ABSTRACT
Cisplatin (CIS) is widely applied for its antihematological malignancies properties and as antisolid tumors drugs. However, it could cause testicular damage related with oxidative stress and testosterone synthesis disorder. Studies reported that grape seed procyanidins extract (GSPE) could improve CIS induced-testes lesion via scavenging free radicals in animals, although its mechanisms were unclear. Therefore, the purpose of the present study was to explore the antagonistic mechanisms of GSPE on CIS-induced testes lesion. Rats were treated with 10 mg/kg by weight CIS by intraperitoneal injection singly on the 11th day, and different doses of GSPE were administrated via intragastric gavage for 15 days consecutively. The results showed that GSPE improved the pathological changes of testicular tissue, and the decreased concentrations of testosterone in serum induced by CIS. GSPE inhibited CIS-induced oxidative/nitrative stress, as well as increased the mRNA and protein levels of testosterone synthetase in rat testes. In conclusion, the main protection exerted by GSPE on CIS-induced testicular toxicity is related to its effects including suppressing oxidative/nitrative stress and up-regulating expression of testosterone synthetase.
Abbreviations: CIS: Cisplatin; GSPE: grape seed procyanidins extract; LH: luteinizing hormone; FSH: follicle-stimulating hormone; STAR: steroidogenic acute regulatory protein; CYP11A1: P450 side chain cleavage enzyme; HSD3B1: 3β-hydroxysteroid dehydrogenase; CYP17A1: 17α-hydroxylase; HSD17B: 17β-hydroxysteroid dehydrogenase; ROS: reactive oxygen species; O2−: superoxide anion; H2O2: hydrogen peroxide; •OH: hydroxyl radicals; SOD: superoxide dismutase; CAT: catalase; GSH-Px: glutathione peroxidase; LPO: lipid peroxidation; 8-OHdG: 8-hydroxy-2-deoxyguanosine; HO-1: heme oxygenase-1; MT-1: metallothionein-1; NO: nitric oxide; ONOO-: peroxynitrite; NOS: nitric oxide synthases; nNOS: neuronal NOS; iNOS: inducible NOS; eNOS: endothelial NOS; MDA: malondialdehyde; GSH: glutathione; T-AOC: total antioxidant capacity; TNOS: total nitric oxide synthases; Lhcgr: luteinizing hormone receptor; Scarb1: lipoprotein-receptor; Cyp19a1: 19α-hydroxylase; ELISA: enzyme linked immunosorbent assay; RT-qPCR: reverse transcription-quantitative polymerase chain reaction; PAS: periodic acid–Schiff; MTs: Metallothioneins; cAMP: cyclic adenosine monophosphate; cDNA: complementary DNA; RIPA: radioimmunoprecipitation buffer; PMSF: phenylmethanesulfonyl fluoride; PVDF: polyvinylidenedifluoride; β-actin: beta-actin
Introduction
Cisplatin (CIS) is one of the most widely applied antitumor drugs to the treatment of hematological malignancies and solid tumors, such as head and neck, lung, testis, ovarian, and bladder cancer. CIS can disturb normal cell cycles and induce oxidative damage in vivo (Ozkok and Edelstein Citation2014). In recent years, studies reported that the use of CIS was limited by various severe side-effects such as acute kidney injury, gastrointestinal toxicity and ototoxicity in clinical practice (Gao et al. Citation2014). Male reproductive toxicity, especially testicular damage was also noted (Malarvizhi and Mathur Citation1996).
The testes secrete male hormones, which play an important role in the development and maturation of male reproductive organs, as well as the emergence of male sexual characteristics (Almasry et al. Citation2017). Aksu et al. (Citation2016) discovered CIS could induce abnormal changes of sperm density and motility, as well as oxidative stress, testicular degeneration and apoptosis. The study reported (Fouad et al. Citation2017) that 10 mg/kg CIS significantly reduced serum testosterone levels in rats. In addition, CIS significantly increased oxidative stress in testicular tissues, which furthermore may lead to testosterone synthesis disorder (Iman et al. Citation2017). CIS can parallel abnormal changes of sperm/testis parameters, as well as levels of testosterone, Luteinizing Hormone (LH) and Follicle Stimulating Hormone (FSH) in rat serum (Fallahzadeh et al. Citation2017).
Testosterone, as an important sex androgen, plays a critical role in growth, reproduction and maintenance of the function of vital organs. It is synthesized in Leydig cells a source of a variety of testosterone biosynthetic enzymes (Tremblay Citation2015). Cholesterol is initial substrate for testosterone synthesis, which is transported by steroidogenic acute regulatory protein (STAR) from its intracellular location into the mitochondrial inner membrane (Ye et al. Citation2011). Thus, STAR is the rate-limiting enzyme for testosterone synthesis (Liu et al. Citation2015a). P450 side chain cleavage enzyme (CYP11A1) is the first-step enzyme in the synthesis of testosterone, converting cholesterol to pregnenolone as it enters the smooth endoplasmic reticulum (Liu et al. Citation2015a). In the smooth endoplasmic reticulum 3β-hydroxysteroid dehydrogenase (HSD3B1) then converts pregnenolone to progesterone, and progesterone is converted into androstenedione via 17α-hydroxylase (CYP17A1) (Liu et al. Citation2015a). 17β-hydroxysteroid dehydrogenase (HSD17B) is the last-step enzyme for conversion androstenedione into testosterone (Garcia et al. Citation2012). Garcia et al. (Citation2012), showed that CIS could lead to increased levels of ROS in testicular Leydig cells while inhibiting the mRNA expression of CYP11A1, which ultimately led to testosterone synthesis disorders in mouse Leydig cells. CIS can induce Leydig cells dysfunction and testicular steroidogenic synthesis disorder in animals, inhibiting testosterone production causing infertility (Adler and Tarras Citation1990; Vawda Citation1994).
Aksu et al. (Citation2016) discovered CIS could induce oxidative stress in testicular tissues. Oxidative stress reflects the imbalance between the oxidative and the antioxidative capacity in tissues. Under physiological conditions, reactive oxygen species (ROS) are continuously produced by mitochondria include superoxide anion (O2−), hydrogen peroxide (H2O2), hydroxyl radicals (•OH), etc (Yin et al. Citation2017). Excess free radicals in vivo react with biological molecules such as unsaturated lipid, protein and DNA, as they induce an oxidative lesion (Aksu et al. Citation2016). The antioxidant enzymatic system of the testicular tissue contains superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GSH-Px). SOD can prevent O2− from participating in the formation of •OH. CAT avoids testes injury by converting H2O2 to H2O (Aitken and Roman Citation2008). The level of lipid peroxidation (LPO) is linked to the production of superoxide radicals (Adejuwon et al. Citation2015). LPO and 8-Hydroxy-2-deoxyguanosine (8-OHdG) are usually detected and indicators of oxidative stress. Heme oxygenase (HO-1) is a sensitive stress-response protein in oxidative damage, and widely used as the biomarker of oxidative stress induced by xenobiotics (Hu et al. Citation2007). Metallothionein-1 (MT-1) can scavenge and even prevent free radical formation to inhibit oxidative stress (Takano et al. Citation2004).
Nitrative stress is that nitric oxide (NO) free radical reacted with O2− generating peroxynitrite (ONOO−) in a short time, resulting in protein tyrosine nitration and cell damage cascade reaction (Radi Citation2004). NO is synthesized by nitric oxide synthases (NOS) which include neuronal NOS (nNOS), inducible NOS (iNOS), and endothelial NOS (eNOS) (Kone Citation2004). Among them, iNOS plays an important role in NO synthesis in rat testes (İsmail et al. Citation2004). The content of NO increases significantly in rat testes after CIS exposure (Eid et al. Citation2016).
