ABSTRACT
Spermatogenesis and steroidogenesis are testicular functions regulated by gonadotrophins as well as other factors, including serotonin. Testicular serotonin acts as an autocrine regulator of testosterone secretion, but studies on its role in spermatogenesis and sperm quality are scarce. Here, we analyzed the effects of intratesticular inhibition of serotonin synthesis on gonadotrophins, testosterone, and sperm quality. Both testicles of 30-day-old rats were injected once with saline solution (SS) or distinct concentrations of p-chloroamphetamine (PCA) (0.03, 0.06, or 0.12 mg). At 65 days of age, rats were euthanized and sperm density, motility, membrane integrity, mitochondrial function, and abnormalities were evaluated in gametes from the vas deferens. Inhibition of synthesis of intratesticular serotonin by PCA diminished the concentrations of testosterone and follicle-stimulating hormone (FSH) but luteinizing hormone (LH) levels were unaltered. Sperm density was not modified in animals injected with the different concentrations of PCA. In contrast, the percentage of sperm with abnormalities increased and the sperm membrane integrity decreased in animals injected at higher PCA concentrations. The functionality of sperm mitochondria in PCA-injected animals decreased only at the highest PCA dose. Our results indicate that testicular serotonin plays a role in testosterone synthesis and in the normal development of sperm, and blocking its effects disrupts the hormonal communication between the testis and hypophysis.
Abbreviations: SS: saline solution; PCA: p-chloroamphetamine; FSH: follicle-stimulating hormone; LH: luteinizing hormone; TPH: tryptophan hydroxylase; MAO: monoamine oxidase; AC: absolute control group; PI: propidium iodide; FLICA: fluorescence inhibitor of caspase; 3β-HSD: 3β-hydroxysteroid dehydrogenase; 17-KSR: 17-ketosteroid reductase; DHT: 5-dihydrotestosterone
Introduction
Testicular functions, spermatogenesis and steroidogenesis, are regulated by gonadotrophins. LH and FSH exert their effects on Leydig cells in the testicular interstice and Sertoli cells in seminiferous epithelium, respectively. Testosterone produced by Leydig cells and growth factors produced by Sertoli cells are needed for proliferation and differentiation of germ cells (Wang et al. Citation2009; O’Shaughnessy et al. Citation2010; O’Shaughnessy Citation2014).
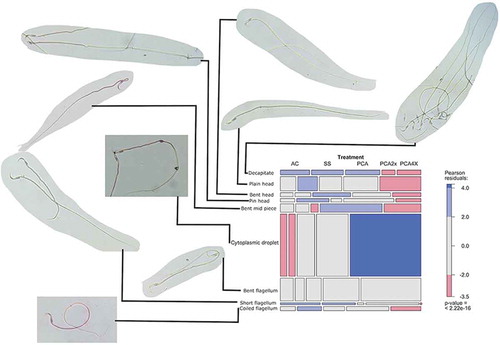
Figure 1. Effects of distinct concentrations of PCA on the proportion of morphological abnormalities. Each tile of the mosaic plot represents the proportion of each abnormality in a treatment group. The width of each tile is proportional to the marginal frequency n+j in each column of the table (Treatment), and the height is proportional to the conditional frequency ni/j for each row (types of abnormalities) for a given column (Treatment), so the area is proportional to cell frequency, and complete independence is shown when all tiles in a row have the same height (Friendly Citation1994). Tiles with positive, negative, or no deviation from independence are filled with blue, red, or gray color, respectively (see the table at the right side of the mosaic). Micrographs (400X) signaled by the lines, to the left of the mosaic plot, are representative of each sperm abnormality.
