the GSH content was assessed by using 5,5′-dithiobis (2-nitrobenzoicacid) (DTNB) by GSH determination kits. In our study, the activity of GSH-Px (p < 0.01) and SOD (p < 0.05) were significantly reduced in the BPA group, while C. militaris could alter such decreases (). C. militaris could also significantly resist the decrease in level of GSH induced by BPA. It likely increases the SOD and GSH-PX activity and the content of GSH.
Levels of a series of hormones were quantified directly from the prepared serum using radioimmunoassay (RIA) kits. BPA significantly reduced the concentration of serum testosterone (T) and luteinizing hormone (LH) (p < 0.05), while C. militaris eliminates such decreases () (p < 0.05). However, there were no significant changes in the level of serum follicle-stimulating hormone (FSH) among each group ().
Star, CYP11A1, 3β-HSD, and CYP17A1 play key roles in catalyzing testosterone synthesis. Our results showed that BPA could reduce the mRNA levels of Star, as well as CYP11A1, 3β-HSD, and CYP17A1 to different degrees. The presence of C. militaris could significantly reduce the negative effect of BPA on the mRNA levels of Star, CYP11A1, 3β-HSD, and CYP17A1 (), especially in the 800 mg/kg C. militaris extract administered (H) group (p < 0.01).
Sperm concentration and motility can be another important indicator for male reproductive health, which could directly affect male fertility. They were microscopically assessed. The results showed there was a significant decrease in the sperm count and sperm motility in the BPA-treated rats (p < 0.05), while sperm motility and sperm count could be significantly improved in the BPA-treated rats in the presence of C. militaris (p < 0.05) ().
Histopathology
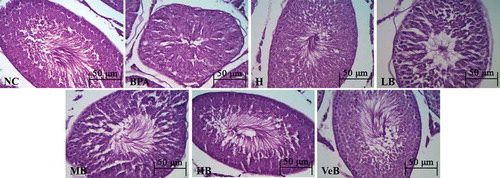
Photomicrographs of testis tissue of the NC and H groups showed normal architecture of testicular tissue with active spermatogenesis in seminiferous tubules. The BPA group revealed an abnormal appearance with respect to the number of mature sperm with obvious loss in seminiferous tubules, sertoli cells, and germ cells arranged disorderly and decreased in quantity, especially for mature sperms and elongated spermatids. Testis tissue of BPA treated rats along with C. militaris (200, 400, and 800 mg/kg, respectively) showed that the number of mature sperm increased significantly in seminiferous tubules with the increase of C. militaris dose, and Sertoli cells and germ cells were arranged orderly (). Testis tissue of the BPA treated rat along with the Ve Group normalized compared to the BPA group.
Figure 3. Histological of rat testis of different experimental groups stained with haematoxylin and eosin (original magnification 400×). Testis from normal control group (NC) and 800 mg/kg Cordyceps militaris (C. militaris) extract administered group (H) group revealed normal testicular morphology for seminiferous tubule architecture. Testis from the bisphenol A (BPA) group revealed germ cells arranged disorderly and decreased in quantity, especially for mature sperms and elongated spermatids. Testis from the BPA+ C. militaris group revealed C. militaris could reverse the opposite effect caused by BPA. LB, MB, and HB: 200 mg/kg BPA + 200 mg/kg, 400 mg/kg, and 800 mg/kg C. militaris administered group, respectively; VeB: 200 mg/kg BPA + 300 mg/kg Vitamin E administered group.
Discussion
The toxic effects of environmental endocrine disruptors on the human reproductive system are of major health concern, which is strongly associated with reproductive dysfunction in both animal and human populations. BPA is one of the potential environmental endocrine disruptors that has serious harmful effects on the reproductive system. In this study, C. militaris enhanced the levels of serum LH and T, but not FSH to raise the sperm count and motility inhibited by BPA (). Histopathology indicated that C. militaris treatment could reverse the BPA-induced abnormal testis morphology. This suggests that C. militaris possesses the potential to prevent BPA-induced reproductive damage.