Grape seed procyanidins extract (GSPE) is a natural plant polyphenols extracted from grape seed, which consists of ~15% of (+)-catechin and (−)-epicatechin, 80% of (−)-epicatechin 3-O-gallate, dimers, trimers, tetramers and their gallates and 5% of pentamers, hexamers, heptamers and their gallates (Gabetta et al. Citation2000). GSPE, as main polyphenols in grapes, has strong antioxidant and free radical scavenging capability (Hassan et al. Citation2013). The antioxidant effects of GSPE were much stronger than vitamin C, E and β-carotene (Boghdady Citation2013). GSPE has a broad spectrum of pharmacological, therapeutic, and chemoprotective properties (Sönmez and Tascioglu Citation2015). Zhao et al. (Citation2014) reported that CIS could reduce the epididymal and testes weight, count and motility of sperms, activities of GSH-Px and SOD, as well as increase malondialdehyde (MDA) content in rat testes, whereas GSPE could significantly antagonize the above toxic effects. Although this study showed that GSPE could counteract CIS-induced oxidative stress damage in rat testes, the mechanism is still incomplete. It is still unclear whether GSPE attenuates CIS-induced testosterone reduction via inhibiting testosterone synthetase in rat testes. Therefore, we hypothesized that GSPE could attenuate testicular toxicity induced by CIS via suppressing oxidative/nitrative stress and up-regulating expression of testosterone synthetase.
In this study, the animal model of CIS-induced testes lesion was established by intraperitoneal injection of CIS (10 mg/kg b.w.) and intragastric administration of GSPE. To test the above hypothesis, we observed changes of testicular pathological, and detected oxidative/nitrative stress-related indictors including •OH, SOD, CAT, Glutathione (GSH), total antioxidant capacity (T-AOC), LPO, 8-OHdG, NO, iNOS, total nitric oxide synthases (TNOS), as well as mRNA expression levels of MT-1 and HO-1 in rat testes. Furthermore, we measured the testosterone concentration in serum, and gene expression levels of testosterone synthases such as luteinizing hormone receptor (Lhcgr), lipoprotein-receptor (Scarb1), Star, Cyp11a1, Cyp17a1, 19α-hydroxylase (Cyp19a1), Hsd3b1, Hsd17b, as well as protein contents of key testosterone synthetase including STAR, CYP11A1 and HSD17B in rat testes. We aimed to elucidate the mechanisms which GSPE attenuates CIS-induced oxidative stress and inhibition of testosterone synthases in rat testes.
Results
GSPE attenuated CIS-induced basic toxicity in male rats
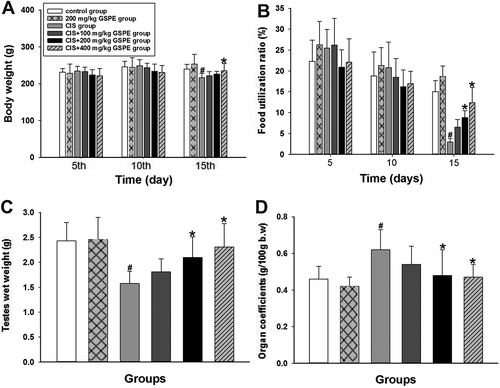
In order to investigate protective effects of GSPE on the basic toxicity induced by CIS, we established the animal model of CIS exposure and administrated rats with different doses GSPE. As shown in , compared with the control group, the weight of rats in CIS group declined significantly by day 15 (p < 0.05), while increased in the CIS + 400 mg/kg GSPE group when compared to the CIS group (p < 0.05). Food utilization rate in CIS group was lower than that of the control group (p < 0.05). Meanwhile, food utilization rate in the CIS group was also lower than that of CIS + 200 mg/kg and 400 mg/kg GSPE groups on the 15th day (, p < 0.05). Compared to the control group, testes weight decreased and organ coefficients increased in CIS group (p < 0.05), but significantly improved in CIS + 200 mg/kg GSPE and CIS + 400 mg/kg GSPE groups with a dose-dependent manner ( and ).
Figure 1. Protective effects of GSPE on CIS-induced basic toxicity in male rats. (A) dynamic changes of the body weight in the 5th day, 10th day and 15th day, (B) dynamic changes of the food utilization rate (the amount of weight gain/food intake × 100%) within 5 days, 10 days and 15 days, (C) alterations of the total wet weight of bilateral testes and (D) alterations of the testes coefficient to body weight (testicular wet weight/body weight). Values are expressed as mean ± standard deviation (n = 8). Data analysis was performed using multiple comparisons (LSD t-test or Dunnett’s t-test). # GSPE, CIS groups versus the control group, * CIS + GSPE groups versus the CIS group (p < 0.05). CIS: Cisplatin, GSPE: grape seed procyanidins extract.
Protection effects of GSPE on CIS-induced histopathological damage in rat testes
To evaluate the protective effect of GSPE from the perspective of pathological damage, the animal model of testicular lesion induced by CIS was established in rats. As shown in and , the seminiferous tubule structure and epithelial cells were normal in control and 200 mg/kg GSPE groups in stages IV–VI of seminiferous epithelium cycle. However, seminiferous epithelium cells were disordered, and some damaged spermatogenic epithelial cells fell into the seminiferous tubule lumen, as well as the seminiferous epithelium became thin in stages IV–VI of the CIS group (). Abnormalities of seminiferous tubule structure and epithelial cells induced by CIS were gradually improved in CIS + 100, 200 and 400 mg/kg GSPE groups, in stages IV–VI of cycle (, , and ).
Figure 2. Histopathological changes by PAS staining in rat testes (×400). (A1–F1) stages IV–VI of spermatogenic epithelium, (A2–F2) stage XI of spermatogenic epithelium, (A3–F3) interstitium. (A1–A3) the control group, (B1–B3) the 200 mg/kg GSPE group, (C1–C3) the CIS group, (D1–D3) the CIS + 100 mg/kg GSPE group, (E1–E3) the CIS + 200 mg/kg GSPE group and (F1–F3) the CIS + 400 mg/kg GSPE group. Damaged spermatogenic epithelial cells fell into the seminiferous tubule lumen, interstitial blood vessel hyperemia as well as vacuoles were observed in the CIS group. (n = 5), ![]()
 represents vacuoles, → represents interstitial blood vessel hyperemia. PAS: periodic acid-Schiff.
represents vacuoles, → represents interstitial blood vessel hyperemia. PAS: periodic acid-Schiff.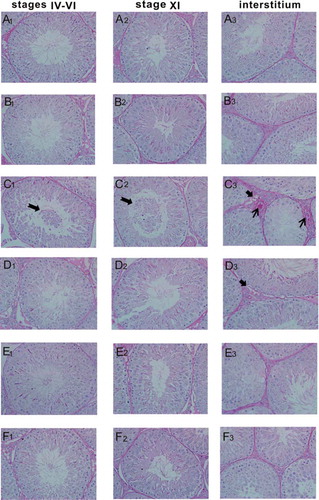
Similarly, the seminiferous tubule structure and epithelial cells were normal in stage XI of control and the 200 mg/kg GSPE groups ( and ). The number of the seminiferous epithelium cells decreased, and the damaged spermatogenic cells were detached to the tubule lumen in the stage XI of the CIS group (). As shown in –, the seminiferous tubule structure and the number of epithelial cells recovered in the CIS + 200 and 400 mg/kg GSPE groups, especially in the CIS + 400 mg/kg GSPE group.