Serotonin is a neurotransmitter produced by neurons in the central nervous system and is also present in organs such as the testis (Campos et al. Citation1990; Tinajero et al. Citation1993; Frungieri et al. Citation1999; Gerendai et al. Citation2007). Serotonin, serotonin receptors, and enzymes involved in serotonin biosynthesis and metabolism, are present in distinct locations of the testis. Serotonin is present in the capsule, mastocytes, platelets, and Leydig cells (Aguilar et al. Citation1995; Campos et al. Citation1990; Tinajero et al. Citation1993; Frungieri et al. Citation1999); testicular serotonin also arises from the spermatic nerve (Campos et al. Citation1990; Frungieri et al. Citation1999). Serotonin receptors 5-HT1 and 5-HT2 are present in Leydig cells (Tinajero et al. Citation1992; Frungieri et al. Citation2002). The enzyme tryptophan hydroxylase (TPH) is the limiting factor in the serotonin biosynthetic pathway and is located in the Leydig cells (Tinajero et al. Citation1992). Monoamine oxidase (MAO) metabolizes serotonin and is present in the wall of seminiferous tubules (Ellis et al. Citation1972).
Serotonin plays a role in testicular steroidogenesis, as demonstrated by in vivo and in vitro studies (Dufau et al. Citation1993; Tinajero et al. Citation1993; Csaba et al. Citation1998; Frungieri et al. Citation2002; Gerendai et al. Citation2007). In Leydig cells, the coupling of serotonin to the receptor 5-HT2 stimulates the production of corticotropin-release factor and inhibits the synthesis of testosterone (Tinajero et al. Citation1992; Dufau et al. Citation1993). In male rats, the effects of serotonin on testosterone production are related to age; for example, serotonin stimulates testosterone production in prepubertal animals but is inhibited in adults (Tinajero et al. Citation1993; Csaba et al. Citation1998). Some reports indicate that testicular serotonin levels could be dysregulated in distinct ways. For example, in the rat, anatomical section of the superior spermatic nerve caused a 34% reduction in serotonin concentrations in the capsule and interstitial fluid (Campos et al. Citation1990), whereas the chemical destruction of serotonergic neurons by 5,7-dihydroxytryptamine (Csaba et al. Citation1998) or 5,6-dihydroxytryptamine (Gerendai et al. Citation1996) reduced secretion of testosterone.
Despite detailed knowledge of serotonin and steroidogenesis, information on the role of serotonin in spermatogenesis is scarce. The experimental induction of continuous serotonin release in adult male rats by a subcutaneous implant diminished testicular weight (Niaraki et al. Citation1982), whereas exposure to increased serotonergic activity, induced by the administration of inhibitors of the serotonin reuptake, induced degeneration of seminiferous tubules (De Oliveira et al. Citation2013). Thus, increased serotonin activity could have an inhibitory effect on testicular functions. Intraperitoneal administration of, 3,4-methylenedioxymethamphetamine, an inhibitor of the serotonergic system, in adult male rats, induced damage in the seminiferous epithelium and decreased the number of testicular spermatids but did not affect either sperm motility or sperm morphology (Barenys et al. Citation2009). In contrast, we have observed that administration of PCA, another inhibitor of the serotonergic system, from 30 days of age to 45 or 65 days of age, induced damage to seminiferous tubules due to the apoptotic death of germ cells, diminished sperm motility and increased the presence of morphological abnormalities in sperm (Aragón et al. Citation2005). These opposing effects of serotonin in the testes could be due to the model used. The effects observed could be due to a direct action of the substances in the testes as we proposed (Aragón et al. Citation2005). In the studies by Barenys et al. (Citation2009) and Aragón et al. (Citation2005), serotonergic inhibitors were administered by a systemic route, thus testicular effects occurred as a result of their action on components of the hypothalamus–hypophysis–testicle axis.
Information on the role of local serotonin in spermatogenesis and sperm quality is scarce. Evaluation of sperm quality, using measures such as motility and viability, is routinely performed to determine the potential fertilizing ability of an individual. The quality of this evaluation is improved when objective assessments, such as those from automated methods, were employed. The objective of this work was to investigate the effect of intratesticular injection of PCA on hormones regulating testicular function and sperm quality.