Oxidative stress is one of the important factors in testicular structural damage and dysfunction. Previous studies reported that BPA causes oxidative stress that is characterized by excessive reactive oxygen species (ROS) and imbalance between ROS and the antioxidant defense system in testis [Kabuto et al. Citation2004]. BPA can be metabolized to semiquinone and/or quinone products by various CYP450s. These products can act catalytically to generate large quantities of ROS, e.g., superoxide anion and hydrogen peroxide yielding in-vivo oxidative stress [Atkinson and Roy Citation1995a; Citation1995b; Gutierrez Citation2000; Kovacic Citation2010]. BPA can directly react with GSH by forming the complex compound to clear from the body [Gualtieri et al. Citation2011]. BPA administered orally to male rats at a dose of 10 mg/kg bwt for 14 days led to a decrease of testicular antioxidant enzymes including GSH, GSH-Px, SOD, and catalase [El-Beshbishy et al. Citation2013]. Our results showed that C. militaris enhanced the activity of antioxidant enzyme (SOD and GSH-Px) and the level of GSH in BPA-treated rats. C. militaris has been used as an antioxidant for a long time. It has been confirmed that the extract of C. militaris could inhibit lipid peroxidation by scavenging free radicals [Reis et al. Citation2013] and C. Sinensis could significantly improve the activity of SOD and GSH-Px to protect the testis from oxidative damage induced by cyclophosphamide in mice [Jin and Chen Citation2008]. These results suggested that C. militaris could alleviate BPA-induced oxidative damage.
Testosterone synthesis is an important indicator of male reproductive health. There are two pathways that mainly regulate testosterone synthesis: the coarse regulation of testicular gonadal axis and the fine regulation of steroid synthesis enzymes. The testicular gonadal axis is primarily regulated through LH (the release from pituitary) and LH receptors on the testicular cell membrane binding surface. Subsequent activation of the cAMP-pkA signaling pathway promotes de novo steroid-related protein synthesis (star, transport of cholesterol into the Leydig cell) [Cooke Citation1999; Lucki and Sewer Citation2010]. Regulation of steroid synthesis is catalyzed by four different enzymes including CYP11A1, 3β-HSD, CYP17A1, and 17β-HSD. CYP11A1 is the first catalytic cholesterol (transported by star) to generate pregnenolone, which is a key and rate-limiting enzyme in the synthesis process. Pregnenolone is synthesized via 3β-HSD with CYP17A1 generating androstenedione. Finally, it is catalyzed to testosterone by 17β-HSD. Recent studies showed that BPA reduced testosterone and LH serum concentration by disrupting the negative feedback mechanisms of hypothalamic–pituitary–gonadal axis [Wisniewski et al. Citation2015]. BPA is considered a potential endocrine disruptor that can inhibit testosterone production by inhibiting CYP11A1, 3β-HSD, CYP17A1, and 17β-HSD [Peretz and Flaws Citation2013; Ye et al. Citation2011]. In our study, C. militaris could rectify the negative effects of BPA-induced degeneration of serum T and LH level (). Previous reports confirmed that C. sinensis could stimulate testosterone production both in-vivo and in-vitro, and it is possible that the polysaccharides and/or glycoproteins in extracts might be structurally similar to LH. These could possess the ability to recognize LH receptors on Leydig cells to stimulate testosterone production [Hsu et al. Citation2003a; Huang et al. Citation2001; Huang et al. Citation2004]. Hsu et al. [Citation2003b] also proved that C. sinensis could activate the cAMP-protein kinase-A signal pathway. Cordycepin, as adenosine analogues, was associated with adenosine receptors to activate cAMP-PKA-Star pathway and steroidogenesis in mouse Leydig cells [Leu et al. Citation2011]. However, Pao et al. [Citation2012] reported that cordycepin could activate intracellular PLC/PKC and MAPK signal transduction pathways, but not PKA and PI3K, to induce MA-10 cell steroidogenesis. The different pathways shown above might be associated with the anti-tumor effect of cordycepin. As the data suggests, C. militaris could directly up-regulate Star, CYP11A1, 3β-HSD, and CYP17A1 expression, thereby attenuating the decline of testosterone synthesis induced by BPA.