There were no abnormalities of testicular interstitium in the control and 200 mg/kg GSPE groups ( and ). The interstitial blood vessels exhibited hyperemia, testicular interstitium had vacuoles and cells decreased in the CIS group (), and the above changes were also observed in the CIS + 100 mg/kg GSPE group (). Testicular interstitial hyperemia and vacuoles declined significantly in CIS + 200 and 400 mg/kg GSPE groups ( and ). No significant pathological changes were observed in other stages of seminiferous epithelium cycle.
As shown in and , our data of quantitative histological analysis showed that the relative area of seminiferous tubule, the relative area of seminiferous epithelium and diameter of seminiferous tubule significantly decreased, and the relative area of tubule lumen and diameter of tubule lumen increased after CIS exposure in stages IV–VI and XI of seminiferous epithelium cycle in rats. In parallel, the relative area of testicular interstitium was reduced in the CIS group, when compared with the control group (p < 0.05). In contrast with the CIS exposure group, the aforementioned indicators were revivified obviously in CIS + 200 and 400 mg/kg GSPE groups as a dose-dependent manner (p < 0.05). There was no statistical difference between the GSPE group and control group (p > 0.05) and in the other stages of seminiferous epithelium cycle in rats.
Table 1. Quantitative histological analysis of testicular tissue in stages IV–VI of seminiferous epithelium cycle in rats.
Table 2. Quantitative histological analysis of testicular tissue in stage XI of seminiferous epithelium cycle in rats.
GSPE alleviated CIS-induced oxidative stress in male rats
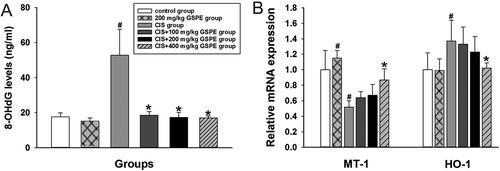
To explore the potential mechanism of GSPE’s protective effects, we examined levels of testicular oxidative stress indicators in CIS and CIS + GSPE groups. As shown in , activities of SOD and CAT were inhibited, and levels of T-AOC decreased, instead ▪OH levels increased significantly in the CIS group, compared with the control group (p < 0.05). Levels of LPO in the testicular tissue and 8-OHdG in serum were dramatically increased, whereas levels of GSH in rat testes were decreased after CIS exposure ( and and ), when compared with the control group (p < 0.05). The up-regulated HO-1 and down-regulated level of MT-1 mRNA were observed in rat testes after CIS administration (). There was no significant difference between the GSPE group and control group of the above indicators (p > 0.05). Levels of ▪OH, LPO and 8-OHdG remarkably declined, whereas levels of SOD, CAT, GSH and T-AOC significantly increased in CIS + 200 and 400 mg/kg GSPE groups (p < 0.05, and ). The abnormal mRNA expression of MT-1 and HO-1 was significantly improved in the CIS + 400 mg/kg GSPE group (p < 0.05, ).
Figure 3. Protective effects of GSPE on CIS-induced oxidative stress in rat testes. (A) •OH levels, (B) activity of SOD, (C) activity of CAT, (D) GSH levels, (E) LPO levels and (F) T-AOC levels. Values were expressed as mean ± standard deviation (n = 8). Data analysis was performed using multiple comparisons (LSD t-test or Dunnett’s t-test). # GSPE, CIS groups versus the control group, * CIS + GSPE groups versus the CIS group (p < 0.05). •OH: hydroxyl radicals, SOD: superoxide dismutase, CAT: catalase, GSH: glutathione, LPO: lipid peroxidation, T-AOC: total antioxidant capacity.
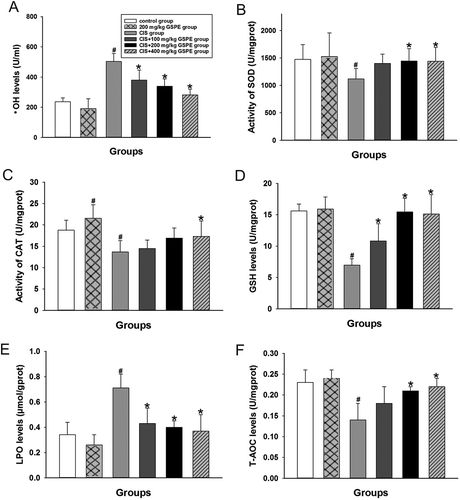
Figure 4. 8-OHdG levels in serum and mRNA levels of MT-1 and HO-1 in rat testes. (A) 8-OHdG levels by ELISA and (B) relative mRNA expression levels of MT-1 and HO-1 by RT-qPCR. Values were expressed as mean ± standard deviation (n = 8). Data analysis was performed using multiple comparisons (LSD t-test or Dunnett’s t-test). # GSPE, CIS groups versus the control group, * CIS + GSPE groups versus the CIS group (p < 0.05). 8-OHdG: 8-hydroxy-2-deoxyguanosine, HO-1: heme oxygenase, MT-1: metallothionein-1, ELISA: enzyme linked immunosorbent assay, RT-qPCR: reverse transcription-quantitative polymerase chain reaction.
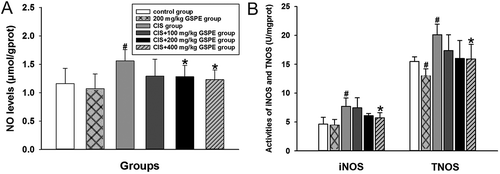
GSPE alleviated CIS-induced nitrative stress in male rat testes
To investigate the role of nitrative stress in the protective effect of GSPE on CIS-induced testicular toxicity, we measured indicators of nitrative stress in rat testes. As shown in , NO, iNOS and TNOS levels were obviously increased in the CIS group, compared with the control group (p < 0.05). There was no significant difference of nitrative stress-related indicators between the GSPE group and control group (p > 0.05). Compared with the CIS group, NO levels shifted down significantly in CIS + 200 and 400 mg/kg GSPE groups (p < 0.05, ); furthermore, activities of iNOS and TNOS declined remarkably in the CIS + 400 mg/kg GSPE group ().
Figure 5. Protective effects of GSPE on CIS-induced nitrative stress in rat testes. (A) NO levels were increased in CIS groups, and (B) activities of iNOS and TNOS were increased in CIS groups. Values were expressed as mean ± standard deviation (n = 8). Data analysis was performed using multiple comparisons (LSD t-test or Dunnett’s t-test). # GSPE, CIS groups versus the control group, * CIS + GSPE groups versus the CIS group (p < 0.05). NO: nitric oxide, iNOS: inducible nitric oxide synthases, TNOS: total nitric oxide synthases.
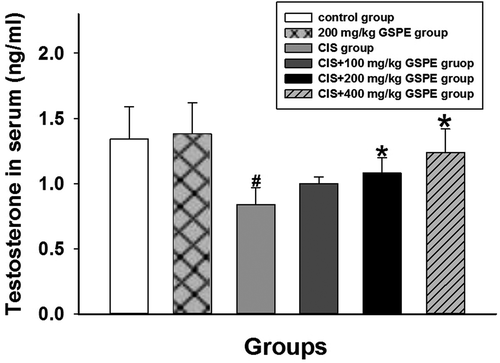
GSPE protected against CIS-induced testosterone synthesis disorder in rats
To determine whether GSPE can reverse the decline of CIS-induced testosterone, we used an enzyme-linked immunosorbent assay (ELISA) to detect testosterone content in serum. As shown in , testosterone concentrations were not statistically different between the 200 mg/kg GSPE group and control group (p > 0.05). Compared with the control group, the testosterone concentrations decreased in the CIS group, whereas testosterone content in CIS + 200 and 400 mg/kg GSPE groups were higher than that of the CIS group (p < 0.05).
