ABSTRACT
The Fas/FasL signaling pathway is one of the major pathways that regulate apoptosis. Increasing studies have shown that the activation of the Fas/FasL signaling pathway is closely associated with testicular cell apoptosis. However, the mechanism involved is still unclear. We discuss recent findings regarding the molecular mechanisms by which environmental toxicants induce testicular pathology via Fas/FasL signaling. These findings suggest that Fas/FasL signaling is employed to impact the sensitivity (a response to external factors) of germ cells, disrupt steroidogenic hormone and cytokine metabolism mediated by Sertoli cells, and elicit the activation of NFAT (nuclear factor of activated T-cells) in Leydig cell apoptosis. Consequently, degeneration of testicular somatic (Sertoli and Leydig) and spermatogenic cells, leads to decreased numbers of mature sperm and subsequently translates into infertility issues. Collectively, these findings illustrate that it is beneficial to develop potential targets for a new generation of new pharmaceutical therapies that would alleviate testicular dysfunctions.
Abbreviations: BTB: blood-testis barrier; DD: death domains; DR3: death receptor 3; DR4: death receptor 4; DR5: death receptor 5; DED: death effector domain; DISC: death-inducing signaling complex; ERα: estrogen receptor alpha; FADD: Fas-associated death domain; FSH: follicle- stimulating hormone; IL-1β: interleukin 1 beta; LH: luteinizing hormone; LPS: lipopolysaccharide; mFas: membrane Fas; MMP2: matrix metalloproteinase-2; MTA1: metastasis-associated protein 1; NAC: N-acetylcysteine; NCCD: the Nomenclature Committee on Cell Death; NFAT: nuclear factor of activated T-cells; NF-kB: nuclear transcription factor-kappaB; NO: nitric oxide; NP: 4-nonylphenol; PCD: programmed cell death; PP1/PP2A: protein phosphatase 1 and 2A; ROS: reactive oxygen species; sFas: soluble Fas; T: testosterone; TGF-β: transforming growth factor-beta; THD: TNF homology domain; TIMP-2: tissue inhibitor of metalloproteinase-2; TNF: tumor necrosis factor; TNF-α: tumor necrosis factor-alpha; TNF-R1: Tumor necrosis factor receptor 1; TNFRSF1A: TNF receptor superfamily member 1A.
Introduction
Global rates of male infertility vary from 2.5% to 12% (Agarwal et al. Citation2016). Male factor contributes to 50% of infertility cases overall (Agarwal et al. Citation2015; Traven et al. Citation2017). Many reasons can be responsible for male infertility, one of which is the stimulation from environmental toxicants (Mathur and D’Cruz Citation2011). When it comes to the statistics of what percentage of male infertility/subfertility is caused by environmental toxicants, unfortunately, accurate data are not found. Nevertheless, there are many studies referring to environmental toxicant-induced male infertility (Wang et al. Citation2016, Citation2017). Environmental toxicants can directly damage testicular tissue, resulting in the disruption of sperm production (John Aitken Citation2013).
Due to the scarce clinical signs, infertility that is caused by testicular toxicity a huge challenge for today’s medical establishment remains. Several studies have focused on understanding the mechanisms involved in tissue damage in the male reproductive system, including the mitochondrial apoptotic pathway and endoplasmic reticulum apoptotic pathways, which have been recognized in recent years. These plausible mechanisms have a palpable effect on the prevention of testicular damage caused by environmental toxins; yet, there is still no progress on the treatment of testicular injury. It is therefore imperative to further explore Fas/FasL signaling as an alternative mechanism involved in testicular injury. Utilizing the latest contributions to the field, this paper has conducted a review on the impact of Fas/FasL signaling on testes (germ cells, Sertoli cells, and Leydig cells). Unraveling the complex molecular mechanisms involved in toxic injury to the testes is essential to provide new insight to develop clinically relevant drugs and improve human reproductive health.
It is well known that cell apoptosis, which is similar to ‘programmed cell death’ (PCD), plays a critical role in regulating the balance of cell death with cell survival (Elmore Citation2007). However, the Nomenclature Committee on Cell Death (NCCD) elaborates unified criteria for the definition of cell death, and specifically defines that PCD and ‘apoptosis’ are not synonyms because cell death, as it occurs during physiological development, can manifest non-apoptotic features (Barkla and Gibson Citation1999; Roach and Clarke Citation2000; Baehrecke Citation2002; Kroemer et al. Citation2009).
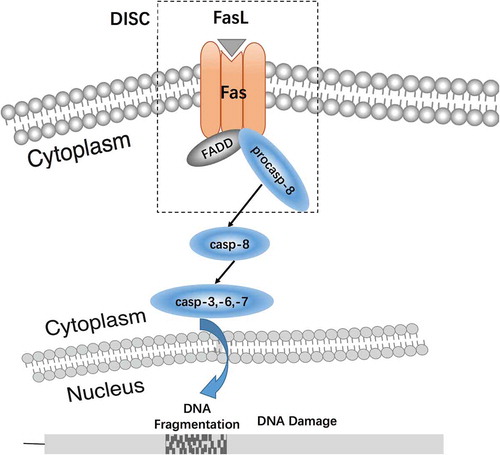
Apoptotic signaling pathways include the mitochondrial pathway, endoplasmic reticulum pathway, and death receptor pathway (Xu et al. Citation2016). Currently, there are five known death receptors (Ashkenazi and Dixit Citation1998): Tumor necrosis factor receptor 1 (TNF-R1), Fas, death receptor 3 (DR3), death receptor 4 (DR4), and death receptor 5 (DR5). ‘Death receptor pathway’ refers to a variety of external factors that promote apoptotic agents, i.e., apoptotic signals mediated through different death receptor signaling systems, eventually causing apoptosis (Ashkenazi and Salvesen Citation2014). Fas/FasL signaling is a key element of the death receptor signaling pathway (). Fas and its ligand FasL have a profound effect on the balance of proliferation and apoptosis (Lavrik Citation2014).The Fas/FasL signaling pathway is one of the major regulatory pathways of apoptosis. FasL induces the formation of the Fas trimer, in which the three death domains (DD) of Fas cluster together to bind to FasL. This Fas trimer recruits and binds to the N-terminal death effector domain (DED) of the cytoplasmic adapter protein, Fas associated death domain (FADD), and transmits the apoptotic signal to procaspase-8. The formation of the death-inducing signaling complex (DISC), which is constituted by the Fas-FasL-FADD-procaspase-8 on the cell membrane, leads to caspase hydrolysis and a series of enzyme-linked reactions. The DNA-degrading enzyme is ultimately activated, which results in degradation of DNA and induces cell apoptosis (Xu et al. Citation2016) ().