Materials and methods
Preparation of C. militaris extracts
C. militaris was obtained as a gift sample from the Boen Pharmaceutical Ltd. Co. (Chifeng City, Inner Mongolia, China) and authenticated by Professor Dr. Yong Wang of the School of Biological Science and Engineering, Shaanxi University of Technology. The crushed C. militaris (10 g) was mixed with 200 mL of distilled water, then boiled for 2 h, and the extract collected. The residue was extracted again with 100 mL of distilled water and boiled for 1 h. Finally, the extracts were combined, lyophilized, and stored at 4°C for further use.
Determination of cordycepin and polysaccharide in C. militaris extracts
C. militaris extract (0.3 g) was diluted with distilled water to 10 mL and filtered through a 0.22 μm membrane filter. The filtered solutions were analyzed by UPLC (Waters Acquity-UPLC, Milford Massachusetts, USA) using a Waters BEH C18 column (1.7 μm, 50 mm × 2.5 mm) and a water–methanol solvent (85:15, v/v) system at a wavelength of 260 nm with flow rate of 0.3 mL/min as described in a previous report [Suo et al. Citation2008]. A standard curve for detecting the cordycepin content in C. militaris extracts was prepared using cordycepin (Shanghai yuanye Bio-Technology Co., Ltd., Shanghai, China) and identified by a comparison of its retention time. The total cordycepin content was expressed as milligrams of cordycepin equivalents per gram of dried C. militaris extracts.
Total polysaccharide content was estimated by the Phenol-sulfuric acid method described by Shi [Citation2012]. Briefly, 0.5 mL of C. militaris extract, 1.5 mL of distilled water, 1 mL of 5% phenol reagent, and 5 mL of sulfuric acid solution were mixed together in 10 mL test tube. The mixture was eventually diluted with distilled water to 10 mL and let stand for 10 min at room temperature. The absorbance of the mixture was measured using a spectrophotometer at 490 nm. A standard curve for total polysaccharide content was prepared using glucose and the total polysaccharide content was expressed as milligrams of glucose equivalents per gram of dried C. militaris extracts.
Animals and experimental design
A total of 35 adult male Sprague-Dawley rats (200.78±14.70 g BW) were purchased from laboratory animal center of the Xi′an Jiaotong University (Shaanxi, China). Animals were housed under normal environmental conditions with 12 h dark/light cycles in polypropylene cages and free access to food and drinking water. The animal experimental procedures were carried out in accordance with the university guidelines for care and use of laboratory animals.
Rats were randomly divided into seven groups (n = 7) with five rats (n = 5) per group. Group 1: normal control group (NC group) without any intervention; Group 2: 200 mg/kg BPA (Gracia Chengdu Chemical Technology Co., Ltd., Chengdu, China; BPA group); Group 3: 800 mg/kg C. militaris (H group); Groups 4, 5, and 6: 200 mg/kg BPA along with 200 mg/kg, 400 mg/kg, and 800 mg/kg C. militaris, respectively (LB, MB, and HB groups); Group 7: 200 mg/kg BPA and 300 mg/kg Vitamin E (Beijing Solarbio Science &Technology Co., Ltd., Beijing, China; VeB group). Rats were treated with C. militaris (dissolved in distilled water) and BPA (dissolved in corn oil) with an interval of one hour, and administered once a day by oral gavage for 28 consecutive days.
Collection of samples
As described in a previous study on BPA rat model construction [Revathy et al. Citation2013], after 28 days of interventions with BPA and/or medicines, the animals were fasted overnight, weighed, carefully anaesthetized (10% chloral hydrate, 0.3 mL/kg), and sacrificed. Blood was collected by cardiac puncture and serum was separated by centrifugation at 3,000 rpm (15 min, 4°C) and stored at -20°C until further analysis. Testes, epididymidis, seminal vesicles, and prostate were weighed after clearing off the adhering tissues. Epididymidis were used for determination of sperm motility and count. Unilateral testicles were fixed in Bouin’s fixative at room temperature for histological analysis. Other testicles were used for biochemical studies and further assays. The organ indexes were calculated by using the following formula:
Antioxidant analysis and lipid peroxidation assessments
The testicle homogenate was prepared with ice-cold saline through an automatic homogenate machine, and then centrifuged at 2,000 rpm for 15 min to obtain the homogenate supernatant for the determination of total superoxide dismutase (T-SOD), glutathione peroxidase (GSH-Px), glutathione (GSH), and malondialdehyde (MDA) by using total superoxide dismutase (T-SOD, A001-1) assay kit, reduced glutathione (GSH, A006-1) assay kit, glutathione peroxidase (GSH-PX, A005) assay kit, and malondialdehyde (MDA, A003-1) assay commercial detection kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s protocol.
