ABSTRACT
The effectiveness of semen preparation using the migration-sedimentation (MS) method was evaluated, and compared to density gradient centrifuge and swim-up combination (DGC+SU). Sperm selection using MS is based on motility, thus, deleterious effects for which centrifugation has been blamed, are believed to be avoided. Normozoospermic male patients who had more than 10% forward progressive motile sperm in their ejaculate were included in the study. Spermatozoa selected by two different methods were investigated and compared according to sperm motility, concentration, morphology, vitality, DNA fragmentation, and presence of persistent histones. The concentration and motility of sperm in the MS group was improved when compared to the DGC+SU group, but the difference between groups was not significant. The proportion of sperm with normal morphology was found to be 12.19 ± 6.45% vs. 10.67 ± 5.44%, vitality rate was 74.09 ± 16.65% vs. 70.45 ± 16.78%, DNA fragmentation rate was 3.91 ± 3.96%, vs. 2.95 ± 3.33%, presence of persistent histone proportion was 10.59 ± 13.40%, vs. 8.86 ± 7.89% in DGC+SU and MS groups respectively, without significance. The simple technique avoids centrifuge-based damage.
Introduction
Sperm preparation is a crucial process in assisted reproductive treatments (ART) to select viable sperm and remove seminal plasma. Briefly, these procedures are liquefaction, seminal evaluation, selection, and washing, and mostly carried out using centrifuge-based methods [De los Santos et al. Citation2016]. Density gradient centrifugation, (DGC) allows elimination of the leukocytes, immature or damaged spermatozoa, spermatogenic cells, infectious agents, epithelial cells, and seminal plasma, where most are expected to stay suspended on the upper layers of the solutions after centrifugation [Michou et al. Citation2012]. The pelleted cohort represents improved morphology [Yamanaka et al. Citation2016], motility [Noguchi et al. Citation2015], and nuclear content [Amiri et al. Citation2012; Matsuura et al. Citation2010; Muratori et al. Citation2016]. A subsequent sperm selection method, called the swim-up technique (SU) is usually used in combination with DGC for the selection of the most active and motile spermatozoa [Mortimer Citation1994; Volpes et al. Citation2016; WHO Citation2010].
It has been reported that centrifugation has harmful effects on the sperm functions especially on motility [Matas et al. Citation2007]. There is a potential of cell damage due to the formation of reactive oxygen species (ROS) caused by lipid peroxidation that can damage mitochondria during centrifugation [Amiri et al. Citation2012; Shekarriz et al. Citation1995]. Alterations in sperm functions and sperm DNA fragmentation were also reported [Matsuura et al. Citation2010], with poor IVF outcome [Simon et al. Citation2013].
Migration-sedimentation (MS) is a sperm selection method based on motility, and by the help of gravity, cells make a sediment in the bottom of a tube where centrifugation is not needed. Thus, deleterious effects for which centrifugation has been attributed, are believed to be avoided. Although MS is an old technique, its usage in in vitro fertilization (IVF) laboratories has only recently became available with the introduction of standardized commercial kits. Nevertheless, the efficacy of the method was not evaluated before, against the most common sperm selection combination, DSG+SU. The MS procedure offers a faster sperm preparation and eliminates the need of a centrifuge; as a result, sperm preparation can be performed more practically for selected patients. The purpose of this study was to compare the efficacy of the sperm selection between MS and DGC+SU, by using many essential tests assessing sperm quality and nuclear content.
Results and discussion
As far as we know our human study is unique as it represents the data on sperm parameters together with nuclear stability, when sperm are selected using the DGC+SU combination versus MS. There are a limited number of publications evaluating the efficacy of MS with or without comparison to other methods. Two papers indicated the superiority of MS by sharing clinical outcome measures, without any comparison to other sperm preparation methods. One represented clinical outcomes of 85% fertilization, 4% polyspermia, and 35.3% pregnancy rate in 17 patients [Lucena et al. Citation1989]. Accordingly, Hauser et al. [Citation1992] evaluated whether sperm performance following separation by migration-sedimentation technique predicts the fertilizing capacity in an IVF and found a significant correlation between the sperm parameters after MS separation and the fertilization rates. Among the studies, comparing MS where cells sediment by gravity to the bottom of a tube () and another sperm selection method, comparisons were made against either DGC or SU, but not to their combination. These papers indicated improved parameters of concentration, motility, and morphology [Ramos et al. Citation2015; Sanchez et al. Citation1996; Yavetz et al. Citation1996; Yener et al. Citation1990].
