ABSTRACT
This study aimed to evaluate sperm chromatin maturity and integrity of that injected into good-quality oocytes in an in vitro fertilization-intra cytoplasmic sperm injection (IVF-ICSI) program. A cut-off value of sperm chromatin maturity and integrity was developed as a function of their correlation to the zygote development, i.e., embryo formation and cleavage rate. The study assessed sperm chromatin maturity using aniline blue (AB) staining, whereas toluidine blue (TB) staining was used to assess sperm chromatin integrity. Ejaculates from 59 patients undergoing ICSI and 46 fertile normozoospermic donors for determination of normal values of sperm chromatin status were used in this study. Embryo formation and cleavage rates were observed for the period of 3 days after ICSI. There was a significant difference in the percentage of sperm with mature chromatin between ejaculate from ICSI patients and fertile donor (p=0.020); while there was no significant difference in sperm chromatin integrity of both samples (p=0.120). There was no significant correlation between sperm chromatin maturity and either embryo formation or cleavage rate; as well as sperm chromatin integrity to both parameters of zygote development (p>0.05). Furthermore, we found that the cut-off value of sperm chromatin maturity and integrity of the fertile normozoospermic ejaculates were 87.2% and 80.2%, respectively. Using the cut-offs, we found that low sperm chromatin maturity at the level of <87% correlated significantly with the cleavage rate of the zygote (p=0.022; r=0.371); whereas poor sperm chromatin integrity at the level of <80% correlated with embryo formation (p=0.048; r=0,485). In conclusion, this study showed that poor maturity and integrity of sperm chromatin (AB<87% and TB<80%, respectively), could affect zygote development following ICSI.
Abbreviations: AB: aniline blue; CMA3: chromomycin A3; ICSI: intra cytoplasmic sperm injection; IVF: in vitro fertilization; PBS: phosphate buffer saline; SPSS: Statistical Package for Social Science; TB: toluidine blue; WHO: World Health Organization
Introduction
A survey data in 2010 estimated that about 48.5 million couples worldwide were infertile [Mascarenhas et al. Citation2012]. As an assisted reproductive program method, intra cytoplasmic sperm injection (ICSI) was successfully applied in 62.5% of all aspiration cycles to the infertile couples in order to gain pregnancies. However, the delivery rate of couples that participate in the assisted reproductive program is still low with the average number of 34.1% in in vitro fertilization (IVF) clinics worldwide [Committee for Assisted Reproductive Technology et al. Citation2013; Ishihara et al. Citation2015]. A successful assisted reproduction program depends on the quality of oocyte and sperm [Committee for Assisted Reproductive Technology et al. Citation2013]. Spermatogenesis must be properly regulated to gain good quality sperm, including the histone replacement process by protamine during spermiogenesis. Mature human spermatozoa contain approximately 85% protamine and 15% histones [Carrell et al. Citation2007; de Lamirande et al. Citation2012; Hammadeh et al. Citation1996]. The proportion of protamines is larger than the histones, playing a critical role in sperm condensation. When packaged in this manner the paternal genome appears to be somewhat protected from nucleases, mutagens, and other factors that may damage the sperm DNA. The sperm nucleus also becomes more hydrodynamic, thus spermatozoa can move faster towards the ovum for fertilization [de Lamirande et al. Citation2012; Oliva Citation2006; Balhorn Citation2011]. Generally, the quality of the sperm chromatin plays an important role in the success of ovum fertilization [de Lamirande et al. Citation2012; Hofmann and Hilscher Citation1991].
Different simple methods based on different staining or fluorochromes have been proposed in recent years to assess sperm chromatin quality, using many staining methods for DNA integrity such as aniline blue (AB), toluidine blue (TB), and chromomycin A3 (CMA3) for protamination and chromatin integrity assay as well as fragmentation assays [de Lamirande et al. Citation2012; Hekmatdoost et al. Citation2009; Hofmann and Hilscher Citation1991; Kazerooni et al. Citation2009; Kim et al. Citation2013; Pizzol et al. Citation2014; Sellami et al. Citation2013; Tsarev et al. Citation2009]. The usefulness of these methods in the evaluation of male infertility and as prognostic markers for successful assisted reproductive programs is still debatable, partly because standardization has not been reached for some of these methods [Pizzol et al. Citation2014]. In this study we evaluated sperm chromatin quality using AB and TB staining of normozoospermic fertile male subjects to standardize the assay and to determine the cut-off value of sperm chromatin quality. As indicated by positive staining results in both methods, they could be used to evaluate the association between sperm chromatin maturity as well as integrity and zygote development in an IVF-ICSI setting.