Hormone level assay
Serum T, FSH, and LH were quantified directly from the prepared serum using radioimmunoassay (RIA) kits (Beijing Sino-UK institute of biological Technology, Beijing, China) as per the manufacturer’s instructions.
RNA extraction and real-time quantitative PCR analysis
The gene expressions of Star, CYP11A1, 3β-HSD, and CYP17A1 were determined with quantitative real time PCR (qRT-PCR). Total RNA was extracted from testicular tissue by using the TianGen Total RNA extraction kit (TianGen Biotech CO., LTD., Beijing, China) according to the manufacturer’s instructions. The cDNA was synthesized by using the HiscriptTM Q Select RT SuperMix for qPCR (+gDNA) Reverse Transcriptase Kit (Vayme Biotech CO., LTD., Nanjing, China). The qRT-PCR was conducted by using the SYBR@ Green PCR Master Mix (Vayme Biotech CO., LTD., Nanjing, China) with 10 μmol of each primer as previously reported () [Feng et al. Citation2012].The qRT-PCR was set up in total 20 μL reaction volume. PCR reactions consisting of 95°C for 5 min (1 cycle), 95°C for 10 s, and 60°C for 30 s (40 cycles) were performed by Two Step method. Data were analyzed and quantified by using the Rotor-Gene Q series Software (QIAGEN, Germany). Relative expression of the studied genes was calculated by using the comparative threshold cycle method. All values were normalized to the β-actin gene.
Table 3. Primer sequences used for real time (RT)-PCR.
Histological studies
The testis was carefully removed, cleaned and fixed in Bouin’s fixative, embedded in paraffin, sectioned at 5 μm thickness, and stained with Hematoxylin and Eosin (H&E) for evaluation by light microscopy at 400× magnifications.
Sperm concentration and motility
Epididymis sperm were obtained by mincing the epididymis with anatomical scissors in 4 mL of physiological saline, and incubated at 37°C for 10 min for obtaining sperm suspension for the detection of semen parameters.
Sperm motility was measured using the previously reported method [WHO Citation2010a]. Briefly, 10μL of sperm suspension was placed on a slide and the motile sperm were counted with a minimum of 200 sperm by using microscope at 400× magnification. Sperm motility was expressed as a percent of motile sperm of the total sperm counted.
Sperm concentration was measured as per the method reported previously [Asadi et al. Citation2014]. Briefly, PBS buffer containing 10% of formaldehyde (450 μL) was added into sperm suspension (50 μL) to induce sperm immobility. Then, 10 μL of sperm suspensions of each sample was placed into the hemocytometer. After 5 min standing, the sperm concentration was counted and expressed as the mean sperm count of five random squares×107/mL.
Statistical analysis
All data were expressed as the mean ± standard deviation and analyzed by using one-way ANOVA procedure of SPSS 19.0 (SPSS Inc., Chicago, IL, USA), *p < 0.05, **p < 0.01, compared with control groups; #p < 0.05, ##p < 0.01, compared with BPA-treated groups, by using one-way ANOVA.
Conclusion
This study showed evidence that C. militaris could significantly protect testicles against oxidative damage caused by BPA and relieve BPA-induced degeneration of serum T and LH concentration by triggering Star, CYP11A1, 3β-HSD, and CYP17A1 expressions.