Results and discussion
We and others previously demonstrated that systemic administration of inhibitors of the serotonergic system induced deleterious effects in the hypothalamus–hypophysis–testicle axis (Aragón et al. Citation2005; Barenys et al. Citation2009). Gross parameters, such as body weight and organ weight were also affected by PCA (Aragón et al. Citation2005). In this work body weight and organ weight were affected by PCA (). No changes were observed in the weight of testicles or seminal vesicles; however, body weight was reduced at PCA4X (p = 0.0116), prostate weight was decreased in PCA2X and PCA4X treatment groups (p = 0.0048 and p = 0.0039, respectively). The weight of the right epididymis was reduced in the PCA4X group (p = 0.0190). Testosterone is necessary for growth and function of the prostate (Mirosevich et al. Citation1999). It stimulates cell proliferation and inhibits cell death (Wright et al. Citation1996; Liu et al. Citation2018), thus the diminution in testosterone levels induced by PCA could lead to impaired prostate function.
Table 1. Effects of intra-testicular injection of distinct concentrations of PCA on body weight and testicles organs weight, grams, mean ± SD.
Hormone concentrations were perturbed at a high dosage of PCA. Gonadotrophin concentrations were less sensitive to the effects of PCA: FSH was reduced at PCA4X (p = 0.0234) but LH levels were not modified. Low concentrations of testosterone were detected after treatment with PCA2X and PCA4X (p = 0.0014 and p = 0.0025, respectively) ().
Table 2. Effects of intra-testicular injection of distinct concentrations of PCA on hormone concentrations and sperm parameters (mean ± SD).
The functionality of Sertoli cells depends on FSH levels. Testosterone and FSH are widely recognized as regulators of spermatogenesis (Abel et al. Citation2008; Ramaswamy and Weinbauer Citation2014). The fact that FSH and testosterone concentrations were significantly reduced after treatment with PCA indicates a clear deregulation of communication in the hypothalamus–hypophysis–testicle axis.
Testosterone is produced in Leydig cells in response to stimulation with LH. In vitro, serotonin stimulates testosterone synthesis (Tinajero et al. Citation1993). The low concentrations of testosterone observed in our work could be attributed to a direct effect of PCA on Leydig cells, which diminished testosterone synthesis, thus lowering circulating concentrations of this hormone. Two observations support our idea; first, the bilateral intratesticular injection of ketanserine, an antagonist of the 5-HT2 serotonin receptor, to prepubertal male rats diminished testosterone concentrations (Csaba et al. Citation1998). Second, we have observed that the systemic and chronic administration of PCA to prepubertal male rats diminished the concentrations of testicular serotonin (unpublished observations). Furthermore, our concept is supported by the fact that in this work PCA treatment did not concomitantly diminish the concentrations of LH and testosterone.
The mechanism underlying the reduction of testosterone levels by PCA is not clear. However, there is evidence pointing to a direct effect of PCA on factors involved in the synthesis of testosterone. Leydig cells in rats exposed to amphetamines in vitro showed diminished activity of the enzymes 3β-hydroxysteroid dehydrogenase (3β-HSD), p450 c17, and 17-ketosteroid reductase (17-KSR), resulting in reduced testosterone synthesis (Tsai et al. Citation1997).
The administration of amphetamines has been shown to reduce distinct parameters of sperm quality. The intraperitoneal administration of methamphetamine induces deleterious changes in sperm quality of adult male mice (Sabour et al. Citation2017). Previously, we demonstrated that intraperitoneal administration of PCA to prepubertal rats reduced sperm quality at the adult stage (Aragón et al. Citation2005).
Here, we observed a reduction in sperm quality in groups of rats treated with direct testicular injection of PCA. Neither sperm density nor motility changed significantly; although motility was slightly reduced at PCA2X and PCA4X (p = 0.1020 and p = 0.1610, respectively) (). The percentage of sperm abnormalities increased after treatment with PCA2X and PCA4X (p = 0.0033 and p = 0.00273, respectively). Cytoplasmic droplets and bent flagella were the most common abnormalities observed in the groups treated with PCA ().