Results and discussion
The ages of the normozoospermic donors were 36.6±5.4 years with 1 to 3 children. Sperm analysis for 46 normozoospermic donors showed mean sperm concentration of 35.9±15.3 million/ml and percentage of motile sperm of 53.0±27.3 in the ejaculate. While the average age of 59 male patients was 34.3±5.5 years and female patients was 31.5±3.3 years, with a sperm concentration of 52.9.7±34.8 million/ml and the percentage of motile sperm 52.7±11.5 in ejaculate.
Sperm concentration and motility parameters in donor sperm as well as in patients showed a normal value, i.e., ≥ 15 million/ml for sperm concentration and ≥ 32% sperm motile in ejaculate according to WHO [Citation2010] laboratory manual for the examination of human semen and sperm-cervical mucus interaction. Most of the female patients had no pregnancy history, a few had offspring or indicated a recurrent abortus history. In this study, 22 female partners had unexplainned infertility, 14 female partners had endometriosis (23.7%), 11 female partners had a tubal disturbance (18.6%), nine female partners had polycystic ovarian syndrome (PCOS) condition (15.2%), two female partners had hyperprolactinemia condition (3.4%), and one female partner had adenomyosis (1.7%). Two to twenty oocytes were retrieved from each women. Despite the gynecological conditions of the female partners of our ICSI patients, we selected only the oocytes of good quality based on the morphological evaluation, such as the appearance of clear of zona pellucida, a small perivitelline space, and single non-fragmented polar body [Ebner et al. Citation2003]. Practically only oocytes with good nuclear and cytoplasm maturation were included in this IVF-ICSI study. Of a total of 689 harvested oocytes, only 586 met the morphological criteria of a good-quality oocyte and thus were selected and fertilized for this study. Only one to three selected cultured embryos were transferred. Using such an inclusion criteria of the oocytes, we would have eliminated or – at least – minimized the effect of other determining factors, such as oocyte quality, which is strongly associated with the female partners’ gynecological condition, on the zygote development. Only then, the interpretation of the correlation between the sperm quality and zygote development as measured in this study could be reliably preserved. The mean percentage of sperm containing mature chromatin in ejaculates from 46 donors was 86.2±3.1% (AB-negative), whereas sperm containing good integrity chromatin was 80.2±6.0% (TB-negative), ().
Figure 1. Aniline blue (AB) and toluidine blue (TB) staining assay for sperm chromatin integrity analysis. (A) AB staining assay for sperm chromatin integrity analysis. A: Sperms with mature chromatin appear with unstained head (AB-negative). Mature chromatin rich in cysteine residues, cannot bind AB dye; B: sperms with immature chromatin that are rich in lysine and arginine residues bind AB and appear with blue stained head (AB-positive). (B) TB staining assay for sperm chromatin integrity analysis. A: Sperms with good chromatin integrity. The phosphate groups of DNA strands in sperms with good chromatin integrity bind just a few TB dye molecules so that the sperms’ heads appear light blue (TB-negative); B: sperms with poor chromatin integrity. TB dye bind tight to the phosphate groups of DNA strands with of sperm chromatin with poor integrity resulting in dark blue (TB-positive) sperms’ heads. Magnification 1000x.