Declaration of interest
This study was supported by the High-end Foreign Experts Recruitment Program (GDW20146100228); Key Construction Program of International Cooperation Base in S&T, (2015SD0018) Shaanxi Province; Construction Project of Shaanxi Collaborative Innovation Center (2015, Shaanxi University of Technology), China. The authors declare that there are no conflicts of interest.
Additional information
Notes on contributors
Jian Wang
Conceived and designed the experiments: JW, ZJ, XZ; Performed the experiments: JW, MW, ZJ, HJ; Analyzed the data: MW, JW; Wrote the article: XZ, JW, CC.
Chen Chen
Conceived and designed the experiments: JW, ZJ, XZ; Performed the experiments: JW, MW, ZJ, HJ; Analyzed the data: MW, JW; Wrote the article: XZ, JW, CC.
Zhihui Jiang
Conceived and designed the experiments: JW, ZJ, XZ; Performed the experiments: JW, MW, ZJ, HJ; Analyzed the data: MW, JW; Wrote the article: XZ, JW, CC.
Meng Wang
Conceived and designed the experiments: JW, ZJ, XZ; Performed the experiments: JW, MW, ZJ, HJ; Analyzed the data: MW, JW; Wrote the article: XZ, JW, CC.
Hai Jiang
Conceived and designed the experiments: JW, ZJ, XZ; Performed the experiments: JW, MW, ZJ, HJ; Analyzed the data: MW, JW; Wrote the article: XZ, JW, CC.
Xiaoying Zhang
Conceived and designed the experiments: JW, ZJ, XZ; Performed the experiments: JW, MW, ZJ, HJ; Analyzed the data: MW, JW; Wrote the article: XZ, JW, CC.
References
- Anjum, S., Rahman, S., Kaur, M., Ahmad, F., Rashid, H., Ansari, R.A., et al. (2011) Melatonin ameliorates bisphenol A-induced biochemical toxicity in testicular mitochondria of mouse. Food Chem Toxicol 49: 2849–2854.
- Asadi, M.H., Zafari, F., Sarveazad, A., Abbasi, M., Safa, M., Koruji, M., et al. (2014) Saffron improves epididymal sperm parameters in rats exposed to cadmium. Nephro-Urol Mon 6: e12125.
- Atkinson, A. and Roy, D. (1995a) In vitro conversion of environmental estrogenic chemical bisphenol A to DNA binding metabolite(s). Biochem Bioph Res Co 210: 424–433.
- Atkinson, A. and Roy, D. (1995b) In vivo DNA adduct formation by bisphenol A. Environ Mol Mutagen 26: 60–66.
- Ch., P.C. (ed.) (2000) Chinese pharmacopoeia 2000 edition. Beijin: Chemical Industry Press: 86.
- Cooke, B.A. (1999) Signal transduction involving cyclic AMP-dependent and cyclic AMP-independent mechanisms in the control of steroidogenesis. Mol Cell Endocrinol 151: 25–35.
- El-Beshbishy, H.A., Aly, H.A. and El-Shafey, M. (2013) Lipoic acid mitigates bisphenol A-induced testicular mitochondrial toxicity in rats. Toxicol Ind Health 29: 875–887.
- El-Missiry, M.A., Othman, A.I., Al-Abdan, M.A. and El-Sayed, A.A. (2014) Melatonin ameliorates oxidative stress, modulates death receptor pathway proteins, and protects the rat cerebrum against bisphenol-A-induced apoptosis.J Neurol Sci 347: 251–256.
- Fan, H.T. and Lin, H.S. (2013) Advances on Cordyceps militaris constituents and pharmacological effect. China J Chinese Mater Med 38: 2549–2551.
- Feng, Y.X., Yin, J., Jiao, Z.H., Shi, J.C., Li, M. and Shao, B. (2012) Bisphenol AF may cause testosterone reduction by directly affecting testis function in adult male rats. Toxicol Lett 211: 201–209.
- Gualtieri, A.F., Iwachow, M.A., Venara, M., Rey, R.A. and Schteingart, H.F. (2011) Bisphenol A effect on glutathione synthesis and recycling in testicular Sertoli cells. J Endocrinol Invest 34: e102–109.
- Gutierrez, P.L. (2000) The metabolism of quinone-containing alkylating agents: free radical production and measurement. Front Biosci 5: D629–638.