The predominant sperm abnormality induced by PCA was the presence of cytoplasmic droplet. Post-testicular maturation of sperm occurs during traverse along the epididymis, where the cytoplasmic droplet is displaced toward a distal position in the flagellum, until its complete removal from the maturing spermatozoa (Cooper Citation2011). The fact that the treatment with PCA diminished the concentrations of testosterone and also increased the percentage of sperm with retention of the cytoplasmic droplet, suggest a role for the lack of testosterone in the induction of this abnormality. The epididymis is an organ dependent on testosterone (Robaire et al. Citation2006), where the metabolite of testosterone, 5-dihydrotestosterone (DHT), maintains epididymal structure and functionality (Henderson et al. Citation2006; Robaire and Hamzeh Citation2011). Furthermore, when DHT is inhibited the proportion of sperm retaining the cytoplasmic droplet increased (Henderson et al. Citation2006).
Testicular germ cells are a less differentiated form of sperm. To identify whether germ cells are sensitive to the effects of PCA, we calculated the time elapsed between the administration of PCA and sperm maturation, on the basis of the known cycle of seminiferous epithelium. The map of the cycle of seminiferous epithelium in the rat has 14 stages and 3.5 rows (Russell et al. Citation1990). Considering 12.9 days as the length of the cycle of seminiferous epithelium (the sum of all stages) (Russell et al. Citation1990) and 9.3 days from stage I to stage VIII (in stage VIII sperm are released from the seminiferous epithelium) plus 10 days during which the sperm traverse the epididymis (Robaire et al. Citation2006), then the germ cells exposed to PCA were pachytene spermatocytes and spermatogonia, in rats exposed at 30 days of age and euthanized at 65 days of age. Given the effects on sperm quality parameters, our results highlight the point that pachytene spermatocytes represent a sensitive stage that requires serotonin signaling to continue normal development toward a normal mature sperm.
In this study, we observed that high concentrations of PCA diminished sperm quality in terms of membrane integrity, mitochondrial functionality and presence of active caspases. The percentage of sperm with membrane integrity, measured with SYBR14, decreased at PCA2X and PCA4X (p = 0.0018 and p = 0.0051, respectively) (), whereas the percentage of sperm with mitochondrial functionality were significantly reduced in the PCA4X group (p = 0.0032). The presence of sperm with active caspases was greater in the PCA group (p = 0.0231).
Table 3. Effects of intra-testicular injection of distinct concentrations of PCA on sperm membrane integrity, mitochondrial functionality and expression of active caspases (mean ± SD).
Our results show clearly that administration of PCA reduced the permeability of the cytoplasmic membrane and altered sperm mitochondrial functionality. The negative effects of intratesticular injection of PCA on sperm quality could have distinct origins: (1) Deregulation of Sertoli cells, which leads to altered communication with developing germ cells; (2) An inhibitory effect on the steroidogenic function of Leydig cells, which leads to diminished testosterone production; (3) A possible alteration in the epididymal environment, which could alter sperm maturation; (4) Multiple effects as the result of a combination of all or some of these factors. It is possible that the low sperm quality observed in PCA groups was due to diminished testosterone production, which primarily affected the Sertoli cells, which in turn affected the developing germ cells. Evidence supporting our idea comes from the fact that binding of testosterone to its receptors in Sertoli cells promotes the survival of germ cells (Hill et al. Citation2004). In addition, testosterone is recognized as an essential factor for the elongation of round sperm during their differentiation toward sperm (O’Shaughnessy Citation2014).
The fact that the lower concentration of PCA increased the percentage of FLICA positive sperm cannot be explained at this time. However, some reports have pointed out the possibility that the FLICA reagent could associate with substances in addition to active caspases (Pozarowski et al. Citation2003; Kuzelová et al. Citation2007).
In summary, our results show that inhibition of testicular serotonin by PCA alters endocrine communication, diminishes circulating concentrations of testosterone and FSH, and leads to a decrease of sperm quality parameters. Substances affecting serotonin synthesis or serotonergic communication are broadly used in children and adolescents (Dobry et al. Citation2013; Sessa Citation2017), thus information generated in this work is relevant due to possible long-term effects in the adult stage.