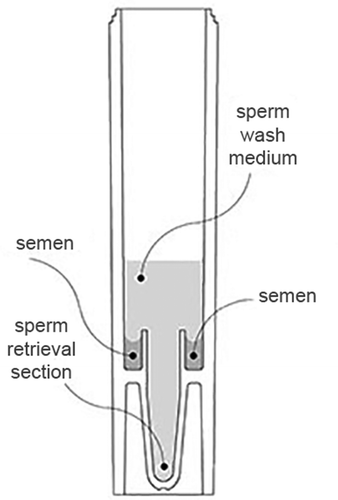
Figure 1. Sectional drawing of a migration-sedimentation (MS) tube and its chambers for loading. Sperm selection was made by the upper circular chamber of the MS tube with 400μl of liquefied semen (marked as “semen”). Then, with a sterile 5 mL syringe and its needle, the central axis of tube was passed and starting from the bottom, 2 mL of sperm washing medium was carefully added until it covered the loaded semen in the circular chamber (marked as “sperm wash medium”). After incubating for 30 minutes at 37ºC, 700 µl of wash medium with the migrated sperms were collected from the very bottom of the MS tube (marked as “sperm retrieval section”) carefully using a micropipette.
In our study, the average age, semen volume, sperm concentration, and sperm motility raw values of the included patients are presented in . As ejaculates from 22 subjects were allocated in each group equally, the sample characteristics were identical.
Table 1. The patients’ average values of age and raw seminal parameters.
Motility and concentration
In natural conception, sperm are actively selected during their passage through the female reproductive track and sperm with low motility and altered potential of fertilization are eliminated [Holt et al. 2016]. Both in natural conception and ART procedures, the quality of sperm cells has a direct impact on embryo development, pregnancy, and ongoing pregnancy rates [Muratori et al. Citation2016]. In the current study, DGC+SU group’s final concentration in processed sperm was 2.80±3.06 million/mL, group ‘a, b, c’ motility were 75.84±29.03%, 8.1±11.29%, and 16.05±27.18%, respectively, according to WHO [Citation2010] criteria. After the MS method, average final spermatozoon concentration was 3.25±2.87 million/mL, groups ‘a, b, c’ motility were 77.38±21.84%, 9.58±7.54%, and 13.05±22.27%, respectively. Both concentration and motility were enhanced in the MS group when compared to DGC+SU but there was no statistical difference (p>0.05) between the two groups (). The increased concentration of sperm in the MS group was unexpected as it is known that, methods using centrifugation yield a higher number of cells [Sanchez et al. Citation1996]. Application of SU after DGC provides an additional selection, based on motility, which may be negating the effect of centrifugation on high sperm yield. It has been shown that centrifugation has harmful effects on sperm function especially on motility [Shekarriz et al. Citation1995]. During centrifugation, mechanical and physical effects occur due to cell membrane damage, which may cause the accumulation of reactive oxygen species (ROS) that have been reported to impair normal sperm functions [Balasuriya et al. Citation2014; Lopes et al. Citation1998; Mortimer Citation1991].
Table 2. Comparative test results following two different methods.
Morphology
A recent publication revealed significantly enhanced morphology of sperm, selected by the combination of DSG+SU [Yamanaka et al. Citation2016]. Our study, using normozoospermic samples, presents comparable outcomes after MS, compared to the combination of DGC and SU (10.67±5.44% vs. 12.19±6.45%, respectively, p=0.220) (; ). Centrifugation of the ejaculate is the most common and effective method used to avoid cellular components in semen. However, the detrimental mechanical effect of centrifugation on sperm is one of the controversial issues in the field [Aitken et al. 1988; Alvarez et al. Citation1993]. It is reported that sperm with significantly better morphology can be selected in MS, when compared to both DGC and SU separately in fertile and subfertile populations [Gabriel and Vawda Citation1993]. Ramos et al. [Citation2015] also found comparable morphology scores when MS was compared to SU only.