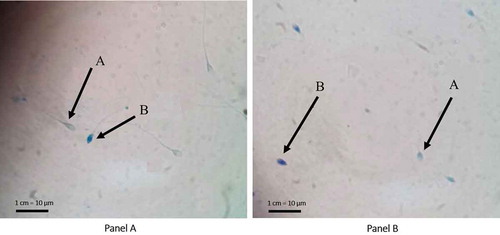
AB staining determined the percentage of sperm chromatin maturity. Sperm with mature chromatin revealed colorless (clear) head (AB-negative), while immature chromatin’s sperm expressed blue colored head (AB-positive) (). The difference was based on the staining results, in that sperm with immature chromatin contains more histones rich in lysine and arginine residues that would bind AB dye staining sperm heads blue. While mature chromatin’s sperm contains more protamine that is rich in cysteine residues not binding the AB dye; thus, the sperm head was colorless (clear). TB staining determined the integrity of sperm chromatin resulting in light blue in the head (TB-negative) for sperm with good integrity, and dark blue or purple (TB-positive) for the poor integrity sperms (). Different TB staining occurs because the TB dye binds to phosphate groups of DNA strands with poor integrity, and only a few good integrity. The mean percentage of sperm with mature chromatin (AB-negative) in our ICSI patients’ ejaculates was 82.1±7.6% and of those with good chromatin integrity (TB-negative) 83.1±8.3%. There was a significant difference in the percentage of sperm with mature chromatin between donor ejaculates and patient ejaculates, p=0.020, but it was not the case with sperm chromatin integrity, p>0.05 ().
Table 1. Comparison between sperm chromatin maturity and integrity between ICSI patients and fertile normozoospermic donors.
Our finding demonstrated that although sperm concentration and motility parameters in patient ejaculates were within the normal values, the percentage of sperm with mature chromatin in patient ejaculates was significantly lower than that in donor ejaculates, suggesting that sperm analysis alone could not ensure the quality of sperm. Our finding suggests that infertility in ICSI patients might be associated with a low level of sperm maturity in their ejaculates. Sellami et al. [Citation2013] reported the degree of sperm chromatin maturity was significantly correlated with the average number of sperm head abnormalities and the acrosome abnormalities in infertile men. Other studies demonstrated significant correlations between sperm chromatin immaturity and the decrease of sperm count and progressive motility [Kazerooni et al. Citation2009; Aoki et al. Citation2005].
Furthermore, we analyzed the correlation between sperm chromatin status in patients’ ejaculates, i.e., maturity and integrity, and the two parameters of zygote development, i.e., embryo development defined as the percentage of oocyte that can develop into a zygote within 72 hours compared to the total sperm-injected oocyte and the cleavage rate defined as the percentage of zygotes divided into more than 8 blastomeres within 72 hours [Prados et al. Citation2012]. The analysis of the overall samples showed that there was no significant correlation between sperm chromatin maturity and either embryo formation (p=0.415) or cleavage rate (p=0.282). We also found that sperm chromatin integrity had no correlation with either embryo formation (p=0.470) or cleavage rate (p=0.147). However, we found different results when we conducted a series of subset analyses using the cut-off values of sperm chromatin maturity and integrity to correlate both variables with the two parameters of zygote development.
The cut off values for a good-quality sperm chromatin were determined by a statistical analysis of the sperm chromatin condition of the fertile normozoospermic donors, resulting in 87.2% for chromatin maturity, and 80.2% for chromatin integrity. We further categorized the sperm chromatin condition of the fertile normozoospermic ejaculates as high and low for maturity and good and poor for integrity, based on the cut off values, and evaluated the correlation of each category of both the maturity and integrity of the sperm chromatin in the ejaculates with zygote development parameters, i.e., embryo formation and cleavage rate ().
Table 2. Correlation analysis between sperm chromatin maturity and integrity and zygote development.
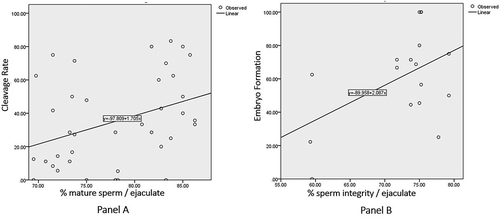
We did not observe low and high sperm chromatin maturity correlating significantly to embryo formation (p>0.05). However, we observed a significant correlation between the low maturity (below the cut-off value, i.e., 87%) and cleavage rate of zygote development, p = 0.022; r = 0.371 (), that was not observed with high maturity (above the cut-off). In contrast sperm chromatin integrity, was correlated with poor integrity (below the cut-off value. i.e., 80%), but not with good integrity (above the cut-off), and was significantly correlated with embryo formation, p = 0.048; r = 0.485 (). However, there was no significant correlation between the good as well as the poor integrity and cleavage rate, p > 0.05.