Materials and methods
Ethical considerations
All experiments were performed following the guidelines established by the Mexican Guidelines for Animal Protection and Treatment. The Committee of the Facultad de Estudios Superiores Zaragoza, UNAM, approved the experimental protocols (Letter 03/01/2012).
Experimental design
Animals and treatment
Thirty-day-old male rats of the CII-ZV strain (n = 8), from our own breeding stock, were maintained under controlled light conditions (lights on from 05:00 to 19:00), with free access to food (Nutri-cubes, Purina S.A., Mexico) and tap water. The males were randomly allocated to different groups: The absolute control group (AC), received a single injection of 20 μl of saline solution (0.9%) (SS) or PCA at a concentration of 0.03 mg (PCA), 0.06 mg (PCA2X) or 0.12 mg (PCA4X) (Sigma-Aldrich, St. Louis, USA) in 20 μl of SS.
The PCA dose was calculated taking into account the testicular weight in 30-day-old rats and the total dosage of 100 mg/kg of total body weight. We have not observed a reduction in testicular serotonin content after systemic administration of a dose of 10 mg/kg body weight of PCA, but serotonin content diminished after 8–10 systemic injections (unpublished data). The previous data and the fact that isoform 1 of TPH, present in peripheral tissues, is more resistant to the effects of amphetamines (Walther and Bader Citation2003) led us to an approach using dosages two (PCA2X) or four times (PCA4X) greater than the initial 0.03 mg dose of PCA. PCA inhibits TPH by decreasing the concentration of serotonin and its principal metabolite, 5-hydroxyindoleacetic acid (5-HIAA), 4 hours after its administration (Sanders-Bush and Sulser Citation1970) and this effect remains for a duration of 14 days (Rothman et al. Citation2003) or more than a month (Sanders-Bush et al. Citation1972). All animals were euthanized at the age of 65 days. The evaluation of different parameters was performed at 65 days of age because that is the age at which sperm are found in the vas deferens (Aragón et al. Citation2005).
Intratesticular injection
For intratesticular injection, rats were anesthetized with sodium ether (Sigma-Aldrich, St. Louis, USA). The scrotal skin was cleaned with surgical soap, and PCA or saline solution was injected into both testes using a 28-gauge needle connected via tubing to a 100 µL Hamilton syringe mounted on a microinjection pump (CMA Microdialysis, North Chelmsford, MA, USA). Another group of rats was injected with the same volume of saline solution into both testes. Injection was carried out at infusion rates of 10 µL/min. To allow diffusion of the PCA into the testicular tissue, the injection needle was held in the testis for one minute.
Autopsy procedure
The animals were euthanized by bleeding under ether anesthesia. The testes, epididymis, prostate, and seminal vesicles were dissected and individually weighed on a precision balance.
Measurement of gonadotrophins and sex steroid hormones
Trunk blood from each animal was collected, allowed to clot at room temperature and centrifuged at 1000 g for 15 min. The serum was separated and stored at –20°C until assayed. FSH and LH concentrations were measured by radioimmunoanalysis (RIA) and expressed as ng/mL as previously reported (Aragón et al. Citation2005). Intra- and interassay coefficients were 5.74% and 7.91% for FSH, and 6.82% and 9.32% for LH, respectively. Serum testosterone concentrations were measured using enzyme-linked immunoassays Kit (AccuBind cat 3725-300, USA) and according to the manufacture protocol. The sensitivity of the assay was 0.038 ng/mL. Testosterone hormone concentrations were expressed in ng/mL. Results are expressed as mean ± SD.