Figure 1. The function of Fas/FasL signaling pathway. The Fas/FasL signaling pathway is one of the major regulatory pathways of apoptosis. FasL induces the formation of the Fas trimer, in which the three death domains (DD) of Fas cluster together and bind to FasL. This Fas trimer recruits and binds to the N-terminal death effector domain (DED) of the cytoplasmic adapter protein, Fas associated death domain (FADD), and transmits the apoptotic signal to procaspase-8. The formation of the death-inducing signaling complex (DISC), which is constituted by the Fas-FasL-FADD-procaspase-8 on the cell membrane, leads to caspase hydrolysis and a series of enzyme-linked reactions. The DNA-degrading enzyme is ultimately activated, which results in degradation of DNA and induces cell apoptosis (Xu et al. Citation2016).
Fas/FasL molecular structure and distribution
The Fas transmembrane receptor (CD95/APO-1) is a member of the TNF receptor superfamily. The human Fas gene is located on chromosome 10q23.31 and consists of 15 exons. There are two forms of Fas protein, membrane Fas (mFas) and soluble Fas (sFas), and Fas primarily exists in the mFas form. The binding of mFas to the corresponding ligands can induce rapid apoptosis. sFas can also be generated from alternative splicing of the gene transcript. However, due to the lack of a transmembrane domain, sFas cannot interact with FasL to induce apoptosis, and it thus plays a regulatory role in apoptosis, such as inhibiting mFas-induced apoptosis (Jee et al. Citation2010).
FasL (CD95L) is a transmembrane protein and pro-apoptotic member of the tumor necrosis factor (TNF) superfamily. The human FasL gene is located on chromosome 1q24.3 and consists of 4 exons. The FasL extracellular region contains a ligand dimer and receptor binding region (TNF homology domain, THD), while the FasL intracellular region is involved in multiple signaling pathways, especially as a T cell receptor co-stimulatory molecule during T-cell activation (Calmon-Hamaty et al. Citation2015).
Fas is expressed in multiple organ tissues, especially peripheral T and B lymphocytes, NK cells, mononuclear cells, fibroblasts, endothelial cells, epithelial cells, etc. However, FasL expression is limited in activated T cells, NK cells, and phagocytic cells of the immune system and testicular Sertoli cells (Chai et al. Citation2008). That ‘FasL as a marker of Sertoli cells’ has been described (Suda et al. Citation1993; Ma et al. Citation2016). However, interestingly, we found that FasL is mainly expressed in sperm cells rather than Sertoli cells according to others (D’Alessio et al. Citation2001; Riccioli et al. Citation2003), which is the opposite of the argument of ‘FasL as a marker of Sertoli cells.’ Additionally, the Fas/FasL pathway is activated in the Leydig cell apoptosis induced by glucocorticoid (Gao et al. Citation2003). In short, the truth is always waiting for people to discover, and in time this will sort out.
Regulation of the Fas/FasL signaling pathway in the reproductive system
Apoptosis, at different developmental stages of the testes, has different functions. During the first wave of spermatogenesis, physiologically the early apoptotic wave is a sporadic event, most of which are directed toward spermatogonia (Allan et al. Citation1992). Each Sertoli cell can only support approximately 30–50 germ cells (Xu et al. Citation2016). Apoptosis is essential for maintaining an optimal proportion of germ cells to Sertoli cells, and eventually guaranteeing successful spermatogenesis and male fertility. In adult life, apoptosis is mainly focused on spermatocytes, and dependent on the balance of BclxL–Bax (Rodriguez et al. Citation1997). Apoptosis in the testis is mediated by closely linked pathways in Sertoli cells, germ cells, Leydig cells and various other signals. Such processes selectively delete germ cells that are damaged by a wide variety of physiological and environmental triggers. Finally, apoptosis efficiently removes senescent moribund spermatozoa by a phagocytotic process (Aitken and Baker Citation2013). Unfortunately, environmental toxicants, such as even low level heavy metal exposure, have had adverse effects on male reproductive function (Wirth and Mijal Citation2010). Recent studies have confirmed that the imbalance between cell survival and apoptosis caused by disease and/or environmental factors seriously affects spermatogenesis and ultimately leads to oligospermia, azoospermia, and hematospermia (Almeida et al. Citation2013).
Regulation of the Fas/FasL signaling pathway in germ cells
In mammalian spermatogenesis, sperm output depends on the coordination of proliferation, differentiation, and gradual maturation of the various types of germ cells (Xu et al. Citation2016). E2F2-induced germ cell apoptosis is particularly evident in the first wave of spermatogenesis (Rotgers et al. Citation2015), while the Fas/FasL system mainly regulates the first wave of spermatogenesis and mediates germ cell apoptosis induced by MEHP (Lin et al. Citation2010). Moreover, caspase-3, −8, and −9 are active in germ cell apoptosis during the first wave of rat spermatogenesis, and Fas/FasL signaling pathway may therefore play an important role in germ cell apoptosis during puberty in the rat (Moreno et al. Citation2006). In the reproductive system, Fas is the TNF/NGFR superfamily type I transmembrane protein receptor expressed on the surface of germ cells (Li et al. Citation2006). Apoptosis is initiated when the intracellular death domain of Fas reacts with FasL receptors on Sertoli cells (Yao et al. Citation2009).