- Hormann, A.M., vom Saal, F.S., Nagel, S.C., Stahlhut, R.W., Moyer, C.L., Ellersieck, M.R., et al. (2014) Holding Thermal Receipt Paper and Eating Food after Using Hand Sanitizer Results in High Serum Bioactive and Urine Total Levels of Bisphenol A (BPA). PLoS One 9: e110509.
- Hsu, C.C., Huang, Y.L., Tsai, S.J., Sheu, C.C. and Huang, B.M. (2003a) In vivo and in vitro stimulatory effects of Cordyceps sinensis on testosterone production in mouse Leydig cells. Life Sci 73: 2127–2136.
- Hsu, C.C., Tsai, S.J., Huang, Y.L. and Huang, B.M. (2003b) Regulatory mechanism of Cordyceps sinensis mycelium on mouse Leydig cell steroidogenesis. Febs Lett 543: 140–143.
- Huang, B.M., Hsu, C.C., Tsai, S.J., Sheu, C.C. and Leu, S.F. (2001) Effects of Cordyceps sinensis on testosterone production in normal mouse Leydig cells. Life Sci 69: 2593–2602.
- Huang, Y.L., Leu, S.F., Liu, B.C., Sheu, C.C. and Huang, B.M. (2004) In vivo stimulatory effect of Cordyceps sinensis mycelium and its fractions on reproductive functions in male mouse. Life Sci 75: 1051–1062.
- Huang, Y.Q., Wong, C.K., Zheng, J.S., Bouwman, H., Barra, R., Wahlstrom, B., et al. (2012) Bisphenol A (BPA) in China: a review of sources, environmental levels, and potential human health impacts. Environ Int 42: 91–99.
- Jin, L. and Chen, S.Z. (2008) The effect of Chinese caterpillar fungus ontestis oxidative damage induced by cyclophosphamide in the mice. Mater Child Health Care China 23: 1858–1860.
- Kabuto, H., Amakawa, M. and Shishibori, T. (2004) Exposure to bisphenol A during embryonic/fetal life and infancy increases oxidative injury and causes underdevelopment of the brain and testis in mice. Life Sci 74: 2931–2940.
- Kloukos, D., Pandis, N. and Eliades, T. (2013) In vivo bisphenol-A release from dental pit and fissure sealants: A systematic review. J Dent 41: 659–667.
- Korkmaz, A., Ahbab, M.A., Kolankaya, D. and Barlas, N. (2010) Influence of vitamin C on bisphenol A, nonylphenol and octylphenol induced oxidative damages in liver of male rats. Food Chem Toxicol 48: 2865–2871.
- Kovacic, P. (2010) How safe is bisphenol A? Fundamentals of toxicity: metabolism, electron transfer and oxidative stress. Med Hypotheses 75: 1–4.
- Leu, S.F., Poon, S.L., Pao, H.Y. and Huang, B.M. (2011) The in vivo and in vitro stimulatory effects of cordycepin on mouse leydig cell steroidogenesis. Biosci Biotech Bioch 75: 723–731.
- Li, Y.J., Song, T.B., Cai, Y.Y., Zhou, J.S., Song, X., Zhao, X., et al. (2009) Bisphenol A exposure induces apoptosis and upregulation of Fas/FasL and caspase-3 expression in the testes of mice. Toxicol Sci 108: 427–436.
- Liu, J., Yang, X., Chen, Z., Liang, M.Y. and Li, J.L. (1994) The calmative and sex hormone-1ike effect of Cordyceps militaris (L) Link (Cantherea Pernyi). J Norman Bethune Univ Med Sci 20: 14–16.
- Lucki, N.C. and Sewer, M.B. (2010) The interplay between bioactive sphingolipids and steroid hormones. Steroids 75: 390–399.
- Manfo, F.P., Jubendradass, R., Nantia, E.A., Moundipa, P.F. and Mathur, P.P. (2014) Adverse effects of bisphenol A on male reproductive function. Rev Environ Contam T 228: 57–82.