Figure 2. The correlation of sperm chromatin maturity in ejaculate of ICSI patients to cleavage rate of zygote development and the correlation of sperm chromatin integrity in ejaculate of ICSI patients to embryo formation of zygote development. (A) The correlation of sperm chromatin maturity in ejaculate of ICSI patients to cleavage rate of zygote development. There was a significant correlation between low sperm chromatin maturity, aniline blue (AB) <87% in ejaculate from ICSI patient to cleavage rate (p= 0.022; r = 0.371). (B) The correlation of sperm chromatin integrity in ejaculate of ICSI patients to embryo formation of zygote development. There was a significant correlation between poor sperm chromatin integrity, AB <80% (A) in ejaculate from ICSI patient to embryo formation (p= 0.048; r = 0.485).
Our results showed that low chromatin maturity and poor integrity could hamper successful development of ICSI derived zygotes. The level of zygote cleavage was the percentage of the products of conception (the oocyte and sperm cells) in a medium undergoing cell division into eight or more blastomeres within 72 hours after ICSI, compared with the total number of injected oocytes. The level of zygote cleavage had a significant correlation with the success rate of embryo implantation and pregnancy rates in the IVF-ICSI programs [Keel Citation2000; Lundin et al. Citation2001; Sakkas et al. Citation1998]. A study conducted by Wong et al. [Citation2008] who also used AB staining to determine the correlation between sperm chromatin maturity with pregnancy rates in the ICSI procedure showed that there was a correlation between sperm chromatin maturity and success rate of pregnancy in women aged 35 years or older.
In this study, a single spermatozoon with apparently normal morphology was randomly selected and injected into the oocyte. Thus, the probability to choose a spermatozoon with immature chromatin and poor integrity for ICSI was higher in sperm chromatin maturity <87% and integrity <80% than in the category of ≥87% for maturity as well as ≥80% for integrity, respectively. The study showed that the higher the percentages of sperm chromatin maturity in the ejaculate, i.e., up to 87%, and of sperm chromatin integrity up to 80%, yielded better zygote development (, ). However, above the cut-off values, i.e., 87% for sperm maturity and 80% for sperm integrity in the ejaculates, both variables did not correlate, thus did not affect zygote development.
Our results also showed a significant relationship between low sperm chromatin maturity (<87%) and poor sperm chromatin integrity (<80%) in patient ejaculates (p=0.009; r=0.610). However, there was no significant relationship (p>0.05) between low maturity and good integrity. In comparison, we did not observe any relationship between high chromatin maturity and either category of chromatin integrity. This likely reflects those ejaculates containing a high percentage (above the cut-off value) of sperms with good chromatin maturity having reached the most optimal condition. Thus at the higher percentages one could not find any strong correlation of the variable with the sperm integrity. It was concluded that immature chromatin i.e., when condensation failed, could lead to their poor integrity ().
Table 3. Relationship between sperm chromatin maturity and integrity.
Immature sperm contains a low level of protamine, such that this relaxed haploid genome may be susceptible to enzymes and an unfavorable microenvironment that could affect infertility treatment [Francis et al. Citation2014]. Another study examined the association of ICSI success with the sperm chromatin status in azoospermic and normozoospermic infertile men using AB and TB [Sadeghi et al. Citation2011]. The percentage of AB- and TB-stained sperm was higher in the group in which sperm was derived from the epididymis rather than in the group with ejaculated sperm. The study did not reveal any correlation between maturity or integrity of sperm chromatin and fertilization rate or embryo quality [Sadeghi et al. Citation2011]. By using three sperm DNA damage assays, i.e., the alkaline comet assay, TUNEL, and flowcytometric chromatin evaluation (FCCE), as well as histone retention using the aniline blue staining method, Simon et al. [Citation2014] reported that histone retention was associated with sperm DNA damage. Further, sperm DNA damage as measured by Comet and TUNEL assays were associated significantly with fertilization rate, embryo quality, and implantation rate in the ART program. A high level of histone retention (>50% AB-stained sperm in ejaculate) was negatively associated with embryo quality on Day 2, whereas absence or low levels of histone retention were positively associated with Day 2 embryo development. Their study is in line with the above; however, the difference is in the cut off value to categorize the histone- or protamine content in an ejaculated patient’s sperm. We gained 87% cut off value based on AB-unstained sperm from fertile normal donors and used this as a standard value and standardization of AB staining method for sperm maturity evaluation in ICSI patients.