Research indicates that increased expression of Fas/FasL induces apoptosis of germ cells on the seminiferous tubule stages VII–VIII and IX–XII when the testes suffer from environmental toxic injury (Zhao et al. Citation2011) as summarized in . In this process, upregulated genes include Fas, FasL, FADD, Apaf-1, caspase-3, caspase-8, and caspase-9 (Xiong et al. Citation2009; Chen et al. Citation2016). Mycrocistines-induced activation of Fas/FasL signaling molecules cause mitotic and meiotic defects by inhibiting protein phosphatase 1 and 2A (PP1/PP2A), disrupting the balance of cell phosphorylation and destroying the cytoskeleton, and subsequently, inducing the activation and differential expression of transcription factors and proteins involved in cell differentiation, proliferation, and tumorigenesis, resulting in abnormal cell proliferation, apoptosis, and necrosis (Chen et al. Citation2016).
Critically, stimulation by common byproducts of physiological processes regulate the Fas/FasL signaling pathway in germ cells. After stimulation with NO or reactive oxygen species (ROS) induced by environmental toxicants, FasL can induce c-FLIP degradation via the ubiquitin-proteasome signaling pathway (Wang et al. Citation2008). In addition, p53 could also promote the degradation of c-FLIP (Chandrasekaran and Richburg Citation2005). Knockout of the mouse FasL gene resulted in the following: (1) the basal germ cell apoptotic index in the testes of adolescent mice was significantly increased; (2) TRAIL (a TNF superfamily receptor) was constitutively expressed; and (3) the testicular seminiferous tubules consisted of only Sertoli cells; there were no germ cells. These results suggested that FasL was crucial for modulating the levels of the death receptor pathway inhibitor, c-FLIP, to regulate germ cell apoptosis (Almeida et al. Citation2013; Lin et al. Citation2010).
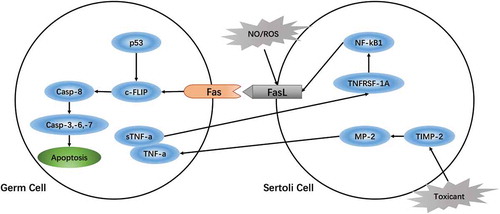
The sensitivity of germ cells is closely related to the Fas/FasL signaling pathway, which may dominate germ cell apoptosis. Embryonic exposure to environmental poisons increases the probability of abnormal spermatogenesis with subsequent exposure to the toxin during adolescence, which would promote germ cell apoptosis by activating Fas/FasL signaling (Traore et al. Citation2016). Toxic substances (such as MEHP, BLCO) activate matrix metalloproteinase-2 (MMP-2) by downregulating Sertoli cell tissue inhibitors of metalloproteinases-2 (TIMP-2) thereby degrading tumor necrosis factor-alpha (TNF-α) (Yao et al. Citation2009; Ebokaiwe et al. Citation2015). TNF-α interacts with the Sertoli cell TNF receptor superfamily member 1A (TNFRSF1A) to activate the nuclear transcription factor-kappa B (NF-kB) signaling pathway (Yao et al. Citation2007), thus stimulating expression of FasL and initiating germ cell apoptosis. Therefore, to some extent, the sensitivity of the germ cell may be regulated by the Sertoli cells via mediating Fas/FasL signaling in germ cell apoptosis (Yao et al. Citation2009) ().
Figure 2. Regulation of the Fas/FasL signaling pathway in germ cells damaged by environmental toxicants. Toxic substances (such as MEHP, BLCO) activate matrix metalloproteinase-2 (MMP-2) by downregulating tissue inhibitors of metalloproteinases-2 (TIMP-2) on Sertoli cells and degrade tumor necrosis factor-alpha (TNF-α) (Yao et al. Citation2009). TNF-α interacts with TNF receptor superfamily member 1A (TNFRSF1A) on Sertoli cells to activate the nuclear transcription factor-kappa B (NF-kB) signaling pathway (Yao et al. Citation2007), thus stimulating expression of FasL and initiating germ cell apoptosis. Therefore, to some extent, the sensitivity of the germ cell may be regulated by the Sertoli cells via mediating Fas/FasL signaling in germ cell apoptosis (Yao et al. Citation2009).
Regulation of the Fas/FasL signaling pathway in the Sertoli cells
Sertoli cells, as the only somatic cells of mammalian testicular seminiferous tubules, not only regulate hormones and growth factors but also play important roles in maintaining and controlling spermatogenesis. These include supporting structural stability, providing nutrients and synthesizing other essential elements for germ cells, and constructing the blood-testis barrier (BTB) (Cheng and Mruk Citation2012). Therefore, any damage to Sertoli cells will severely impact spermatogenesis (Xu et al. Citation2015).
Sertoli cell apoptosis mainly involves FasL-dependent, direct or indirect regulation of the intrinsic and extrinsic apoptotic pathways (Xu et al. Citation2015). On the one hand, FasL induces caspase-8 activation of the extrinsic apoptotic pathway, followed by activation of the caspase-3, −6, −7 cascade reaction, leading to cell apoptosis; on the other hand, activation of caspase-8 contributes to the Bax/Bcl-2 reaction of the intrinsic apoptosis pathway and initiates caspase-9, caspase-3 signal to induce apoptosis (Hengartner Citation2000).