Figure 6. Concentrations of testosterone in serum by ELISA. Concentrations of testosterone decreased in CIS groups, while GSPE could reverse CIS-induced adverse effect and with a dose dependence manner. Values were expressed as mean ± standard deviation (n = 8). Data analysis was performed using multiple comparisons (LSD t-test). # GSPE, CIS groups versus the control group, * CIS + GSPE groups versus the CIS group (p < 0.05).
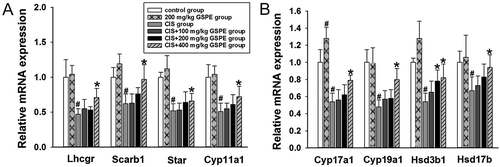
To further confirm whether GSPE can improve down-regulated gene expression levels of testosterone synthetase induced by CIS, mRNA levels of testosterone synthases were analyzed by reverse transcription-quantitative polymerase chain reaction (RT-qPCR) in rat testes. As shown in , we found that levels of relative mRNA expression of Lhcgr, Scarb1, Star, Cyp11a1, Cyp17a1, Cyp19a1, Hsd3b1 and Hsd17b were distinctly down-regulated in the CIS group, when compared to the control group (p < 0.05). However, compared to the CIS group, mRNA levels of testosterone synthetase were up-regulated in the CIS + 400 mg/kg GSPE group (p < 0.05).
Figure 7. Effects of GSPE on CIS-induced the down-regulation of testosterone synthetase mRNA levels in rat testes. (A,B) relative mRNA expression levels of Lhcgr, Scarb1, Star, Cyp11a1, Cyp17a1, Cyp19a1, Hsd3b1, and Hsd17b by RT-qPCR. Values were expressed as mean ± standard deviation (n = 8). Data analysis was performed using multiple comparisons (LSD t-test or Dunnett’s t-test). # GSPE, CIS groups versus the control group, * CIS + GSPE groups versus the CIS group (p < 0.05). Lhcgr: luteinizing hormone receptor, Scarb1: lipoprotein-receptor, Star: steroidogenic acute regulatory protein, Cyp11a1: P450 side chain cleavage enzyme, Cyp17a1: 17α-hydroxylase, Cyp19a1: 19α-hydroxylase, Hsd3b1: 3β-hydroxysteroid dehydrogenase, Hsd17b: 17β-hydroxysteroid dehydrogenase.
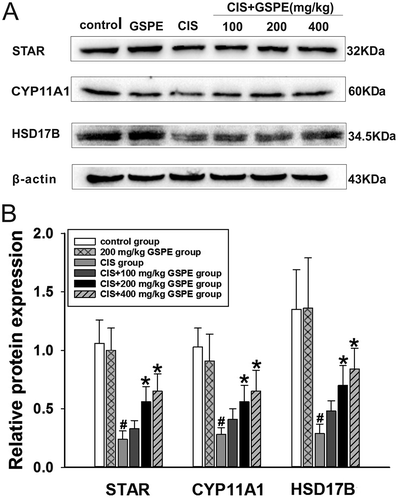
In order to further explore possible mechanisms, we selected three key enzymes of testosterone synthesis including STAR, CYP11A1 and HSD17B to explore the role of GSPE in CIS-induced testosterone synthesis disorder in rats. As shown in and , compared to the control group, the protein contents of STAR, CYP11A1 and HSD17B were reduced in the CIS group (p < 0.05), whereas there was no change in the GSPE group (p > 0.05). In contrast, compared to the CIS group, GSPE could up-regulate the three protein expression in a dose-dependent manner (p < 0.05), especially in CIS + 200 and 400 mg/kg GSPE groups.
Figure 8. GSPE up-regulated protein expression levels of the key testosterone synthetase in rat testes. (A) STAR, CYP11A1 and HSD17B contents determined by Western blot, and (B) semiquantitative analysis of protein expression levels by IPP. Values were expressed as mean ± standard deviation (n = 5). Data analysis was performed using multiple comparisons (LSD t-test or Dunnett’s t-test). # GSPE, CIS groups versus the control group, * CIS + GSPE groups versus the CIS group (p < 0.05). STAR: steroidogenic acute regulatory protein, CYP11A1: P450 side chain cleavage enzyme, HSD17B: 17β-hydroxysteroid dehydrogenase.
Discussion
CIS, an inorganic anticancer agent, has been recommended for clinical use due to its curative effect. CIS chemotherapy is widely accepted regimen for treatment of testicular malignancies (Krege et al. Citation2008). However, toxic effects of CIS acting as a chemotherapeutic agent have been reported in recent years, with the increasing incidence of testicular cancer in fertile men (Aminsharifi et al. Citation2017). Many studies have reported that CIS could trigger the reproductive toxicity in male animals. After a single dose of CIS (10 mg/kg) was administrated to male rats by intraperitoneal injection, results showed that CIS decreased the testes weight, sperm motility, sperm density, sperm count, and levels of testosterone, LH and FSH in serum (Iman et al. Citation2017). In rats, CIS significantly increased oxidative stress of testicular tissues, with increased MDA and NO levels, and significant reduction of GSH levels and CAT activity (Heeba et al. Citation2016). GSPE is recognized as the most effective natural antioxidant to remove free radicals in vivo. Isabel et al. (Citation2008) found that GSPE could scavenge free radicals and reduce the peroxidation induced by free radicals. GSPE could alleviate significant nephrotoxicity caused by CIS, which related with GSPE-inhibiting free radical production and lipid peroxidation induced by CIS (Saad et al. Citation2009). Therefore, we chose GSPE as a protective agent against CIS-induced testicular toxicity. GSPE at adosage of 400 mg/kg b.w could reverse CIS-induced oxidative stress in rat testes, similar to GSPE being shown to have an anti-oxidative function in normal rats. Therefore, in order to explore whether 200 mg/kg GSPE had the antioxidative function for normal rats, we chose 200 mg/kg GSPE as the single dose treatment group (Zhao et al. Citation2014).
In the present study, the body weight, food utilization rate, testes weight significantly decreased after treatment with CIS in rats. These results were consistent with our earlier study by Liu et al. (Citation2015b), and the reported literature (Ateşşahin et al. Citation2006; Ilbey et al. Citation2009a). Others (Ilbey et al. Citation2009b) have suggested that CIS (10 mg/kg) could cause irregular seminiferous tubules, reduction of seminiferous epithelial layers and germ cells in rats. In our study, we used Periodic acid–Schiff (PAS) staining for testicular tissue sections and divided the testicular spermatogenic epithelium into 14 stages. Our results suggest that histopathological alternations of rat testes induced by CIS mainly occurred in the stages IV–VI and XI of seminiferous epithelium cycle. The seminiferous epithelium was characterized as appeared disordered, damaged spermatogenic cells fell off into the seminiferous tubule lumen, and the seminiferous epithelium became thin. Quantitative histological analysis revealed that with CIS, the diameters of seminiferous tubule decreased along with the area of seminiferous tubule and seminiferous epithelium. This occurred with an increased diameter and area of tubule lumen. Furthermore, CIS could induce the testicular interstitial lesions, such as the interstitial vascular congestion and the decreased interstitial area, consistent with Eid et al. (Citation2016). Nevertheless, our results indicated testicular histopathological changes were apparently improved in CIS when combined with different doses GSPE in a dose-dependent manner. The above results indicated that the rat model of testicular lesion induced by CIS was successfully established and GSPE had protective effects on CIS-induced basic toxicity.