Replacement of histones by protamines in the spermatid during spermiogenesis condenses the chromatin such that integrity is better maintained as it becomes resistant to deleterious substances or an unfavorable microenvironment [Dadoune Citation1995; Hekmatdoost et al. Citation2009]. In addition, aspects of sperm chromatin integrity also play a role in forming the nucleus to be more hydrodynamic, thus sperm can move faster towards the ovum for fertilization [Golan et al. Citation1997; Oliva Citation2006].
Sperm chromatin maturity and integrity assays as well as a sperm DNA damage assay can be used to examine the fertility status in men using advanced sperm analysis. It has been known that at least 20% of infertility in men is idiopathic. In part this could be caused by low sperm chromatin maturity and poor sperm chromatin integrity. This study showed that ICSI patients who have normal values in sperm concentration and motility might have a low percentage of sperm chromatin maturity in their ejaculate; this condition correlated with zygote development after ICSI program.
Some studies question the usefulness of particular methods in evaluating male infertility and as prognostic markers for natural fertility and assisted reproductive program success. This partly reflects that standardization has not been reached for some of these methods [Erenpreiss et al. Citation2006; Pizzol et al. Citation2014]. To counteract these conditions and address this issue, we developed AB and TB staining methods for the evaluation of sperm chromatin maturity and integrity status. This was standardized by applying these methods in ejaculated sperm from 46 normozoospermic fertile donors. Both methods offer different benefits, e.g., cost and simplicity to execute. Moreover, in general, studies all over the world have also utilized both methods [Boe-Hansen et al. Citation2006; Erenpreiss et al. Citation2001; Erenpreiss et al. Citation2006; Hammadeh et al. Citation1996]. With this strategy, we suggest the percentage of sperm with mature chromatin and good integrity in normal ejaculate that might be used as a reference for evaluation of sperm chromatin quality in patient ejaculates.
Materials and methods
Subjects
In this study, 46 ejaculated sperm samples from normozoospermic fertile donors and 59 sperm samples obtained from patients undergoing the IVF-ICSI program in Yasmin Fertility Clinic, Cipto Mangunkusumo Hospital (Jakarta, Indonesia) were used. The evaluation of sperm chromatin maturity (AB assay) and integrity (TB assay) in ejaculates obtained from normozoospermic fertile men was done to determine the average value of AB-stained sperm and TB-stained sperm in the ejaculates. The study protocol was approved and the ethical clearance was obtained from the Ethics Committee of Faculty of Medicine, University of Indonesia. Informed consent was given by each participant.
The semen samples of both groups were collected by masturbation after 3-4 d of abstinence. After complete liquefaction, semen analysis of each sample was performed according to World Health Organization (WHO) [Citation2010] guidelines.
AB staining
The staining process began when a drop of raw semen was spread on aglass slide. Air-dried smear was fixed in 3% glutaraldehyde solution in phosphate buffer saline (PBS) for 30 min. Then, the slide was dipped twice in PBS solution for 5 min and then air-dried. After being dried, the slide was stained with 5% aqueous AB mixed with acetic acid solution to control pH (3.5) for 7 min. The stained slide was washed with PBS solution, then air-dried, and covered by the cover glass. Examination of AB-stained slides as well as the calculation of negative- and positive-stained sperm was carried out by using a light microscope with 1,000 times magnification. Sperm heads with mature chromatin were not stained while sperm heads with immature chromatin were stained blue [Erenpreiss et al. Citation2001]. Observations were made by two observers through the determination of the color of the sperm head randomly from 200 sperm.