Several studies have shown that Fas/FasL and p38MAPK signaling pathways are jointly involved in Sertoli cell apoptosis. Zhang et al. (Citation2010) confirmed for the first time that cadmium significantly inhibited proliferation of piglet primary Sertoli cells and caused DNA damage. Interestingly, the p38MAPK inhibitor SB202190 significantly alleviated the cadmium-induced toxicity of the Sertoli cells (Zhang et al. Citation2010). Similarly, the expression of Fas, FasL, and caspase-3 were significantly increased in rat Sertoli cells treated with bisphenol A and the phosphorylation level of JNK/p38MAPK was enhanced. NF-KB was transported into the nucleus, which indicated that the Fas/FasL and JNK/p38MAPK signaling pathway induced Sertoli cell apoptosis (Qi et al. Citation2014). However, the mechanism involved in how FasL interacts with the p38MAPK signal transduction pathway to control Sertoli cell apoptosis is undefined. Further investigation of the crosstalk between the two signaling pathways may prove useful in developing antagonists to rescue abnormal Sertoli cell apoptosis.
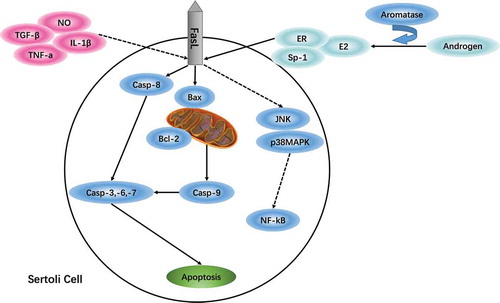
The role of Fas/FasL signaling in Sertoli cell apoptosis involves a variety of cytokines and hormone regulation. On one hand, proinflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin-1B (IL-1B), nitric oxide (NO), and transforming growth factor-beta (TGF-β) were elevated when lipopolysaccharide (LPS) were added to immature cultured porcine Sertoli cells. FasL expression levels were increased and cell viability decreased in a time- and dose-dependent manner (Wang et al. Citation2015). It is well known that aromatase converts androgen into estrogen, which promotes FasL expression through estrogen receptor alpha (ERα) and Sp-1 protein (Cooke et al. Citation2017). A study by Duan et al. (Citation2016b) suggested that NP could remarkably reduce serum follicle- stimulating hormone (FSH), luteinizing hormone (LH), and testosterone (T) in pubertal SD male rats (Duan et al. Citation2016b). Meanwhile, Fas/FasL signaling was reported to be involved in the NP-induced apoptosis (Duan et al. Citation2017), and FasL expression was conducive to maintaining immune privilege and achieving immune protection in Sertoli cells treated with estradiol (Catalano et al. Citation2007). Together, these observations have shown that FasL plays a regulatory role on the cytokines and hormones during the environmental toxicants-induced Sertoli cell apoptosis.
In addition, a recent study has shown that NP has an estrogen-like effect on Sertoli cells, which is mediated by reactive oxygen species (ROS) regulating AMPK/AKT-mTOR and JNK signaling (Fas-dependent signaling) (Huang et al. Citation2016). N-acetylcysteine or autophagy inhibitor 3-MA pretreatment can effectively antagonize autophagy, apoptosis, and necrosis (Duan et al. Citation2016a). Most likely, regulating autophagy can be an effective Fas/FasL-dependent mechanism to inhibit apoptosis and necrosis.
Surprisingly, we have noticed that not all environmental toxicants are mediated by Fas/FasL signaling to elicit Sertoli cell apoptosis. Fluoride influences testicular immune privilege by disturbing the Fas/FasL signaling pathway in Sertoli cells (Sun et al. Citation2017), and p,p’-DDE induces Sertoli cell DNA damage in rat testes and destroys spermatogenesis through the Fas/FasL signaling pathway, that results in oligozoospermia (Song and Yang Citation2006). However, certain common environmental toxicants do not lead to Sertoli cell apoptosis. For example, during gestation and lactation, nicotine exposure affects offspring Sertoli cell structure rather than changing the number of apoptotic cells or the Fas/FasL expression levels (Paccola and Miraglia Citation2016). Additionally, it was noteworthy that the RNAi-knockdown of metastasis-associated protein 1 (MTA1) in SCs was shown to inhibit MEHP-induced early activation of the NF-kB pathway and abolish the recruitment of NF-kB to the FasL promoter, which consequently diminished the MEHP-triggered FasL induction (Chen et al. Citation2013). MTA1 may be a way to impede certain environmental toxicant-induced Sertoli cell apoptosis via surpressing FasL expression ().
Figure 3. Regulation of the Fas/FasL signaling pathway in Sertoli cells damaged by environmental toxicants. Fas/FasL and p38MAPK signaling pathways are jointly involved in Sertoli cell apoptosis; the crosstalk between the two signaling pathways may prove useful in developing antagonists to rescue abnormal Sertoli cell apoptosis. Also, the role of Fas/FasL signaling in Sertoli cell apoptosis involves a variety of cytokines and hormone regulation; and not all environmental toxicants are mediated by Fas/FasL signaling to elicit Sertoli cell apoptosis. Regulating autophagy may be an effective Fas/FasL-dependent mechanism to inhibit apoptosis and necrosis induced by environmental toxicants; and MTA1 may be a way to obstruct certain environmental toxicants-induced Sertoli cell apoptosis via suppressing FasL expression.
Regulation of the Fas/FasL signaling pathway in Leydig cells
Testicular Leydig cells are important for the synthesis of testosterone. Abnormal Leydig cells alter hormone levels and consequently cause widespread germ cell apoptosis (Diaz de Barboza et al. Citation2014). Early research indicates that Leydig cell apoptosis is mediated by Fas receptor and its ligand, but Bcl-2 family proteins do not seem to be involved in the process (Taylor et al. Citation1999). Several studies relate Ca2+-dependent signaling to corticosterone-induced Leydig cell apoptosis in rats (Gao et al. Citation2003). Ca2+-induced FasL expression is critical in cell apoptosis.