Oxidative stress was a negative effect of free radicals in the body and considered to be an important factor in causing aging and disease (Amin and Hamza Citation2006). Studies have confirmed (Hamza et al. Citation2016) that oxidative stress is a critical mechanism of testicular damage in CIS-exposed animals. CIS could decrease levels of SOD, GSH-Px and GSH, while increased content MDA in rat testes (Zhao et al. Citation2014). Our results showed that CIS inhibited activities of antioxidant enzymes such as SOD and CAT, and induced over production of •OH. GSH, as a nonenzymatic antioxidant, is widely found in biological tissues that has the effect of scavenging free radicals and singlet oxygen (Tremellen Citation2008). In our study, we showed that CIS could induce a decline of GSH and T-AOC in rat testicular tissues. In addition to LPO and 8-OHdG as ultimate points of oxidative stress, elevated levels were observed after CIS exposure. Metallothioneins (MTs), small cysteine-rich molecules are involved in many protective stress responses in the organism (Ruttkaynedecky et al. Citation2013). Whereas, as a stress-inducible enzyme, HO-1 can induce a variety of stimulus that are beneficial to oxidative stress, such as H2O2, NO, heavy metals and growth factors (Agarwal and Nick Citation2000). Heeba et al. (Citation2016) showed that the activity and protein expression of HO-1 in testicular tissues significantly increased with CIS exposure in rats. Results of our study showed CIS down-regulated mRNA expression of MT-1 and up-regulated the HO-1 mRNA level, revealing that CIS can cause testicular toxicity via triggering oxidative stress in rats.
Zhao et al. (Citation2014) reported that GSPE (200, 400 mg/kg) could improve CIS-induced reductions of SOD, GSH-PX, GSH, as well as increase of MDA in rat testes. Our results illustrated that contents of SOD, CAT, T-AOC, and GSH were elevated, while •OH, LPO, and 8-OHdG were decreased in CIS + 200 and 400 mg/kg GSPE groups, as well as mRNA expression levels of MT-1 were up-regulated and HO-1 was down-regulated in the CIS +400 mg/kg GSPE group. These results indicated that GSPE could protect testes from oxidative damage induced by CIS.
Eid et al. (Citation2016) reported that a signal dose of 10 mg/kg CIS remarkably augmented NO contents and iNOS protein expression in rat testicular tissues. In our study, we found that CIS increased levels of NO, iNOS and TNOS, which implied that CIS resulted in nitrative stress in rat testes. However, indicators of nitrative stress including NO, iNOS and TNOS were reduced in the CIS + 400 mg/kg GSPE group. Results suggested that GSPE had an intervention effect via reducing the nitrative stress induced by CIS in rat testes.
An earlier study indicated (Lonare et al. Citation2016) that oxidative stress is responsible for the damage to the reproductive system in rats. ROS could impair LHCGR signaling and down-regulate levels of cyclic adenosine monophosphate (cAMP) in Leydig cells, leading to testosterone synthesis disorder (Midzak et al. Citation2009). In the present study, we also showed that testosterone levels in serum were significantly decreased by CIS-induced oxidative stress, but antioxidant effects of GSPE increased the testosterone production, especially in CIS + 200 and 400 mg/kg GSPE groups. This result indicated that GSPE could promote the testosterone secretion of Leydig cells via antagonizing of oxidative stress induced by CIS in rat testes.
Kong et al. (Citation2017) reported testosterone synthesis was a complex process requiring a range of enzymes involved. LHCGR, as a specific receptor on plasma membrane of Leydig cells, is bound by pituitary gonadotropin luteinizing hormone, resulting in cAMP release and protein kinase A activation, which initiates a series of enzyme cascade reactions (Liu et al. Citation2015a). Cholesterol, as the raw material for testosterone synthesis, is scavenged into Leydig cells via SCARB1, subsequently transported into mitochondrial inner membrane by STAR and cAMP, which was catalyzed by CYP11A1 to produce pregnenolone (Gao et al. Citation2012). HSD3B1 is responsible for converting pregnenolone to progesterone. CYP17A1 could convert progesterone into androstenedione, which is eventually metabolized by HSD17B to form testosterone (Clewell et al. Citation2010).
CIS exerts its inhibitory action on testosterone synthesis by modulating Cyp11a1 gene expression in mouse testes interstitial cells (Garcia et al. Citation2012). CIS combined with bleomycin and etoposide down-regulates the gene expression of Star, Cyp17a1 and Cyp19a1 in rat testes (Al-Bader and Kilarkaje Citation2015). Our current study has been suggested that CIS could down-regulate the mRNA expression of testosterone synthetase including Lhcgr, Scarb1, Star, Cyp11a1, Cyp17a1, Cyp19A1, Hsd3b1, and Hsd17b, as well as protein contents of STAR, CYP11A1, and HSD17B. However, mRNA levels of the testosterone synthetase and protein contents of three key enzymes were up-regulated in the CIS + GSPE groups with a dose dependent manner. Our results indicated that GSPE could increase testosterone concentrations by rescuing the down-regulated expression of testosterone synthetase induced by CIS in rats.
An animal model of CIS-induced testicular toxicity and GSPE intervention was established in the present study. Results indicated CIS could lead to testicular toxicity via triggering oxidative/nitrative stress and inhibiting testosterone synthase in rats. This study provides some evidences that GSPE has protective effects on the testicular toxicity induced by CIS, which included basic toxicity, oxidative stress/nitrative stress, and testosterone synthesis disorder. However, whether GSPE can be used as an agent of adjuvant therapy requires further study and independent confirmation.
Materials and methods
Chemicals
CIS (5 mg/mL, Code H20040813) was purchased from Jiangsu Hansoh pharmaceutical Group Co., Ltd. (Jiangsu, China). GSPE (Code HX20160815) was obtained from Baoji Haoxiang Bio-technology Co., Ltd. (Shanxi, China). According to the manufacture, these grape seed extracts contained proanthocyanidins (≧95% pure), polyphenol (≧80% pure) and oligomeric procyanidin (≧70% pure).
Experimental animals
Adult male Wistar rats (180–220 g) were purchased from the Lanzhou Institute of Veterinary Medicine, Chinese Academy of Agricultural Sciences. Animals were kept in sterilized polypropylene rat cages with wood chip bedding under a 12 h dark/light cycle at 22 ~ 24°C. Food and water were provided ad libitum. Rats were housed one week prior to experimental use. From then on, they received a standard experimental diet until the end of the experiment. All experiments in this study were based on the ethics guidance of the Institutional Animals Ethics Committee of Gansu province (China), Permit No. (2017-03).
Experimental design and treatment
Total of 48 rats were randomly divided into six groups: control group, GSPE (200 mg/kg b.w) group, CIS group (10 mg/kg b.w), CIS + 100 mg/kg b.w GSPE group, CIS + 200 mg/kg b.w GSPE group and CIS + 400 mg/kg b.w GSPE group. Distilled water was administrated to the control group and CIS group by gavage for 15 days consecutively. Meanwhile, 200, 100, 200 and 400 mg/kg b.w GSPE were administrated to GSPE, CIS + 100, 200 and 400 mg/kg GSPE groups for 15 days, respectively (Zhao et al. Citation2014). In order to demonstrate the significant adverse effects of CIS in rat testes, we selected 10 mg/kg as CIS exposure dose according to Ciftci et al. (Citation2011) study. Normal saline was singly administrated via intraperitoneal injection to control and GSPE groups on 11th day. In addition, rats of other four groups were treated singly with 10 mg/kg b.w CIS by intraperitoneal injection.