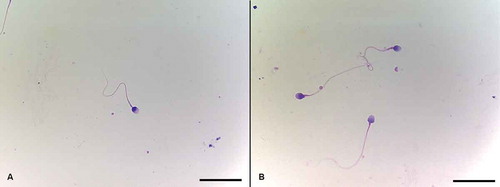
Figure 2. Sperm morphology assessment using Diff Quick Staining. Morphology assessment of both groups was performed under 1,000x magnification according to Kruger’s strict criteria. Normal spermatozoa were scored if the head was smooth, regularly contoured, and generally oval in shape with a well-defined acrosomal region comprising 40–70% of the head area, the midpiece was slender, regular, and about the same length as the sperm head, and the principal piece was uniform along its length, thinner than the midpiece, and approximately 45 µm long (about 10 times the head length). A) Spermatozoon with normal head, acrosome, midpiece, and tail morphology. B) Spermatozoa with slightly abnormal midpiece. Diff Quick staining. Scale bars indicate 25 µm.
Evaluation of vitality
Sperm vitality rate was 74.09±16.65% in the DGC+SU group while it was 70.45±16.78% in the MS group with no significant difference between the groups (p=0.333) (; ). Two publications, comparing sperm vitality after MS to other methods point out significant improvement [Engelmann et al. Citation1989; Sanchez et al. Citation1996] although they did not compare combined methods.
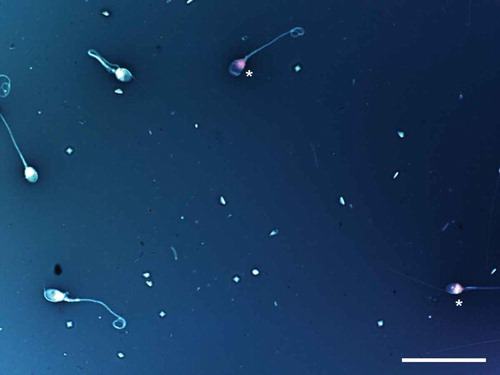
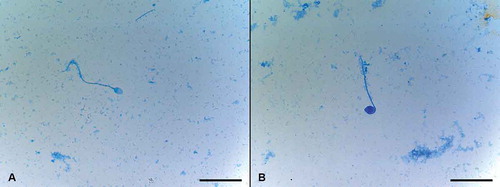
Figure 3. Sperm vitality assessment by one-step eosin-nigrosin staining technique. One-step eosin-nigrosin staining technique is used to detect the percentage of viability in a given sperm population. Eosin is used to mark dead cells which uptake the stain through damaged, porous membranes and appear red or pinkish (asterisk), where live spermatozoa that hinder eosin to penetrate head region because of their intact membrane, appear white. Nigrosin stains the background to increase the contrast between stained and unstained cells providing a dark contrast background. Eosin-nigrosin staining. Scale bar indicates 50 µm.
Sperm DNA fragmentation assessment
Formation of ROS may cause sperm DNA fragmentation and lipid peroxidation on sperm membrane [Williams and Ford Citation2005]. It is known that the DGC method improves the DNA fragmentation index [Wang et al. Citation2014; Xue et al. Citation2014], however Muratori et al. [Citation2016] stated that DGC itself leads to increased DNA fragmentation in sperm causing ROS production. The Muratori et al. [Citation2016] study showed that DGC use in sperm selection could cause sperm DNA fragmentation. More importantly, when DNA fragmentation occurs, couples have a 50% lower chance of achieving pregnancy and especially oocytes of women of late reproductive age may not engage their potential to repair sperm DNA damage [Jaroudi and SenGupta Citation2007; Menezo et al. Citation2010]. Aitken et al. [Citation2014] recently reported that transition metals in discontinuous colloidal silicon gradients may intensify the levels of oxidative DNA damage in human spermatozoa after DGC [Aitken et al. Citation2014]. For this reason additional supplementation of antioxidants is recommended during centrifugation [Banihani et al. Citation2012], although this approach is experimental and not recommended either by the WHO or the society guidelines. This topic is open to advanced investigation especially in patients with poor sperm quality. It is remarkable in our study that sperm selected by the MS method had a DNA fragmentation rate of 2.95±3.33%, which was not significantly different from those selected using DGC+SU (3.91±3.96%) (p=0.213) (; ). That implies that the MS technique is as effective as DGC+SU on selecting sperm with normal DNA among normozoospermic patients.