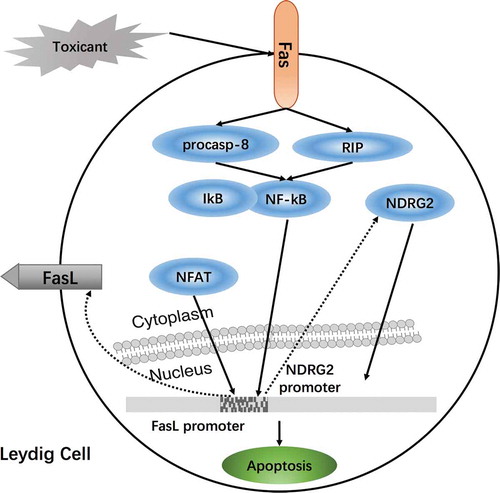
The transcriptional regulation of FasL is a complex process involving a variety of transcription factors, mainly dependent on the nuclear factor of activated T cells (NFAT) in T lymphocytes. Chai et al. (Citation2008) demonstrated that activated NFAT is transported from the cytoplasm to the nucleus, which then specifically binds to the NFAT target gene located in the −201 to +71 region of the FasL promoter sequence; NFAT directly promotes FasL transcription in stromal tumor cells, which results in Leydig cell apoptosis (Chai et al. Citation2008). NF-kB and Fas, both pro-apoptotic factors, are closely linked to Leydig cell apoptosis. NDRG2, which is located in the cytoplasm of Leydig cells, is involved in cell differentiation, development, and apoptosis. After Leydig cells are exposed to toxicants, activated Fas sequesters NF-kB from IkB via RIP and pro-caspase-8. NF-kB, migrates into the nucleus and binds to the NDRG2 promoter. NDRG2 is upregulated when it is stimulated by apoptosis and migrates back to the nucleus. In conjunction, NF-kB receives feedback from the signal to regulate downstream genes (Li et al. Citation2012). N-acetylcysteine (NAC) appears to effectively antagonize the activation of the Fas/FasL signaling pathway in Leydig cell apoptosis (Aggarwal et al. Citation2012) ().
Figure 4. Regulation of the Fas/FasL signaling pathway in Leydig cells damaged by environmental toxicants. Nuclear transcription factor-kappaB (NF-kB) and Fas, both pro-apoptotic factors, are closely linked to Leydig cell apoptosis. NDRG2, which is located in the cytoplasm of Leydig cells, is involved in cell differentiation, development, and apoptosis. After Leydig cells are exposed to toxicants, activated Fas sequesters NF-kB from IkB via RIP and pro-caspase-8. NF-kB, migrating into the nucleus, binds to the NDRG2 promoter. NDRG2 is upregulated when it is stimulated by apoptosis and migrates back to the nucleus; meanwhile, NF-kB receives feedback from the signal to regulate downstream genes (Li et al. Citation2012). Environmental toxicants induced testicular injury via Fas/FasL signaling to elicit nuclear factor of activated T-cells (NFAT) in Leydig cell apoptosis; it may be a new way to regulate Leydig cell apoptosis by mediated Fas/FasL signaling and NFAT.
Conclusions
Our literature review has summarized the evidence that the Fas/FasL signaling pathway plays an important role in regulating testicular cell apoptosis induced by environmental toxicants. We have given specific examples of the environmental toxins in . It is well known that apoptosis is a natural physiological process required for normal spermatogenesis and maintenance of the mature and aging testis. Similarly, it is important for apoptosis to control cell populations and timing. Fas/FasL signaling is one of the major pathways driving this process. Together this shows that apoptotic pathways, especially Fas/FasL signaling, is vital to normal spermatogenesis, which guarantees male fertility.
Table 1. Environmental toxicants- induced testicular cell apoptosis via Fas/FasL signaling.
As previously stated, many of studies revealed that avariety of environmental toxicants, including organic and inorganic pollutants, candamage the testes and result in decreased numbers of mature sperm, subsequently translating into infertility. It is imperative to understand the molecular mechanism.
The field is moving from an overview on Fas/FasL signaling in apoptosis, to structure and function, to the role of this pathway in germ cells, Sertoli cells, and finally Leydig cells. Testicular cell apoptosis is part of a multifaceted complex mechanism that is reflected in the sensitivity of germ cells thatmay be regulated by the Sertoli cells via mediating Fas/FasL signaling in germ cell apoptosis.
The Sertoli cell Fas/FasL signaling pathway can be damaged by environmental pollutants. Regulating autophagy may be an effective Fas/FasL-dependent mechanism to inhibit apoptosis and necrosis induced by environmental toxicants.
Not all environmental toxicants are mediated by Fas/FasL signaling to elicit Sertoli cell apoptosis.Fas/FasL signaling in Sertoli cell apoptosis uses a variety of cytokines and hormone regulation.
It is impacted by external factors (toxins) that negatively influence sperm production via Fas/FasL mediated apoptosis. Environmental toxicant induced testicular injury via Fas/FasL signaling to elicit NFAT in Leydig cell apoptosis may be a new way to regulate Leydig cell apoptosis
The Fas/FasL signaling pathway presents an intricate molecular mechanism of environmental toxicant-induced testicular cell apoptosis. This offers a new opportunity to develop potential targets for the next generation of new pharmaceutical therapies that would alleviate testicular dysfunctions and will certainly prove interesting as we intercede to minimize the effects of environmental toxicants with male reproduction.
Acknowledgments
The authors thank the Editor and Reviewers for their significant contributions during the revision period.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Notes on contributors
Mei Wang
Collected the data from publications, developed the database, and wrote the manuscript: MW; Designed the study and edited the final text: MW, PS.
Ping Su
Collected the data from publications, developed the database, and wrote the manuscript: MW; Designed the study and edited the final text: MW, PS.
References
- Agarwal A, Mulgund A, Hamada A, Chyatte MR. 2015. A unique view on male infertility around the globe. Reprod Biol Endocrinol. 13:37.