TB staining
The staining process began when a drop of raw semen was spread on a glass slide. Air-dried smear was fixed in 96% ethanol-acetone (1:1) at 4°C for 30 min. Then, the slide was hydrolyzed in 0.1 N HCl at 4°C for 5 min. Furthermore, slide was rinsed 3 times using distilled water each for 2 min and air-dried. The slide was stained with 0.05% TB solution, which consists of 50% citrate phosphate (McIlvain buffer at pH 3.5) for 10 min then rinsed with distilled water. It was dehydrated using t-butanol twice at 37°C, 3 min for each, and dipped in xylol twice for 3 min. The slide was covered with a cover glass. Examination of the slide as well as calculation of negative- and positive-stained sperm was carried out by using a light microscope with 1,000 times magnification. Sperm heads with good integrity of sperm chromatin were stained light blue or clear while sperm heads with poor integrity of sperm chromatin were stained purple or violet [Erenpreiss et al. Citation2001]. Observations were made by two observers through the determination of the color of the sperm head randomly from 200 sperm.
ICSI procedure
Fifty-nine sperm samples obtained from patients who underwent the IVF-ICSI program were analyzed according to WHO [Citation2010] guidelines. All sperm samples were processed for isolation of viable spermatozoa using the density gradient method. After centrifugation (1,500 rpm) over a discontinuous gradient of two layers of Supra Sperm (MediCult Origio, Denmark), sperm pellets were washed using Sperm Preparation Medium (MediCult Origio) by resuspension and centrifugation. Then spermatozoa were allowed to swim up.
Two hours before ICSI, oocytes were denuded and examined to assess their maturity. Only oocytes that had reached metaphase II (MII) and extruded the first polar body were injected (ICSI). Matured oocytes used in this study were derived from follicles with diameter 18 mm, from women aged <35 y. Before that, these women underwent a short recombinant follitropin alfa (Merck Serono, Germany) protocol and choriogonadotropin alfa 250 microgram (Merck Serono) for final oocyte maturation with the protocol as previously described [Hatzi et al. Citation2011].
Immediately before injection, sperm suspension was added to a micro droplet of 10% Polyvinylpyrrolidone /PVP (MediCult Origio) in an ICSI dish. Besides a micro droplet of sperm in an ICSI dish, there were also some micro droplets of GMOPS plus medium (Vitrolife, Sweden) to place oocytes that were going to be injected (ICSI). Then those micro droplets were covered with liquid paraffin (MediCult, Origio).
A single motile spermatozoon with apparently normal morphology was immobilized by touching its tail with the injection pipette and then aspirated into the injection pipette.
After securing the oocyte with the polar body at the 6 or 12 o’clock position onto the holding pipette, the micropipette was pushed through the zona pellucida and the oolemma into the ooplasm at the 3 o’clock position. When penetration of the oolemma was verified by aspirating some cytoplasm, the spermatozoon was slowly ejected. The injection pipette was withdrawn gently and the oocyte was released from the holding pipette. The ICSI procedure was repeated until all MII oocytes were injected. Those injected oocytes were then put into a culture dish containing micro droplets of culture medium ISM1 (MediCult, Origio) and stored at 37°C in a 5% O2, 6% CO2 incubator for 3 d. These embryos were checked and documented for their development once every day.
ICSI outcome measures
The embryo formation and the stage of embryo cleavage were assessed from the percentage of fertilized oocytes that underwent cell division into eight or more blastomeres on day 3 after ICSI compared with the total number of oocytes used in the IVF-ICSI program [Sherban Citation2015; Veeck Citation1988].
Statistical analysis
The data were analyzed using Statistical Package for Social Science (SPSS) version 22 for Windows. The association between two categories of sperm chromatin maturity and integrity with the level of cleavage zygotes and the embryo formation were analyzed using Pearson’s test. Significance level of statistical analysis was p<0.05.
Conclusion
This study showed that poor maturity and integrity of sperm chromatin, with the cut-off value of AB <87% and TB <80%, respectively, could affect zygote development after ICSI treatment. Therefore, we suggest that sperm chromatin maturity and integrity examination should be implemented in the standard sperm analysis for ICSI program in clinical practice.
Declaration of interest
This study was supported by International Collaborative Research Fund, in Directorate for Higher Education of Indonesian Ministry for Education and Culture of Republic of Indonesia. The authors report no declarations of interest.
Acknowledgments
The authors thank Amalia Shadrina, MD from the Faculty of Medicine University of Indonesia - Cipto Mangunkusumo Hospital for help in editing the manuscript, and Hans-Joachim Freisleben from the Faculty of Medicine University of Indonesia, and Liana W. Susanto, MBiomed, of Dexa Medica, Indonesia, for help revising the manuscript.