Sample collection
Rats were placed in a static inhalation cabinets contained diethyl ether. After several minutes, rats were taken out and fixed on the console when rats were breathing slowly. Next, we collected blood by exsanguination via hearts, then rats were killed and testes were quickly removed to weight for calculating testicular organ coefficients (testicular wet weight/body weight, g/100g b.w). We received bilateral testes from eight rats of each group, and left testes were used to histopathologic examinations. Right testes were preserved in −20°C for total RNA and protein isolation, as well as the detection of biochemical indicators.
Evaluation of food utilization rate
The food utilization rate was equal to the amount of weight gain divided by food intake of rats for different experimental interval and multiplied by 100%.
Histopathological examinations and quantitative analysis
A standard protocol was used to prepare testicular tissue sections by PAS staining. Left testes of each group were fixed in Bouin’s solution for 24 h. And then prepared 4 μm pathologic sections were stained by PAS to observe pathological changes by Olympus light microscope (Olympus AX-70, Japan) with attached camera (Olympus Camedia C-5050, Japan) under 400 × magnification.
According to Clermont and Russell’s methods (Clermont and Perey Citation1957; Russell et al. Citation1993) about criteria for different stages of the epithelial cycle by PAS staining, the seminiferous epithelium cycle was divided into 14 stages in rat, and we modified slightly. Subsequently, we measured the relative area of whole seminiferous tubule, seminiferous epithelium, tubule lumen, interstitium, as well diameters of seminiferous tubule and tubule lumen by pathological tissue quantitative analysis software of iCALIBUR Bio Life Pro (Beijing Daqi Zongheng Tech. Co. Ltd., China). Seminiferous tubule (%) = A(seminiferous tubule,μm2)/A(whole vision,μm2) × 100%, Seminiferous epithelium (%) = A(seminiferous epithelium,μm2)/A(seminiferous tubule,μm2) × 100%, Tubule lumen (%) = A(Tubule lumen,μm2)/A(whole vision,μm2) × 100%, Interstitium (%) = A(interstitium,μm2)/S(whole vision,μm2) × 100%. A represents the area under the microscope (magnification × 400).
Oxidative/nitrative stress in testicular tissues
To detect levels of oxidative/nitrative stress-related markers, testicular tissues were homogenized in sterile normal saline with ice bath, and centrifuged at 1200 × g for 20 min at 4°C to collect the supernatant. Levels of •OH, CAT, T-AOC, SOD, GSH, LPO, NO, iNOS, and TNOS in supernatant were determined by using commercially available kits according to the manufacturer’s instructions (Nanjing Jiancheng, China). Results were normalized to the concentration of total protein which was measured by the Bicinchoninic Acid Total Protein Quantification Kit (Thermo Scientific, USA).
Determination of serum testosterone and 8-OHdG levels
Levels of testosterone and 8-OHdG in serum were measured with the ELISA kits for rats (Elabscience, China). Concentrations of testosterone and 8-OHdG in serum were calculated by standard curve.
RNA isolation and RT-qPCR
To explore whether CIS and GSPE induced gene expression changes of oxidative stress and enzymes of testosterone synthesis, approximately 95 mg testicular tissue were used to measure the mRNA expression levels of MT-1, HO-1, Lhcgr, Scarb1, Star, Cyp11a1, Cyp17a1, Cyp19a1, Hsd3b1 and Hsd17b. Total RNA of testicular tissues were isolated using TRIzol reagent (Invitrogen, USA). Concentrations of RNA were determined at 260 nm and 280 nm absorbance wavelengths by NanoDrop 2000C spectrophotometer (Thermo Scientific, USA). Selected total RNA with the A260/A280 ratio between 1.8 and 2.2 to reverse transcript PCR. RNA was reversely transcribed into complementary DNA (cDNA) using total RNA (1 μg) by Fast Quant RT kit (Takara, China). RT-qPCR was carried out in 25 μL reaction volume with SYBR Green detection system (Bio-Rad, USA). The steps of the program were described by Liu et al. (Citation2017). The sequences of primers were designed and synthetized by Takara Biotechnology Co., Ltd. (Shanghai, China) and listed in . β-actin was used as the internal reference. We used the 2–ΔΔct relative quantitation formula to quantify mRNA expression levels (Li et al. Citation2017).
Table 3. Primer sequences and theoretical amplification length.
Western blot
To evaluate whether protein contents of the testosterone related enzymes were altered, approximately 130 mg of right testicular tissues were homogenized in 1.0 mL radioimmunoprecipitation buffer (RIPA) containing 10 μL phenylmethanesulfonyl fluoride (PMSF) and 10 μL β-glycerophosphoric acid disodium salt (phosphatase inhibitor), then centrifuged at 1,2000 × g for 10 min at 4°C. The supernatant was obtained to quantify protein concentration with the Bicinchoninic Acid Total Protein Quantification Kit. Western blot analysis was performed as described (Sönmez et al. Citation2016; Liu et al. Citation2017). The protein samples (30 μg) were run on 10% SDS-PAGE, transferred on polyvinylidenedifluoride (PVDF) membranes (Millipore, USA). Subsequently, membranes were blocked with 5% skimmed milk about 3 h at room temperature, and washed in TBST buffer 10 min three times. Membranes were respectively incubated with beta-actin (β-actin), STAR, CYP11A1 and HSD17B (1:500 dilution) primary antibody (Signal Antibody, USA) overnight at 4°C. Then, membranes were incubated in goat anti-rabbit secondary antibody (1:5000 dilution). Target proteins were detected by enhanced chemiluminescence system (Thermo Science, USA) and exposed in Molecular Imager ChemiDoc XRS System (Bio-Rad, USA). Finally, quantification of protein bands was performed with IPP (Media Cybernetics Inc., China). Relative protein expression levels were expressed as the ratio of band intensity of target protein compared to β-actin.
Statistical analysis
Results were expressed as mean ± standard deviation. Multiple comparisons (LSD t-test or Dunnett’s t-test) were performed using one way ANOVA with SPSS 20.0 statistical program (SPSSnc., Chicago, IL, USA). Difference was considered significant at p < 0.05.
Acknowledgment
This work was financially supported by the Science and Technology Bureau, Chengguan District, Lanzhou, Gansu, China (Grant No. 2016-7-12), the Fundamental Research Funds for the Central Universities of China (lzujbky-2017-it36) and National Undergraduate Training Programs for Innovation and Entrepreneurship (201710730161).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Minmin Tian
Conceived and designed the experiments: YS, XC. Performed the experiments: MT, FL, HL, QZ, LL, XH, JZ, SL. Analyzed the data: MT, FL, YS. Contributed reagents/materials/analysis tools: YS, XC. Wrote the manuscript: MT, FL, YS.
Fangfang Liu
Conceived and designed the experiments: YS, XC. Performed the experiments: MT, FL, HL, QZ, LL, XH, JZ, SL. Analyzed the data: MT, FL, YS. Contributed reagents/materials/analysis tools: YS, XC. Wrote the manuscript: MT, FL, YS.
Han Liu
Conceived and designed the experiments: YS, XC. Performed the experiments: MT, FL, HL, QZ, LL, XH, JZ, SL. Analyzed the data: MT, FL, YS. Contributed reagents/materials/analysis tools: YS, XC. Wrote the manuscript: MT, FL, YS.