- Agarwal A, Roychoudhury S, Bjugstad KB, Cho CL. 2016. Oxidation-reduction potential of semen: what is its role in the treatment of male infertility. Ther Adv Urol. 8(5):302–318.
- Aggarwal A, Misro MM, Maheshwari A, Sehgal N. 2012. Differential modulation of apoptotic gene expression by N-acetyllcysteine in Leydig Cells Stimulated Persistently with HCG in Vivo. Mol Cell Endocrinol. 348:155–164.
- Aitken RJ, Baker MA. 2013. Causes and consequences of apoptosis in spermatozoa; contributions to infertility and impacts on development. Int J Dev Biol. 57(2–4):265–272.
- Allan DJ, Harmon BV, Robert SA. 1992. Spermatogonial apoptosis has three morphologically recognizable phases and shows no circadian rhythm during normal spermatogenesis in the rat. Cell Prolif. 25(3):241–250.
- Almeida C, Correia S, Rocha E, Alves A, Ferraz L, Silva J, Sousa M, Barros A. 2013. Caspase signaling Pathways in Human Spermatogenesis. J Assist Reprod Gen. 30(4):487–495.
- Ashkenazi A, Dixit VM. 1998. Death receptors: signaling and modulation. Science. 281:1305–1308.
- Ashkenazi A, Salvesen G. 2014. Regulated cell death: signaling and mechanisms. Annu Rev Cell Dev Biol. 30:337–356.
- Baehrecke EH. 2002. How death shapes life during development. Nat Rev Mol Cell Bio. 3(10):779–787.
- Barkla DH, Gibson PR. 1999. The fate of epithelial cells in the human large intestine. Pathology. 31:230–238.
- Calmon-Hamaty F, Audo R, Combe B, Morel J, Hahne M. 2015. Targeting the Fas/Fasl system in rheumatoid arthritis therapy: promising or risky? Cytokine. 75(2):228–233.
- Catalano S, Rizza P, Gu G, Barone I, Giordano C, Marsico S, Casaburi I, Middea E, Lanzino M, Pellegrino M, et al. 2007. Fas ligand expression in Tm4 Sertoli cells is enhanced by estradiol “in Situ” production. J Cell Physiol. 211:448–456.
- Chai WR, Chen Y, Wang Q, Gao HB. 2008. Mechanism of nuclear factor of activated T-cells mediated Fasl expression in corticosterone-treated mouse Leydig tumor cells. BMC Cell Biol. 9:31.
- Chandrasekaran Y, Richburg JH. 2005. The p53 protein influences the sensitivity of testicular germ cells to Mono-(2-Ethylhexyl) Phthalate-induced apoptosis by increasing the membrane levels of Fas and DR5 and decreasing the intracellular amount of c-Flip. Biol Reprod. 72:206–213.
- Chen L, Chen J, Zhang XZ, Xie P. 2016. A review of reproductive toxicity of microcystins. J Hazard Mater. 301:381–399.
- Chen S, Dong Y, Xu C, Jiang L, Chen Y, Jiang C, Hou W, Li W. 2013. Involvement of a chromatin modifier in response to Mono-(2-Ethylhexyl) Phthalate (MEHP)-induced Sertoli cell injury: probably an indirect action via the regulation of NF-kappa B/FasL circuitry. Biochem Biophys Res Commun. 440(4):749–755.
- Cheng CY, Mruk DD. 2012. The blood-testis barrier and its implications for male contraception. Pharmacol Rev. 64(1):16–64.
- Cooke PS, Nanjappa MK, Ko C, Prins GS, Hess RA. 2017. Estrogens in male physiology. Physiol Rev. 97(3):995–1043.
- D’Alessio A, Riccioli A, Lauretti P, Padula F, Muciaccia B, De Cesaris P, Filippini A, Nagata S, Ziparo E. 2001. Testicular FasL is expressed by sperm cells. P Natl Acad Sci USA. 98(6):3316–3321.
- Diaz de Barboza G, Rodriguez V, Ponce R, Theiler G, Maldonado C, Tolosa de Talamoni N. 2014. Association of cellular and molecular alterations in Leydig cells with apoptotic changes in germ cells from testes of graomys griseoflavusxgraomys centralis male hybrids. Acta Histochem. 116(6):1037–1045.
- Duan P, Hu C, Quan C, Yu T, Zhou W, Yuan M, Shi Y, Yang K. 2016a. 4-Nonylphenol induces apoptosis, autophagy and necrosis in Sertoli cells: involvement of ROS-mediated AMPK/Akt/m-TOR and JNK pathways. Toxicology. 341-343:28–40.
- Duan P, Hu CH, Butler HJ, Quan C, Chen W, Huang W, Tang S, Zhou W, Yuan M, Shi Y, et al. 2016b. Effects of 4-Nonylphenol on spermatogenesis and induction of testicular apoptosis through oxidative stress-related pathways. Reprod Toxicol. 62:27–38.
- Duan P, Hu CH, Butler HJ, Quan C, Chen W, Huang WT, Tang S, Zhou W, Yuan M, Shi Y, et al. 2017. 4-Nonylphenol induces disruption of spermatogenesis associated with oxidative stress-related apoptosis by targeting p53-Bcl-2/Bax-Fas/FasL signaling. Environ Toxicol. 32(3):739–753.
- Ebokaiwe AP, D’Cruz CS, Jubendradass R, Amala Rani JS, Mathur PP, Farombi EO. 2015. Nigerian bonny-light crude oil induces alteration in testicular stress response proteins and caspase-3 dependent apoptosis in albino wistar rats. Environ Toxicol. 30(2):242–252.
- Elmore S. 2007. Apoptosis: A review of programmed cell death. Toxicol Pathol. 35(4):495–516.
- Gao HB, Tong MH, Hu YQ, You HY, Guo QS, Ge RS, Hardy MP. 2003. Mechanisms of glucocorticoid-induced Leydig cell apoptosis. Mol Cell Endocrinol. 199(1–2):153–163.