Sperm quality
Motility, morphology, and sperm density
The vas deferens and its contents were obtained and placed in 2 mL Hank’s buffered salt solution (Sigma Chemical Co., St. Louis, MO, USA), supplemented as previously reported (Seed et al. Citation1996), at 37°C during 30 min and mixed to homogenize the sperm suspension. For sperm motility, 15 µL of sperm suspension was put on a prewarmed slide and observed at 10X magnification in a phase-contrast microscope B3 CLINLAB (Motic, British Columbia, Canada) equipped with a customized stage prewarmed at 37°C. For each sperm sample, three or four image sequences were taken at 100X magnification (200 spermatozoa (Linder et al. Citation1992; Seed et al. Citation1996). Image sequences were captured with a Stingray model F 033B camera (Allied Vision Technologies Inc., Exton, PA, USA) and stored in a computer until analysis. Each image sequence was taken at 60 frames per second with a duration of one second; image sequences were captured with Micro-Manager version 1.4 software and analyzed with ImageJ version 1.50d (Rasband Citation2005) in a MacBook with Mac OS X version 10.11.5. The percentage of motile sperm was defined as the ratio of sperm showing movement to the total number of cells × 100 (Meyer et al. Citation2014). Data are presented as the percentage of motile sperm by treatment.
Fifteen microliters of sperm suspension were placed on a slide, stained with hematoxylin, washed with running water and air dried. For each animal, 200 sperm were evaluated under a light microscope at 100X and 400X magnification. The morphological abnormalities in the head, mid piece, and flagellum were recorded and included the following features: decapitated sperm, plain head, bent head, pin head, bent mid piece, presence and location of the cytoplasmic droplet, bent flagellum, short flagellum, and coiled flagellum. Bright-field micrographs were captured with the App Camera of an Apple IPhone (A1549), attached to the eyepiece of the microscope with a customized adapter. Sperm density was determined in a Neubauer chamber (PROPER Lumicyte 1/100 mm depth).
Mitochondria functional assessment
Metabolic function of sperm was evaluated by measuring mitochondrial dehydrogenase activity. Briefly, 100 µL of sperm suspension were incubated with 1 µL of 50 mM resazurin for 15 min at 37°C. Resazurin was from the LIVE/DEAD cell viability assay kit (Molecular Probes Inc., Eugene, OR, USA). Just before evaluation, samples were adjusted to 500 µL with FACS Flow buffer.
Membrane integrity assay
Membrane integrity was evaluated using the SYBR14 dye in conjunction with propidium iodide (PI). Aliquots of 25 µL of sperm suspension were stained with SYBR14 and PI contained in the LIVE/DEAD® Sperm Viability Kit (Molecular Probes) according to the manufacturer’s instructions. Final concentrations of SYBR14 and PI were 100 nmol/L and 12 µmol/L, respectively, and samples were incubated for 10 min before measurement by flow cytometry.
Caspase activity measurement
Caspase-3 and -7 activity was evaluated in aliquots of sperm samples with the Fluorescence Inhibitor of Caspase Assay (FLICA), provided in the Image-iT LIVE Red Caspase-3 and -7 Detection Kit (Molecular Probes), according to the manufacturer’s instructions.
Flow cytometry evaluation of sperm
Sperm mitochondria function and membrane integrity were assessed using a FACSAria II flow cytometer (Becton Dickinson Immunocytometry BioSciences, San Jose, CA, USA). Flow cytometer settings were as previously reported (Ajf et al. Citation2015). Results are presented as mean ± SD.
Statistics
The normality of the data was corroborated graphically by checking residuals. Hormone concentrations and parameters of sperm quality in experimental groups were compared with controls using one-way ANOVA for unbalanced models. Orthogonal contrasts of means were performed for each ANOVA model. Results are presented as mean ± SD. P < 0.05 was considered significant. The proportion of each type of morphological abnormality in each treatment was tested for independence by the Pearson Chi-squared test. Statistical analyses were performed with R base and the VCD package version 1.3–2 (Meyer et al. Citation2014) and car version 2.1–0 (Fox and Weisberg Citation2011). All analyses were performed with R version 3.2.4 (Team Citation2011) running on a MacBook with Mac OS X version 10.10.5. Results are expressed as mean ± SD.
Acknowledgments
This work was supported by grant [grant number UNAM-DGAPA-PAPIIT-IN226017]. The gift of reagents for FSH and LH RIA measurements by the National Institutes of Health (Bethesda MD, USA) is gratefully acknowledged. We want to thank the Posgrado en Ciencias Biológicas, UNAM and CONACyT, México for the support given in the realization of this study. This work is a requirement for obtaining the degree of Doctor of Biological Sciences.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Juan Díaz-Ramos
Devised the study, planned the experiments, participated in the analysis and discussion of the results: MEA-E, AA-M; Participated in the evaluation of sperm quality, analysis of the results, and the measurement of hormones: JAD-R; Participated in the measurement of hormones: MF-F. All authors read and approved the final manuscript.