Figure 4. Sperm DNA fragmentation assessment by Sperm Chromatin Scattering Test. Sperm Chromatin Scattering Test is based on a controlled DNA denaturation process to facilitate the subsequent removal of the nuclear proteins contained in each spermatozoon. In this way, normal spermatozoa create halos formed by loops of DNA at the head of the sperm, which are not present in those with damaged DNA. A) Image shows spermatozoa in different scattering patterns with normal and fragmented DNA (asterisk). Scale bar indicates 10 µm. B) Spermatozoon with normal scattering pattern reveals unfragmented DNA. Scale bar indicates 50 µm. C and D) Spermatozoa with normal and fragmented DNA (asterisk). Scale bar indicates 50 µm.
Evaluation of persistent histones with aniline blue staining
Eliminating sperm with persistent histones is another goal of sperm preparation in ART. Persisted histones express an immaturity of DNA packaging which may have epigenetic consequences [Gannon et al. Citation2014; Siklenka et al. Citation2015; Urdinguio et al. Citation2015] and increased risk of DNA fragmentation [Hamad et al. Citation2014]. Colleu et al. [Citation1996] demonstrated that sperm with advanced nuclear maturation were successfully selected following both DGC and SU, but more effectively after DGC. In a comprehensive study, Sánchez et al. [1994] compared SU, DGC, glass wool column filtration and MS methods, evaluating nuclear maturation of sperm by aniline blue staining and detected that glass wool column filtration and MS methods obtain the lowest percentage of sperm with persistent histones. In our study we show, using MS, sperm with persistent histones () were effectively eliminated in a way comparable to the combination of DGC+SU. The rate of persistent histones in the prepared sperm was 10.59±13.40% in the DGC+SU group, while 8.86±7.89% in the MS group (n=22). There was no significant difference between groups for persistent histones (p=0.613) ().
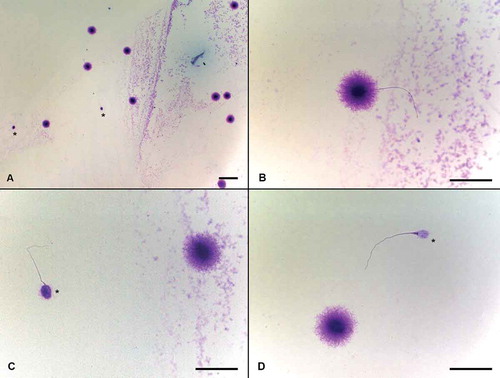
Figure 5. Evaluation of persistent histones with aniline blue staining. Aniline blue staining is used for exposure of sperm chromatin condensation situation and is based on the detection of lysine residues as a measure of persistent histones remaining bound to the sperm DNA which actually needed to be replaced by protamines during spermiogenesis. The spermatozoon that was stained deep with aniline blue was counted as positive for the presence of persistent histones and their percentage was calculated (positive stained sperm/total counted sperm x 100). A) Light stained spermatozoon with no persistent histones, was evaluated as normal. B) The spermatozoon that was stained deep with aniline blue was assessed as positive for the presence of persistent histones. Scale bars indicate 50 µm.
Beyond their potential physical damage via ROS production, centrifugation based techniques can be considered more expensive and time consuming as they need to be performed in a laboratory set up with the usage of a centrifuge device. Correspondingly, MS has an advantage that it can easily be conducted without a centrifuge particularly for the patients referred to intrauterine insemination (IUI). IUI is mostly planned for couples with men who have normal semen parameters [Van Voorhis et al. Citation2001], while centrifuge based methods would yield a higher number of total sperm in oligozoospermic men [Sanchez et al. Citation1996]. Nevertheless, according to our data, if the sperm motility were normal, the final concentration of collected sperm in MS would not be lower when compared to the DGC+SU method (3.25 x106/mL vs. 2.80 x106/mL, respectively, p=0.190).