- Geng J, Fan J, Jiang HW, Fang ZJ, Wang X, Sun JL, Ding Q, Chen G. 2009. Elevated Serum Soluble Fas Ligand Is a Promising Marker of Testicular Toxicity Induced by Epirubicin in Rats. Toxicol Lett. 186(2):96–103.
- Hengartner MO. 2000. The biochemistry of apoptosis. Nature. 407(6805):770–776.
- Huang WT, Quan C, Duan P, Tang S, Chen W, Yang KD. 2016. Nonylphenol induced apoptosis and autophagy involving the Akt/mTOR pathway in prepubertal sprague-dawley male rats in vivo and in vitro. Toxicology. 373:41–53.
- Jee Y, Noh EM, Cho ES, Son HY. 2010. Involvement of the Fas and Fas Ligand in testicular germ cell apoptosis by zearalenone in rat. J Vet Sci. 11(2):115–119.
- John Aitken R. 2013. Falling sperm counts twenty years on: where are we now? Asian J Androl. 15(2):204–207.
- Kajihara T, Okagaki R, Ishihara O. 2006. LPS-induced transient testicular dysfunction accompanied by apoptosis of testicular germ cells in mice. Med Mol Morphol. 39(4):203–208.
- Kijima K, Toyosawa K, Yasuba M, Matsuoka N, Adachi T, Komiyama M, Mori, C. 2004. Gene expression analysis of the rat testis after treatment with Di(2-Ethylhexyl) Phthalate using CDNA microarray and real-time PCR. Toxicol Appl Pharmacol. 200(2):103–110.
- Kroemer G, Galluzzi L, Vandenabeele P, Abrams J, Alnemri ES, Baehrecke EH, Blagosklonny MV, El-Deiry WS, Golstein P, Green DR, et al. 2009. Classification of cell death: recommendations of the nomenclature committee on cell death 2009. Cell Death Differ. 16(1):3–11.
- Lahijani MS, Farivar S, Sarhady M, Amiri M. 2012. Quinazolinone exposure induces apoptosis and changes expressions of Fas/FasL and c-Flip in embryonic mice testicles. Indian J Exp Biol. 50(4):247–255.
- Lavrik IN. 2014. Systems biology of death receptor networks: live and let die. Cell Death Dis. 5:e1259.
- Li MW, Xia W, Mruk DD, Wang CQ, Yan HH, Siu MK, Lui WY, Lee WM, Cheng CY. 2006. Tumor Necrosis Factor-alpha reversibly disrupts the blood-testis barrier and impairs sertoli-germ cell adhesion in the seminiferous epithelium of adult rat testes. J Endocrinol. 190(2):313–329.
- Li YJ, Song TB, Cai YY, Zhou JS, Song X, Zhao X, Wu XL. 2009. Bisphenol a exposure induces apoptosis and upregulation of Fas/Fasl and caspase-3 expression in the testes of mice. Toxicol Sci. 108(2):427–436.
- Li T, Hu J, He GH, Li Y, Zhu CC, Hou WG, Zhang S, Li W, Zhang JS, Wang Z, et al. 2012. Up-regulation of NDRG2 through NF-Kappa B is required for Leydig cell apoptosis in both human and murine infertile testes. Biochim Biophys Acta. 1822(2):301–313.
- Lin YC, Yao PL, Richburg JH. 2010. FasL gene-deficient mice display a limited disruption in spermatogenesis and inhibition of Mono-(2-Ethylhexyl) Phthalate-induced germ cell apoptosis. Toxicol Sci. 114(2):335–345.
- Ma C, Song H, Guan K, Zhou J, Xia X, Li F. 2016. Characterization of swine testicular cell line as immature porcine Sertoli cell line. In Vitro Cell Dev Biol Anim. 52(4):427–433.
- Mathur PP, D’Cruz SC. 2011. The effect of environmental contaminants on testicular function. Asian J Androl. 13(4):585–591.
- Moreno RD, Lizama C, Urzua N, Vergara SP, Reyes JG. 2006. Caspase activation throughout the first wave of spermatogenesis in the rat. Cell Tissue Res. 325(3):533–540.
- Paccola CC, Miraglia SM. 2016. Prenatal and lactation nicotine exposure affects Sertoli cell and gonadotropin levels in rats. Reproduction. 151(2):117–133.
- Qi S, Fu W, Wang C, Liu C, Quan C, Kourouma A, Yan M, Yu T, Duan P, Yang K. 2014. BPA-induced apoptosis of rat Sertoli cells through Fas/FasL and JNKs/p38 MAPK pathways. Reprod Toxicol. 50:108–116.
- Ren L, Zhang J, Zou Y, Zhang L, Wei J, Shi Z, Li Y, Guo C, Sun Z, Zhou X. 2016. Silica nanoparticles induce reversible damage of spermatogenic cells via RIPK1 signal pathways in c57 mice. Int J Nanomed. 11:2251–2264.
- Riccioli A, Salvati L, D’Alessio A, Starace D, Giampietri C, De Cesaris P, Filippini A, Ziparo E. 2003. The Fas system in the seminiferous epithelium and its possible extra-testicular role. Andrologia. 35(1):64–70.
- Roach HI, Clarke NMP. 2000. Physiological cell death of chondrocytes in vivo is not confined to apoptosis. J Bone Joint Surg Br. 82:601–613.
- Rodriguez I, Ody C, Araki K, Garcia I, Vassalli P. 1997. An early and massive wave of germinal cell apoptosis is required for the development of functional spermatogenesis. Embo J. 16(9):2262–2270.
- Rotgers E, Nurmio M, Pietila E, Cisneros-Montalvo S, Toppari J. 2015. E2F1 controls germ Cell apoptosis during the first wave of spermatogenesis. Andrology-Us. 3(5):1000–1014.