Qiong Zhang
Conceived and designed the experiments: YS, XC. Performed the experiments: MT, FL, HL, QZ, LL, XH, JZ, SL. Analyzed the data: MT, FL, YS. Contributed reagents/materials/analysis tools: YS, XC. Wrote the manuscript: MT, FL, YS.
Lei Li
Conceived and designed the experiments: YS, XC. Performed the experiments: MT, FL, HL, QZ, LL, XH, JZ, SL. Analyzed the data: MT, FL, YS. Contributed reagents/materials/analysis tools: YS, XC. Wrote the manuscript: MT, FL, YS.
Xiangbo Hou
Conceived and designed the experiments: YS, XC. Performed the experiments: MT, FL, HL, QZ, LL, XH, JZ, SL. Analyzed the data: MT, FL, YS. Contributed reagents/materials/analysis tools: YS, XC. Wrote the manuscript: MT, FL, YS.
Jianxin Zhao
Conceived and designed the experiments: YS, XC. Performed the experiments: MT, FL, HL, QZ, LL, XH, JZ, SL. Analyzed the data: MT, FL, YS. Contributed reagents/materials/analysis tools: YS, XC. Wrote the manuscript: MT, FL, YS.
Sheng Li
Conceived and designed the experiments: YS, XC. Performed the experiments: MT, FL, HL, QZ, LL, XH, JZ, SL. Analyzed the data: MT, FL, YS. Contributed reagents/materials/analysis tools: YS, XC. Wrote the manuscript: MT, FL, YS.
Xuhong Chang
Conceived and designed the experiments: YS, XC. Performed the experiments: MT, FL, HL, QZ, LL, XH, JZ, SL. Analyzed the data: MT, FL, YS. Contributed reagents/materials/analysis tools: YS, XC. Wrote the manuscript: MT, FL, YS.
Yingbiao Sun
Conceived and designed the experiments: YS, XC. Performed the experiments: MT, FL, HL, QZ, LL, XH, JZ, SL. Analyzed the data: MT, FL, YS. Contributed reagents/materials/analysis tools: YS, XC. Wrote the manuscript: MT, FL, YS.
References
- Adejuwon SA, Femi-Akinlosotu OM, Omirinde JO. 2015. Cisplatin-induced testicular dysfunction and its amelioration by Launaea taraxacifolia leaf extract. Andrologia. 47(5):553–559.
- Adler ID, Tarras A. 1990. Clastogenic effects of cis-diamminedichloroplatinum. II. Induction of chromosomal aberrations in primary spermatocytes and spermatogonial stem cells of mice. Mutat Res. 243(3):173–178.
- Agarwal A, Nick HS. 2000. Renal response to tissue injury: lessons from heme oxygenase-1 gene ablation and expression. J Am Soc Nephrol. 11(5):965–973.
- Aitken RJ, Roman SD. 2008. Antioxidant systems and oxidative stress in the testes. Oxid Med Cell Longev. 1(1):15–24.
- Aksu EH, Kandemir FM, Altun S, Küçükler S, Çomaklı S, Ömür AD. 2016. Ameliorative effect of carvacrol on cisplatin-induced reproductive damage in male rats. J Biochem Mol Toxicol. 30(10):513–520.
- Al-Bader M, Kilarkaje N. 2015. Effects of bleomycin, etoposide and cisplatin treatment on Leydig cell structure and transcription of steroidogenic enzymes in rat testis. Eur J Pharmacol. 747:150–159.
- Almasry SM, Hassan ZA, Elsaed WM, Elbastawisy YM. 2017. Structural evaluation of the peritubular sheath of rat’s testes after administration of ribavirin: a possible impact on the testicular function. Int J Immunopathol Pharmacol. 30(3):282–296.
- Amin A, Hamza AA. 2006. Effects of roselle and ginger on cisplatin-induced reproductive toxicity in rats. Asian J Androl. 8(5):607–612.
- Aminsharifi A, Hekmati P, Noorafshan A, Karbalay-Doost S, Nadimi E, Aryafar A, Hosseinabadi OK, Naseri MM, ZarePoor M. 2017. Scrotal cooling to protect against cisplatin-induced spermatogenesis toxicity: preliminary outcome of an experimental controlled trial. Urol. 197(1):233–234.
- Ateşşahin A, Sahna E, Türk G, Çeribaşi T, Yılmaz S, Yüce A, Bulmuş Ö. 2006. Chemoprotective effect of melatonin against cisplatin-induced testicular toxicity in rats. J Pineal Res. 41(1):21–27.
- Boghdady NA. 2013. Antioxidant and antiapoptotic effects of proanthocyanidin and ginkgo biloba extract against doxorubicin-induced cardiac injury in rats. Cell Biochem Funct. 31(4):344–351.
- Ciftci O, Beytur A, Cakir O, Gurbuz N, Vardiet N. 2011. Comparison of reproductive toxicity caused by cisplatin and novel platinum-N-heterocyclic carbene complex in male rats. Basic Clin Pharmacol Toxicol. 109(5):328–333.
- Clermont Y, Perey B. 1957. The stages of the cycle of the seminiferous epithelium of the rat: practical definitions in PA-Schiff-hematoxyl in and hematoxylin-eosin stained sections. Rev Can Biol. 16(4):451–462.
- Clewell RA, Campbell JL, Ross SM, Gaido KW, Clewell HJ, Andersen ME. 2010. Assessing the relevance of in vitro measures of phthalate inhibition of steroidogenesis for in vivo response. Toxicol in Vitro. 24(1):327–334.
- Eid AH, Abdelkader NF, El-Raouf OM, Fawzy HM, EI-Denshary ES. 2016. Carvedilol alleviates testicular and spermatological damage induced by cisplatin in rats via modulation of oxidative stress and inflammation. Arch Pharm Res. 39(12):1693–1702.
- Fallahzadeh AR, Rezaei Z, Rahimi HR, Barmak MJ, Sadeghi H, Mehrabi S, Rabani SM, Kashani IR, Barati V, Mahmoudi R. 2017. Evaluation of the effect of pentoxifylline on cisplatin-induced testicular toxicity in rats. Toxicol Res. 33(3):255–263.
- Fouad AA, Qutub HO, Fouad AE, Audeh AM, Al-Melhim WN. 2017. Epigallocatechin-3-gallate counters cisplatin toxicity of rat testes. Pharm Biol. 55(1):1710–1714.
- Gabetta B, Fuzzati N, Griffini A, Lolla E, Pace R, Ruffilli T, Peterlongo F. 2000. Characterization of proanthocyanidins from grape seeds. Fitoterapia. 71(2):162–175.
- Gao H, Yallampalli U, Yallampalli C. 2012. Gestational protein restriction reduces expression of Hsd17b2 in rat placental labyrinth. Biol Reprod. 87(3):1–7.
- Gao Z, Liu G, Hu Z, Li X, Yang X, Jiang B, Li X. 2014. Grape seed proanthocyanidin extract protects from cisplatin-induced nephrotoxicity by inhibiting endoplasmic reticulum stress-induced apoptosis. Mol Med Rep. 9(3):801–807.
- Garcia MS, Acquier A, Suarez G, Gomez NV, Gorostizaga A, Mendez CF, Paz C. 2012. Cisplatin inhibits testosterone synthesis by a mechanism that includes the action of reactive oxygen species (ROS) at the level of P450scc. Chem Biol Interact. 199(3):185–191.
- Hamza AA, Elwy HM, Badawi AM. 2016. Fenugreek seed extract attenuates cisplatin-induced testicular damage in Wistar rats. Andrologia. 48(2):211–221.