Additional information
Notes on contributors
Asmarinah
All authors contributed similarly, while each had a different main responsibility. Conceived and designed the study as well as drafted the manuscript: A; Performed the study and analyzed the data: AS, LAU; Recruited the subjects and carried out semen analysis: SWL; Recruited the subjects and performed ICSI program: EM, AH; Recruited the subjects for IVF program and helped in drafting the manuscript: AH. Participated in study design and developed the sperm chromatin assay: AP-D.
References
- Aoki, V.W., Liu, L. and Carrell, D.T. (2005) Identification and evaluation of a novel sperm protamine abnormality in a population of infertile males. Hum Reprod 10:1298–1306.
- Balhorn, R. (2011) Sperm Chromatin: An Overview. In Sperm Chromatin. Biological and Clinical Applications in Male Infertility and Assisted Reproduction, ed. Zini, A. and Agarwal, A., pp. 3-18. Springer Science+Business Media LLC.
- Boe-Hansen, G.B., Fedder, J., Ersboll, A.K. and Christensen, P. (2006) The sperm chromatin structure assay as a diagnostic tool in the human fertility clinic. Hum Reprod 21:1576–1582.
- Carrell, D.T., Emery, B.R. and Hammoud, S. (2007) Altered protamine expression and diminished spermatogenesis: what is the link? Hum Reprod Update 13:313–327.
- Committee for Assisted Reproductive Technology, Korean Society of Obstetrics and Gynecology, Choi, Y.M., Chun, S.S., Han, H.D., Hwang, J.H., et al. (2013) Current status of assisted reproductive technology in Korea, 2009. Obstet Gynecol Sci 56:353–361.
- Dadoune, J.P. (1995) The nuclear status of human sperm cells. Micron 26:323–345.
- de Lamirande, E., San Gabriel, M.C. and Zini, A. (2012) Human sperm chromatin undergoes physiological remodeling during in vitro capacitation and acrosome reaction. J Androl 33:1025–1035.
- Ebner. T., Moser, M., Sommergruber, M. and Tews, G. (2003) Selection based on morphological assesment of oocytes and embryos at different stages of preimplantation development: a review. Hum Reprod Update 9:251-262.
- Erenpreiss, J., Bars, J., Lipatnikova, V., Erenpreisa, J. and Zalkalns, J. (2001) Comparative study of cytochemical tests for sperm chromatin integrity. J Androl 22:45-53.
- Erenpreiss, J., Spano, M., Erenpreisa, J., Bungum, M. and Giwercman, A. (2006) Sperm chromatin structure and male fertility: biological and clinical aspects. Asian J Androl 8:11-29.
- Francis, S., Yelumalai, S., Jones, C., Coward, K. (2014) Aberrant protamine content in sperm and consequential implications for infertility treatment. Hum Fertil (Camb) 17:80-89.
- Golan, R., Shochat, L., Weissenberg, R., Soffer, Y., Marcus, Z., Oschry, Y., et al. (1997) Evaluation of chromatin condensation in human spermatozoa: a flow cytometric assay using acridine orange staining. Mol Hum Reprod 3:47-54.
- Hammadeh, M.E., al-Hasani, S., Stieber, M., Rosenbaum, P., Kupker, D., Diedrich, K., et al. (1996) The effect of chromatin condensation (aniline blue staining) and morphology (strict criteria) of human spermatozoa on fertilization, cleavage and pregnancy rates in an intracytoplasmic sperm injection programme. Hum Reprod 11:2468-2471.
- Hatzi, E., Bouba, I., Galidi, A., Lazaros, L., Xita, N., Sakaloglou, P., et al. (2011) Association of serum and follicular fluid SHBG levels and SHBG (TAAAA)n polymorphism with follicle size in women undergoing ovarian stimulation. Gynecol Endocrinol 27:27-32.
- Hekmatdoost, A., Lakpour, N. and Sadeghi, M.R. (2009) Sperm chromatin integrity: etiologies and mechanisms of abnormality, assays, clinical importance, preventing and repairing damage. Avicenna J Med Biotechnol 1:147-160.
- Hofmann, N. and Hilscher, B. (1991) Use of aniline blue to assess chromatin condensation in morphologically normal spermatozoa in normal and infertile men. Hum Reprod 6:979-982.