References
- Abel MH, Baker PJ, Charlton HM, Monteiro A, Verhoeven G, De Gendt K, Guillou F, O’Shaughnessy PJ. 2008. Spermatogenesis and sertoli cell activity in mice lacking sertoli cell receptors for follicle-stimulating hormone and androgen. Endocrinology. 149:3279–3285.
- Aguilar R, Antón F, Bellido C, Aguilar E, Gaytan F. 1995. Testicular serotonin is related to mast cells but not to Leydig cells in the rat. J Endocrinol. 146:15–21.
- Vázquez AJF, Cedillo MJ, Quezada VJ, Rivas AC, Morales ECL, Ayala EME, Hernández MJ, González RA, Aragón MA. 2015. Effects of repeated electroejaculations on kinematic sperm subpopulations and quality markers of Mexican creole goats. Anim Reprod Sci. 154:29–38.
- Aragón MA, Ayala ME, Marín M, Avilés A, Mensumura D, Domínguez R. 2005. Serotoninergic system blockage in the prepubertal rat inhibits spermatogenesis development. Reproduction. 129:717–727.
- Barenys M, Macia N, Camps L, de Lapuente J, Gomez-Catalan J, Gonzalez-Linares J, Borras M, Rodamilans M, Llobet JM. 2009. Chronic exposure to MDMA (ecstasy) increases DNA damage in sperm and alters testes histopathology in male rats. Toxicol Lett. 191:40–46.
- Campos MB, Vitale ML, Calandra RS, Chiocchio SR. 1990. Serotonergic innervation of the rat testis. J Reprod Fertil. 88:475–479.
- Cooper TG. 2011. The epididymis, cytoplasmic droplets and male fertility. Asian J Androl. 13:130–138.
- Csaba Z, Csernus V, Gerendai I. 1998. Intratesticular serotonin affects steroidogenesis in the rat testis. J Neuroendocrinol. 10:371–376.
- De Oliveira WM, de Sá IR, de Torres SM, de Morais RN, Andrade AM, Maia FCL, Tenorio BM, da Silva Junior VA. 2013. Perinatal exposure to fluoxetine via placenta and lactation inhibits the testicular development in male rat offspring. Syst Biol Reprod Med. 59:244–250.
- Dobry Y, Rice T, Sher L. 2013. Ecstasy use and serotonin syndrome: a neglected danger to adolescents and young adults prescribed selective serotonin reuptake inhibitors. Int J Adolesc Med Health. 25:193–199.
- Dufau ML, Tinajero JC, Fabbri A. 1993. Corticotropin-releasing factor: an antireproductive hormone of the testis. FASEB J Off Publ Fed Am Soc Exp Biol. 7:299–307.
- Ellis LC, Jaussi AW, Baptista MH, Urry RL. 1972. Correlation of age changes in monoamine oxidase activity and androgen synthesis by rat testicular minced and teased-tubular preparations in vitro. Endocrinology. 90:1610–1618.
- Fox J, Weisberg S. 2011. An R companion to applied regression [Internet]. 2nd edn. Thousand Oaks (CA, USA): Sage. http://socserv.socsci.mcmaster.ca/jfox/Books/Companion.
- Friendly M. 1994. Mosaic displays for multi-way contingency tables. Journal of the American Statistical Association. 89:190–200.
- Frungieri MB, Gonzalez-Calvar SI, Rubio M, Ozu M, Lustig L, Calandra RS. 1999. Serotonin in golden hamster testes: testicular levels, immunolocalization and role during sexual development and photoperiodic regression-recrudescence transition. Neuroendocrinology. 69:299–308.