- Shi YQ, Wang YP, Song Y, Li HW, Liu CJ, Wu ZG, Yang KD. 2010. P,P’-Dde Induces Testicular Apoptosis in Prepubertal Rats Via the Fas/Fasl Pathway. Toxicol Lett. 193(1):79–85.
- Song Y, Yang KD. 2006. Effect of P, P’-DDE on DNA damage and expression of FasL gene of rat Sertoli cell in vitro. Wei Sheng Yan Jiu. 35(3):261–263.
- Suda T, Takahashi T, Golstein P, Nagata S. 1993. Molecular cloning and expression of the fas ligand, a novel member of the tumor necrosis factor family. Cell. 75:1169–1178.
- Sun Z, Nie Q, Zhang L, Niu R, Wang J, Wang S. 2017. Fluoride reduced the immune privileged function of mouse Sertoli cells via the regulation of Fas/FasL system. Chemosphere. 168:318–325.
- Taylor MF, de Boer-Brouwer M, Woolveridge I, Teerds KJ, Morris ID. 1999. Leydig cell apoptosis after the administration of ethane dimethanesulfonate to the adult male rat is a Fas-mediated process. Endocrinology. 140(8):3797–3804.
- Traore K, Martinez-Arguelles DB, Papadopoulos V, Chen H, Zirkin BR. 2016. Repeated exposures of the male sprague dawley rat reproductive tract to environmental toxicants: do earlier exposures to Di-(2-Ethylhexyl) Phthalate (DEHP) alter the effects of later exposures? Reprod Toxicol. 61:136–141.
- Traven E, Ogrinc A, Kunej T. 2017. Initiative for standardization of reporting genetics of male infertility. Syst Biol Reprod Med. 63(1):58–66.
- Villa-Morales M, Fernandez-Piqueras J. 2012. Targeting the Fas/FasL signaling pathway in cancer therapy. Expert Opin Ther Tar. 16:85–101.
- Wang K. 2014. Molecular mechanisms of hepatic apoptosis. Cell Death Dis. 5:e996.
- Wang L, Azad N, Kongkaneramit L, Chen F, Lu Y, Jiang BH, Rojanasakul Y. 2008. The Fas death signaling pathway connecting reactive oxygen species generation and flice inhibitory protein down-regulation. J Immunol. 180:3072–3080.
- Wang Y, Zhang JJ, Yang WR, Luo HY, Zhang JH, Wang XZ. 2015. Lipopolysaccharide-induced expression of Fas ligand in cultured immature boar Sertoli cells through the regulation of pro-inflammatory cytokines and mir-187. Mol Reprod Dev. 82(11):880–891.
- Wang YX, Zeng Q, Sun Y, Yang P, Wang P, Li J, Huang Z, You L, Huang YH, Wang C, et al. 2016. Semen phthalate metabolites, semen quality parameters and serum reproductive hormones: A cross-sectional study in China. Environ Pollut. 211:173–182.
- Wang YX, Wang P, Feng W, Liu C, Yang P, Chen YJ, Sun L, Sun Y, Yue J, Gu LJ, et al. 2017. Relationships between seminal plasma metals/metalloids and semen quality, sperm apoptosis and DNA integrity. Environ Pollut. 224:224–234.
- Wirth JJ, Mijal RS. 2010. Adverse effects of low level heavy metal exposure on male reproductive function. Syst Biol Reprod Med. 56(2):147–167.
- Xiong Q, Xie P, Li HY, Hao L, Li GY, Qiu T, Liu Y. 2009. Involvement of Fas/Fasl System in Apoptotic Signaling in Testicular Germ Cells of Male Wistar Rats Injected I.V. With Microcystins. Toxicon. 54(1):1–7.
- Xu ML, Hu J, Guo BP, Niu YR, Xiao C, Xu YX. 2015. Exploration of intrinsic and extrinsic apoptotic pathways in zearalenone-treated rat Sertoli cells. Environ Toxicol. 31(12):1731–1739.
- Xu YR, Dong HS, Yang WX. 2016. Regulators in the apoptotic pathway during spermatogenesis: killers or guards? Gene. 582(2):97–111.
- Yao PL, Lin YC, Sawhney P, Richburg JH. 2007. Transcriptional regulation of FasL expression and participation of STNF alpha in response to Sertoli cell injury. J Biol Chem. 282(8):5420–5431.
- Yao PL, Lin YC, Richburg JH. 2009. TNF alpha-mediated disruption of spermatogenesis in response to Sertoli cell injury in rodents is partially regulated by MMP2. Biol Reprod. 80(3):581–589.
- Zhang M, He Z, Wen L, Wu J, Yuan L, Lu Y, Guo C, Zhu L, Deng S, Yuan H. 2010. Cadmium suppresses the proliferation of piglet Sertoli cells and causes their DNA damage, Cell Apoptosis and Aberrant Ultrastructure. Reprod Biol Endocrinol. 8:97.
- Zhang PH, Chen DA, Dong L, Li GS, Yin J, Qu XW, You YD, Chang DG. 2016. Inhibitory effect of qiangjing tablets on the Fas/FasL pathway of cell apoptosis in male SD rats with infertility. Zhonghua Nan Ke Xue. 22(3):246–251.
- Zhang Z, Zhang X, Sun Z, Dong H, Qiu L, Gu J, Zhou, J, Wang X, Wang SL. 2013. Cytochrome p450 3al mediates 2,2’,4,4’-Tetrabromodiphenylether-induced reduction of spermatogenesis in adult rats. Plos One. 8(6):e66301.
- Zhao XF, Wang Q, Ji YL, Wang H, Liu P, Zhang C, Zhang Y, Xu DX. 2011. Fenvalerate induces germ cell apoptosis in mouse testes through the Fas/FasL signaling pathway. Arch Toxicol. 85:1101–1108.