The main limitation of this study is comparing two different sperm selection protocols without comparing them to each sample’s raw values. Samples included in this study were separated in each group equally, so each sample acted as its in-patient control. Using the MS method, sperm concentration, nuclear maturation, DNA fragmentation, and motility showed a better trend of improvement when compared to the DGS+SU protocol, but the changes were insignificant, therefore similar studies with higher numbers of patients may yield significant changes.
In conclusion, for patients with normal sperm parameters, the MS method is preferable in the clinical practice especially where a centrifuge is not available. Also, structural and functional damage in spermatozoa can electively be avoided due to the formation of ROS during centrifugation. Our results validate that MS has the potential to select spermatozoa of similar quality with the combination of conventional semen processing methods in normozoospermic men. Evaluation of a larger number of patients and comparison of different semen parameters will clearly identify restrictions and significant benefits about the technique.
Materials and methods
Patients with semen samples with more than 10% forward progressive motile spermatozoa who applied to Ankara University Centre for Assisted Reproduction between August 2015 and January 2016 were included in the study. Individual raw samples of the same patients were allocated into two experimental groups equally (n=22 for both groups) and due to this approach, seminal parameters were kept identical in both groups (). Ankara University Medical School local ethics committee approval was taken (decision number of 12-504-15 and date 30.07.2015), and consent forms were obtained from each object.
Semen samples were collected in sterile, capped containers by masturbation and liquefaction was allowed at 37°C for 30 min. Liquefaction was assessed using a Pasteur pipette by observing the length of seminal thread. The liquefied semen samples were evaluated with a Makler counting chamber (Sefi Medical Instruments, Haifa, Israel) for determination of concentration and motility.
Collected semen samples were divided into two groups:
Density gradient centrifuge followed by swim-up: DGC+SU, and
Migration-sedimentation: MS.
For the DGC+SU group, 0.5 mL of each gradient medium containing 80% and 40% silica particles (Puresperm 40/80, Nidacon, Gothenburg, Sweden) were prepared as columns into the 15 mL centrifuge tubes. Liquefied semen was carefully layered on top of the gradient column and centrifuged at 400g for 10 min. After centrifugation, the supernatant was discarded carefully, and 2 mL of sperm wash medium (SpermRinse, Vitrolife, Gothenburg, Sweden) was added onto the pellet. Pellet was re-suspended by gentle pipetting and centrifuged again at 400g for 10 min to wash spermatozoa. After the second centrifugation, supernatant was discarded and 1 mL of sperm wash medium was added onto the pellet slowly without disturbing the pellet and the preparation was incubated for 30 min at 37ºC to allow spermatozoa to swim up. At the end of this procedure, 700 µl of the suspension was transferred to a clean tube with the help of a micropipette and used for further examinations.
For the MS group, migration sedimentation tube (RI MSC, Research Instruments, Cornwall, United Kingdom) was prepared by filling the upper circular chamber of the MS tube with 400μl of liquefied semen (marked as “semen” in ). Then, with a sterile 5 mL syringe and its needle, central axis of the tube was passed and starting from the bottom, 2 mL of sperm washing medium was carefully added until it covered the level of loaded semen in the circular chamber (marked as “sperm wash medium” in ). After incubating for 30 min at 37ºC, 700 µl of wash medium with the migrated sperms was collected from the very bottom of the MS tube carefully using a micropipette (marked as “sperm retrieval section” in ). Suspension was collected in a clean tube for further examinations.
The following tests were performed in both groups and compared statistically:
Motility and concentration assessment by using Makler counting chamber,
Morphology assessment by Diff Quick rapid staining,
Eosin-nigrosine staining for vitality assay,
DNA fragmentation by the sperm chromatin scattering test,
Persistent histone assessment by aniline blue staining.
Evaluation of motility and concentration by Makler chamber
Ten microliters of each sample were used to fill a Makler counting chamber, creating a 10 µm thick layer under the cover slip. By using a phase-contrast light microscope (Axio Scope, Zeiss, Jena, Germany) under magnification of 200x, spermatozoa were counted within 10 successive squares in counting frame. This counting was repeated for three different rows and an average was taken to detect the concentration in millions of sperm per millilitre. Then motility determination was performed as described before [WHO Citation2010].