- Ishihara, O., Adamson, G.D., Dyer, S., de Mouzon, J., Nygren, K.G., Sullivan, E.A., et al. (2015) International committee for monitoring assisted reproductive technologies: world report on assisted reproductive technologies, 2007. Fertil Steril 103:402-413 e411.
- Kazerooni, T., Asadi, N., Jadid, L., Kazerooni, M., Ghanadi, A., Ghaffarpasand, F., et al. (2009) Evaluation of sperm’s chromatin quality with acridine orange test, chromomycin A3 and aniline blue staining in couples with unexplained recurrent abortion. J Assist Reprod Genet 26:591-596.
- Keel, B.A., May J.V. and De Jonge, C.J. (2000) Handbook of assisted reproduction laboratory. CRC Press, Boca Raton, FL, USA.
- Kim, H.S., Kang, M.J., Kim, S.A., Oh, S.K., Kim, H., Ku, S.Y., et al. (2013) The utility of sperm DNA damage assay using toluidine blue and aniline blue staining in routine semen analysis. Clin Exp Reprod Med 40:23-28.
- Lundin, K., Bergh, C. and Hardarson, T. (2001) Early embryo cleavage is a strong indicator of embryo quality in human IVF. Hum Reprod 16:2652-2657.
- Mascarenhas, M.N., Flaxman, S.R., Boerma, T., Vanderpoel, S. and Stevens, G.A. (2012) National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLoS Med 9:e1001356.
- Oliva, R. (2006) Protamines and male infertility. Hum Reprod Update 12:417-435.
- Pizzol, D., Ferlin, A., Garolla, A., Lenzi, A., Bertoldo, A. and Foresta, C. (2014) Genetic and molecular diagnostics of male infertility in the clinical practice. Front Biosci (Landmark Ed) 19:291-303.
- Prados, F.J., Debrock, S., Lemmen, J.G. and Agerholm, I. (2012) The cleavage stage embryo. Hum Reprod 27 Suppl 1:i50–71.
- Sadeghi, M.R., Lakpour, N., Heidari-Vala, H., Hodjat, M., Amirjannati, N., Hossaini Jadda, H., et al. (2011) Relationship between sperm chromatin status and ICSI outcome in men with obstructive azoospermia and unexplained infertile normozoospermia. Rom J Morphol Embryol 52:645-651.
- Sakkas, D., Shoukir, Y., Chardonnens, D., Bianchi, P.G. and Campana, A. (1998) Early cleavage of human embryos to the two-cell stage after intracytoplasmic sperm injection as an indicator of embryo viability. Hum Reprod 13:182-187.
- Sellami, A., Chakroun, N., Ben Zarrouk, S., Sellami, H., Kebaili, S., Rebai, T., et al. (2013) Assessment of chromatin maturity in human spermatozoa: useful aniline blue assay for routine diagnosis of male infertility. Adv Urol 2013:578631.
- Sherbahn, R. (2015) IVF embryo quality and day 3 embryo grading after in vitro fertilization, cleavage stage embryo grading. Advanced Fertility Center of Chicago Website: http://www.advancedfertility.com/embryoquality.htm 27 July 2015.
- Simon, L., Liu, L., Murphy, K., Ge, S., Hotaling, J., Aston, K.I., et al. (2014) Comparative analysis of three sperm DNA damage assays and sperm nuclear protein content in couples undergoing assisted reproduction treatment. Hum Reprod 29:904–917.
- Tsarev, I., Bungum, M., Giwercman, A., Erenpreisa, J., Ebessen, T., Ernst, E., et al. (2009) Evaluation of male fertility potential by Toluidine Blue test for sperm chromatin structure assessment. Hum Reprod 24:1569-1574.
- Veeck, L.L. (1988) Oocyte assessment and biological performance. Ann N Y Acad Sci 541:259-274.
- WHO (2010) WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction. 5th editition. World Health Organization.
- Wong, A., Chuan, S.S., Patton, W.C., Jacobson, J.D., Corselli, J. and Chan, P.J. (2008) Addition of eosin to the aniline blue assay to enhance detection of immature sperm histones. Fertil Steril 90:1999-2002.