- Hassan HA, Isa AM, El-kholy WM, Nour SE. 2013. Testicular disorders induced by plant growth regulators: cellular protection with proanthocyanidins grape seeds extract. Cytotechnology. 65(5):851–862.
- Heeba GH, Hamza AA, Hassanin SO. 2016. Induction of heme oxygenase-1 with hemin alleviates cisplatin-induced reproductive toxicity in male rats and enhances its cytotoxicity in prostate cancer cell line. Toxicol Lett. 264:38–50.
- Hu C-M, Lin H-H, Chiang M-T, Chang P-F, Chau L-Y. 2007. Systemic expression of heme oxygenase-1 ameliorates type 1 diabetes in NOD mice. Diabetes. 56(5):1240–1247.
- Ilbey YO, Ozbek E, Cekmen M, Simsek A, Otunctemur A, Somay A. 2009a. Protective effect of curcumin in cisplatin-induced oxidative injury in rat testis: mitogen-activated protein kinase and nuclear factor-kappa B signaling pathways. Hum Reprod. 24(7):1717–1725.
- Ilbey YO, Ozbek E, Simsek A, Otunctemur A, Cekmen M, Somay A. 2009b. Potential chemoprotective effect of melatonin in cyclophosphamide- and cisplatin-induced testicular damage in rats. Fertil Steril. 92(3):1124–1132.
- Iman M, Araghi M, Heidari T, Mohammadi V. 2017. Melissa of cinalis and vitamin E as the potential therapeutic candidates for reproductive toxicity caused by anti-cancer drug, cisplatin, in male rats. Recent Pat Anticancer Drug Discov. 12(1):73–80.
- Isabel S, Sun B, Mateus M, Freitas V, Ricardo-da-Silva JM. 2008. Chemical characterization and antioxidant activities of oligomeric and polymeric procyanidin fractions from grape seeds. J Food Chem. 108(2):519–532.
- İsmail TK, Erdoğru T, Gülkesen H, Sezer C, Usta M, Ctiftcioglu A, Baykara M. 2004. The potential role of inducible nitric oxide synthase (iNOS) activity in the testicular dysfunction associated with varicocele: an experimental study. Int Urol Nephrol. 36(1):67–72.
- Kone BC. 2004. Nitric oxide synthesis in the kidney: isoforms, biosynthesis, and functions in health. Semin Nephrol. 24(4):299–315.
- Kong D, Zhang J, Hou X, Tan J, Chen Y, Yang W, Zeng J, Han Y, Liu X, Xu D, et al. 2017. Acetamiprid inhibits testosterone synthesis by affecting the mitochondrial function and cytoplasmic adenosine triphosphate production in rat Leydig cells. Biol Reprod. 96(1):254–265.
- Krege S, Beyer J, Souchon R, Krege S, Albrecht W, Algaba F, Bamberg M, Bodrogi I, Bokemeyer C, Cavallin-Stahl E, et al. 2008. European consensus conference on diagnosis and treatment of germ cell cancer: a report of the second meeting of the European germ cell cancer consensus group (EGCCCG): part II. Eur Urol. 53(3):497–513.
- Li Z, Zheng X, Li P, Itoua ES, Moukassa D, Andely FN. 2017. Effects of acupuncture on mRNA levels of apoptotic factors in perihematomal brain tissue during the acute phase of cerebral hemorrhage. Med Sci Monit. 23:1522–1532.
- Liu F, Chang X, Tian M, Zhu A, Zou L, Han A, Su L, Li S, Sun Y. 2017. Nano NiO induced liver toxicity via activating the NF-κB signaling pathway in rats. Toxico Res. 6(2):107–113.
- Liu H, Zhu D, Wang C, Guan H, Li S, Hu C, Chen Z, Hu Y, Lin H, Lian Q, et al. 2015a. Effects of etomidate on the steroidogenesis of rat immature Leydig cells. Plos One. 10(11):1–18.
- Liu Z, Sun Y, Su L, Sun Y, Kong S, Chang X, Guo F, Li W, Guo J, Li J. 2015b. Effects of cisplatin on testicular enzymes and Sertoli cell function in rats. Fundamental Toxicol Sci. 2(4):137–145.
- Lonare M, Kumar M, Raut S, More A, Doltade S, Badgujar P, Telang A. 2016. Evaluation of ameliorative effect of curcumin on imidacloprid-induced male reproductive toxicity in wistarrats. Environ Toxicol. 31(10):1250–1263.
- Malarvizhi D, Mathur PP. 1996. Effects of cisplatin on testicular functions in rats. Indian J Exp Biol. 34(10):995–998.
- Midzak AS, Chen H, Papadopoulos V, Zirkin BR. 2009. Leydig cell aging and the mechanisms of reduced testosterone synthesis. Mol Cell Endocrinol. 299(1):23–31.
- Ozkok A, Edelstein CL. 2014. Pathophysiology of cisplatin-induced acute kidney injury. Biomed Res Int. 2014(10):1–17.
- Radi R. 2004. Nitric oxide, oxidants, and protein tyrosine nitration. Proc Natl Acad Sci USA. 101(12):4003–4008.
- Russell LD, Ettlin RA, Hikim A, Clegg ED. 1993. Histological and histopathological evaluation of the testis. Int J Androl. 16(1):83.
- Ruttkaynedecky B, Nejdl L, Gumulec J, Zitka O, Masarik M, Eckschlager T, Stiborova M, Adam V, Kizek R. 2013. The role of metallothionein in oxidative stress. Int J Molec Sci. 14(3):6044–6066.
- Saad AA, Youssef MI, Elshennawy LK. 2009. Cisplatin induced damage in kidney genomic DNA and nephrotoxicity in male rats: the protective effect of grape seed proanthocyanidin extract. J Food Chem Toxicol. 47(7):1499–1506.
- Sönmez MF, Kılıç E, Karabulut D, Çilenk K, Deligönül E, Dündar M. 2016. Nitric oxide synthase in diabetic rat testicular tissue and the effects of pentoxifylline therapy. Syst Biol Reprod Med. 62(1):22–30.
- Sönmez MF, Tascioglu S. 2015. Protective effects of grape seed extract on cadmium-induced testicular damage, apoptosis, and endothelial nitric oxide synthases expression in rats. Toxicol Ind Health. 32(8):1486–1494.
- Takano H, Inoue K, Yanagisawa R, Sato M, Shimada A, Morita T, Sawada M, Nakamura K, Sanbongi C, Yoshikawa T. 2004. Protective role of metallothionein in acute lung injury induced by bacterial endotoxin. Thorax. 59(12):1057–1062.
- Tremblay JJ. 2015. Molecular regulation of steroidogenesis in endocrine Leydig cells. Steroids. 103:3–10.
- Tremellen K. 2008. Oxidative stress and male infertility – a clinical perspective. Hum Reprod Update. 14(3):243–258.
- Vawda AI. 1994. Effect of testosterone on cisplatin-induced testicular damage. Arch Androl. 32(1):53–57.
- Ye L, Su Z, Ge R. 2011. Inhibitors of testosterone biosynthetic and metabolic activation enzymes. Molecules. 16(12):9983–10001.
- Yin B, Barrionuevo G, Batinic-Haberle I, Sandberg M, Weber SG. 2017. Differences in reperfusion-induced mitochondrial oxidative stress and cell death between hippocampal CA1 and CA3 subfields are due to the mitochondrial thioredoxin system. Antioxid Redox Signal. 27(9):534–549.
- Zhao Y, Gao L, Zhang H, Guo J, Guo P. 2014. Grape seed proanthocyanidin extract prevents DDP-induced testicular toxicity in rats. J Food Funct. 5(3):605–611.