Evaluation of morphology
Morphology assessment of both groups was performed under 1,000x magnification according to Kruger’s strict criteria [Menkveld et al. Citation1990] after staining the smears of 20 µL suspension using Diff Quick staining (MGG staining kit, catalogue number: 500 101, Medico Chemical Ltd, Istanbul, Turkey). Slides were covered by a cover slide, at least 200 spermatozoa were evaluated for each and percentage of morphologically normal spermatozoa was calculated (number of normal spermatozoa/total number of counted spermatozoa x 100) ().
Determination of vitality
Sperm vitality test was performed by one-step eosin-nigrosin staining technique as defined before [Bjorndahl et al. Citation2003]. Air-dried samples were examined under 1,000x magnification and 200 spermatozoon were counted from each group. Live spermatozoa where their intact membrane hinders eosin to penetrate head region, appear white on dark nigrosine stained background. Non-viable sperm appear red or pinkish (). Percentages were calculated and noted in terms of live spermatozoa.
Determination of sperm DNA fragmentation
Sperm DNA fragmentation was detected using sperm chromatin scattering test (Halotech, Halosperm kit, Fina Biotech, Madrid, Spain) as instructed by the test kit. Preparations were examined under 1,000x magnification. From each group, 200 sperm were evaluated (). The percentage of spermatozoa that did not show sperm chromatin scattering (with DNA fragmentation) were calculated.
Evaluation of persistent histones by aniline blue staining
Aniline blue stain preparation and staining procedure were performed as described before [Kim et al. Citation2013]. Preparations were examined at 1,000x magnification. From each group, 200 spermatozoa were evaluated. The spermatozoon that was stained deep with aniline blue was counted as positive for the presence of persistent histones () and their percentage was calculated (positive stained sperm/total counted sperm x 100).
Statistical analysis
Statistical analysis was performed using Wilcoxon signed-rank test using SPSS version 11 (SPSS Inc. Chicago, IL, USA).
Declaration of interest
The authors report no declarations of interest.
Additional information
Notes on contributors
Sinan Ozkavukcu
Performed of the experiments and wrote the manuscript: SK; Contributed in the experimental design: MY; Performed the statistical analysis: KK; Designed the study, performed the experiments, and wrote the manuscript: SO.
References
- Aitken, R.J. and Clarkson, J.S. (1988) Significance of reactive oxygen species and antioxidants in defining the efficacy of sperm preparation techniques. J Androl 9:367–376.
- Aitken, R.J., Finnie, J.M., Muscio, L., Whiting, S., Connaughton, H.S., Kuczera, L., et al. (2014) Potential importance of transition metals in the induction of DNA damage by sperm preparation media. Hum Reprod 29:2136–2147.
- Alvarez, J.G., Lasso, J.L., Blasco, L., Nunez, R.C., Heyner, S., Caballero, P.P., et al. (1993) Centrifugation of human spermatozoa induces sublethal damage; separation of human spermatozoa from seminal plasma by a dextran swim-up procedure without centrifugation extends their motile lifetime. Hum Reprod 8:1087–1092.
- Amiri, I., Ghorbani, M. and Heshmati, S. (2012) Comparison of the DNA fragmentation and the sperm parameters after processing by the density gradient and the swim up methods. J Clin Diagn Res 6:1451–1453.
- Balasuriya, A., Serhal, P., Doshi, A. and Harper, J.C. (2014) Processes involved in assisted reproduction technologies significantly increase sperm DNA fragmentation and phosphatidylserine translocation. Andrologia 46:86–97.
- Banihani, S., Sharma, R., Bayachou, M., Sabanegh, E. and Agarwal, A. (2012) Human sperm DNA oxidation, motility and viability in the presence of l-carnitine during in vitro incubation and centrifugation. Andrologia 44 Suppl1: 505–512.
- Bjorndahl, L., Soderlund, I. and Kvist, U. (2003) Evaluation of the one-step eosin-nigrosin staining technique for human sperm vitality assessment. Hum Reprod 18:813–816.
- Colleu, D., Lescoat, D. and Gouranton, J. (1996) Nuclear maturity of human spermatozoa selected by swim-up or by percoll gradient centrifugation procedures. Fertil Steril 65:160–164.