- Meeker, J.D., Ehrlich, S., Toth, T.L., Wright, D.L., Calafat, A.M., Trisini, A.T., et al. (2010) Semen quality and sperm DNA damage in relation to urinary bisphenol A among men from an infertility clinic. Reprod Toxicol 30: 532–539.
- Michałowicz, J. (2014) Bisphenol A–Sources, toxicity and biotransformation. Environ Toxicol Phar 37: 738–758.
- Ng, T.B. and Wang, H.X. (2005) Pharmacological actions of Cordyceps, a prized folk medicine. J Pharm Pharmacol 57: 1509–1519.
- Ni, H., Li, H.H., Huang, W.F. and Li, L. (2007) Research and Product Development of Cordyceps militaris and Its Bioactive Substances. Sci Technol Rev 25: 75–79.
- Pao, H.Y., Pan, B.S., Leu, S.F. and Huang, B.M. (2012) Cordycepin Stimulated Steroidogenesis in MA-10 Mouse Leydig Tumor Cells through the Protein Kinase C Pathway. J Agr Food Chem 60: 4905–4913.
- Peretz, J. and Flaws, J.A. (2013) Bisphenol A down-regulates rate-limiting Cyp11a1 to acutely inhibit steroidogenesis in cultured mouse antral follicles. Toxicol Appl Pharm 271: 249–256.
- Reis, F.S., Barros, L., Calhelha, R.C., Ciric, A., van Griensven, L.J., Sokovic, M., et al. (2013) The methanolic extract of Cordyceps militaris (L.) Link fruiting body shows antioxidant, antibacterial, antifungal and antihuman tumor cell lines properties. Food Chem Toxicol 62: 91–98.
- Revathy, R., Langeswaran, K., Vijayaprakash, S., Tamilselvan, P. and Balasubramanian, M. (2013) Anti-infertility significance of aqueous extract of I pomoea batatas (L.) Lam. against exposure of bisphenol A (BPA) promoted testicular toxicity in male Sprague Dawley rats. Asian Pac J Reprod 2: 263–271.
- Shi, J. (2012) Study on cornel polysaccharide extraction and scavenging free radicals. Jiangsu Agr Sci 40: 289–291.
- Spanier, A.J., Fiorino, E.K. and Trasande, L. (2014) Bisphenol A Exposure Is Associated with Decreased Lung Function. J Pediatr 164: 1403–1408.
- Suo, F.Y., Su, J., Jiang, J.C. and Cheng, S.Z. (2008) Comparation for determination of adenosine and cordyceps between Xinjiang Cordyceps militaris and cordyceps sinensis by HPLC. J Med Pharm Chinese Minorities 14: 65–67.
- Welshons, W.V., Nagel, S.C. and vom Saal, F.S. (2006) Large effects from small exposures. III. Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure. Endocrinology 147: s56–s69.
- WHO (2010a) The 5th edition of WHO Laboratory Manual for the examination and processing of human semen. Beijing: People’s Medical Publishing House: pp.16–20.
- WHO (2010b) Joint FAO/WHO expert meeting to review toxicological and health aspects of bisphenol A. Geneva: World Health Organization.
- Wisniewski, P., Romano, R.M., Kizys, M.M., Oliveira, K.C., Kasamatsu, T., Giannocco, G., et al. (2015) Adult exposure to bisphenol A (BPA) in Wistar rats reduces sperm quality with disruption of the hypothalamic–pituitary–testicular axis. Toxicology 329: 1–9.
- Wolstenholme, J.T., Rissman, E.F. and Connelly, J.J. (2011) The role of Bisphenol A in shaping the brain, epigenome and behavior. Horm Behav 59: 296–305.
- Ye, L.P., Zhao, B.H., Hu, G.X., Chu, Y.H. and Ge, R.S. (2011) Inhibition of human and rat testicular steroidogenic enzyme activities by bisphenol A. Toxicol Lett 207: 137–142.
- Zhu, J.S., Halpern, G.M. and Jones, K.J. (1998) The scientific rediscovery of an ancient Chinese herbal medicine: Cordyceps sinensis Part I. J Altern Complem Med 4: 289–303.