- De los Santos, M.J., Apter, S., Coticchio, G., Debrock, S., Lundin, K., Plancha, C.E., et al. (2016) Revised guidelines for good practice in ivf laboratories (2015). Hum Reprod 31:685–686.
- Engelmann, U., Parsch, E.M. and Schill, W.B. (1989) Modern techniques of sperm preparation–do they influence the sex of offspring? Andrologia 21:523–528.
- Gabriel, L.K. and Vawda, A.I. (1993) Preparation of human sperm for assisted conception: A comparative study. Arch Androl 30:1–6.
- Gannon, J.R., Emery, B.R., Jenkins, T.G. and Carrell, D.T. (2014) The sperm epigenome: Implications for the embryo. Adv Exp Med Biol 791:53–66.
- Hamad, M.F., Shelko, N., Kartarius, S., Montenarh, M. and Hammadeh, M.E. (2014) Impact of cigarette smoking on histone (h2b) to protamine ratio in human spermatozoa and its relation to sperm parameters. Andrology 2:666–677.
- Hauser, R., Homonnai, Z.T., Paz, G.F., Yavetz, H., Amit, A., Lessing, J.B., et al. (1992) Migration sedimentation technique as a predictive test for the fertilizing capacity of spermatozoa in an in-vitro fertilization programme. Int J Androl 15:498–503.
- Holt, W.V. and Fazeli, A. (2016) Sperm selection in the female mammalian reproductive tract. Focus on the oviduct: Hypotheses, mechanisms, and new opportunities. Theriogenology 85:105–112.
- Jaroudi, S. and SenGupta, S. (2007) DNA repair in mammalian embryos. Mutat Res 635:53–77.
- Kim, H.S., Kang, M.J., Kim, S.A., Oh, S.K., Kim, H., Ku, S.Y., et al. (2013) The utility of sperm DNA damage assay using toluidine blue and aniline blue staining in routine semen analysis. Clin Exp Reprod Med 40:23–28.
- Lopes, S., Jurisicova, A., Sun, J.G. and Casper, R.F. (1998) Reactive oxygen species: Potential cause for DNA fragmentation in human spermatozoa. Hum Reprod 13:896–900.
- Lucena, E., Lucena, C., Gomez, M., Ortiz, J.A., Ruiz, J., Arango, A., et al. (1989) Recovery of motile sperm using the migration-sedimentation technique in an in-vitro fertilization-embryo transfer programme. Hum Reprod 4:163–165.
- Matas, C., Decuadro, G., Martinez-Miro, S. and Gadea, J. (2007) Evaluation of a cushioned method for centrifugation and processing for freezing boar semen. Theriogenology 67:1087–1091.
- Matsuura, R., Takeuchi, T. and Yoshida, A. (2010) Preparation and incubation conditions affect the DNA integrity of ejaculated human spermatozoa. Asian J Androl 12:753–759.
- Menezo, Y., Dale, B. and Cohen, M. (2010) DNA damage and repair in human oocytes and embryos: A review. Zygote 18:357–365.
- Menkveld, R., Stander, F.S., Kotze, T.J., Kruger, T.F. and van Zyl, J.A. (1990) The evaluation of morphological characteristics of human spermatozoa according to stricter criteria. Hum Reprod 5:586–592.
- Michou, V., Liarmakopoulou, S., Thomas, D., Tsimaratou, K., Makarounis, K., Constantoulakis, P., et al. (2012) Herpes virus infected spermatozoa following density gradient centrifugation for ivf purposes. Andrologia 44:174–180.
- Mortimer, D. (1991) Sperm preparation techniques and iatrogenic failures of in-vitro fertilization. Hum Reprod 6:173–176.
- Mortimer, D. (1994) Sperm recovery techniques to maximize fertilizing capacity. Reprod Fertil Dev 6:25–31.
- Muratori, M., Tarozzi, N., Cambi, M., Boni, L., Iorio, A.L., Passaro, C., et al. (2016) Variation of DNA fragmentation levels during density gradient sperm selection for assisted reproduction techniques: A possible new male predictive parameter of pregnancy? Medicine (Baltimore) 95:e3624.
- Noguchi, M., Yoshioka, K., Hikono, H., Iwagami, G., Suzuki, C. and Kikuchi, K. (2015) Centrifugation on percoll density gradient enhances motility, membrane integrity and in vitro fertilizing ability of frozen-thawed boar sperm. Zygote 23:68–75.
- Ramos, V.B., Cipriani Dda, C., Araujo, E.S., Salvador, R.A., Senn, A.P., Frajblat, M., et al. (2015) Sperm selection using three semen processing techniques. JBRA Assist Reprod 19:223–226.
- Sanchez, R., Stalf, T., Khanaga, O., Turley, H., Gips, H. and Schill, W.B. (1996) Sperm selection methods for intracytoplasmic sperm injection (ICSI) in andrological patients. J Assist Reprod Genet 13:228–233.
- Sanchez, R., Villagran, E., Risopatron, J. and Celis, R. (1994) Evaluation of nuclear maturity in human spermatozoa obtained by sperm-preparation methods. Andrologia 26:173–176.
- Shekarriz, M., DeWire, D.M., Thomas, A.J., Jr. and Agarwal, A. (1995) A method of human semen centrifugation to minimize the iatrogenic sperm injuries caused by reactive oxygen species. Eur Urol 28:31–35.
- Siklenka, K., Erkek, S., Godmann, M., Lambrot, R., McGraw, S., Lafleur, C., et al. (2015) Disruption of histone methylation in developing sperm impairs offspring health transgenerationally. Science 350: aab2006.
- Simon, L., Proutski, I., Stevenson, M., Jennings, D., McManus, J., Lutton, D., et al. (2013) Sperm DNA damage has a negative association with live-birth rates after IVF. Reprod Biomed Online 26:68–78.
- Urdinguio, R.G., Bayon, G.F., Dmitrijeva, M., Torano, E.G., Bravo, C., Fraga, M.F., et al. (2015) Aberrant DNA methylation patterns of spermatozoa in men with unexplained infertility. Hum Reprod 30:1014–1028.
- Van Voorhis, B.J., Barnett, M., Sparks, A.E., Syrop, C.H., Rosenthal, G. and Dawson, J. (2001) Effect of the total motile sperm count on the efficacy and cost-effectiveness of intrauterine insemination and in vitro fertilization. Fertil Steril 75:661–668.
- Volpes, A., Sammartano, F., Rizzari, S., Gullo, S., Marino, A. and Allegra, A. (2016) The pellet swim-up is the best technique for sperm preparation during in vitro fertilization procedures. J Assist Reprod Genet 33:765–770.
- Wang, M., Sun, J., Wang, L., Gao, X., Lu, X., Wu, Z., et al. (2014) Assessment of density gradient centrifugation (DGC) and sperm chromatin dispersion (SCD) measurements in couples with male factor infertility undergoing ICSI. J Assist Reprod Genet 31:1655–1663.
- WHO (2010) Who laboratory manual for the Examination and processing of human semen, 5th Edition. World Health Organization, Switzerland.
- Williams, A.C. and Ford, W.C. (2005) Relationship between reactive oxygen species production and lipid peroxidation in human sperm suspensions and their association with sperm function. Fertil Steril 83:929–936.
- Xue, X., Wang, W.S., Shi, J.Z., Zhang, S.L., Zhao, W.Q., Shi, W.H., et al. (2014) Efficacy of swim-up versus density gradient centrifugation in improving sperm deformity rate and DNA fragmentation index in semen samples from teratozoospermic patients. J Assist Reprod Genet 31:1161–1166.
- Yamanaka, M., Tomita, K., Hashimoto, S., Matsumoto, H., Satoh, M., Kato, H., et al. (2016) Combination of density gradient centrifugation and swim-up methods effectively decreases morphologically abnormal sperms. J Reprod Dev:599–606.
- Yavetz, H., Hauser, R., Homonnai, Z.T., Paz, G.F., Lessing, J. B., Amit, A., et al. (1996) Separation of sperm cells by sedimentation technique is not suitable for in vitro fertilization purposes. Andrologia 28:3–6.
- Yener, C., Mathur, S. and Parent, B. (1990) Comparison of two sperm preparation techniques using automated sperm motion analysis: Migration sedimentation versus swim-up. Arch Androl 25:17–20.