- Frungieri MB, Zitta K, Pignataro OP, Gonzalez-Calvar SI, Calandra RS. 2002. Interactions between testicular serotoninergic, catecholaminergic, and corticotropin-releasing hormone systems modulating cAMP and testosterone production in the golden hamster. Neuroendocrinology. 76:35–46.
- Gerendai I, Banczerowski P, Csernus V, Halász B. 2007. Innervation and serotoninergic receptors of the testis interact with local action of interleukin-1beta on steroidogenesis. Auton Neurosci Basic Clin. 131:21–27.
- Gerendai I, Csaba Z, Csernus V. 1996. Testicular injection of 5,6-dihydroxytryptamine or vasectomy interferes with the local stimulatory effect of oxytocin on testicular steroidogenesis in immature rats. Neuroendocrinology. 63:284–289.
- Henderson NA, Cooke GM, Robaire B. 2006. Region-specific expression of androgen and growth factor pathway genes in the rat epididymis and the effects of dual 5alpha-reductase inhibition. J Endocrinol. 190:779–791.
- Hill CM, Anway MD, Zirkin BR, Brown TR. 2004. Intratesticular androgen levels, androgen receptor localization, and androgen receptor expression in adult rat Sertoli cells. Biol Reprod. 71:1348–1358.
- Kuzelová K, Grebenová D, Hrkal Z. 2007. Labeling of apoptotic JURL-MK1 cells by fluorescent caspase-3 inhibitor FAM-DEVD-fmk occurs mainly at site(s) different from caspase-3 active site. Cytom A. 71:605–611.
- Linder RE, Strader LF, Slott VL, Suarez JD. 1992. Endpoints of spermatotoxicity in the rat after short duration exposures to fourteen reproductive toxicants. Reprod Toxicol. 6:491–505.
- Liu R-F, Fu G, Li J, Yang Y-F, Wang X-G, Bai P-D, Chen Y-D. 2018. Roles of autophagy in androgen-induced benign prostatic hyperplasia in castrated rats. Exp Ther Med. 15:2703–2710.
- Meyer D, Zeileis A, Hornik K. 2014. vcd: visualizing categorical data. Thousand Oak (CA, USA): Sage.
- Mirosevich J, Bentel JM, Zeps N, Redmond SL, D’Antuono MF, Dawkins HJ. 1999. Androgen receptor expression of proliferating basal and luminal cells in adult murine ventral prostate. J Endocrinol. 162:341–350.
- Niaraki MA, Subramanian MG, Moghissi KS. 1982. Effects of serotonin on reproductive hormone levels and testis morphology in adult male rats. Proc Soc Exp Biol Med Soc Exp Biol Med N Y N. 170:464–470.
- O’Shaughnessy PJ. 2014. Hormonal control of germ cell development and spermatogenesis. Semin Cell Dev Biol. 29:55–65.
- O’Shaughnessy PJ, Monteiro A, Verhoeven G, De Gendt K, Abel MH. 2010. Effect of FSH on testicular morphology and spermatogenesis in gonadotrophin-deficient hypogonadal mice lacking androgen receptors. Reprod Camb Engl. 139:177–184.
- Pozarowski P, Huang X, Halicka DH, Lee B, Johnson G, Darzynkiewicz Z. 2003. Interactions of fluorochrome-labeled caspase inhibitors with apoptotic cells: a caution in data interpretation. Cytom A. 55:50–60.
- Ramaswamy S, Weinbauer GF. 2014. Endocrine control of spermatogenesis: role of FSH and LH/testosterone. Spermatogenesis. 4:e996025.
- Rasband WS. 2005. ImageJ (computer program). Bethesda (MD): US National Institutes of Health.
- Robaire B, Hamzeh M. 2011. Androgen action in the epididymis. J Androl. 32:592–599.
- Robaire B, Hinton BT, Orgebin-Crist M-C. 2006. The epididymis. In: Knobil E, Neill JD, editors. Physiology of reproduction. 3rd ed. Vol. 2. New York (USA): Elsevier, Inc; p. 1071–1148.
- Rothman RB, Jayanthi S, Wang X, Dersch CM, Cadet JL, Prisinzano T, Rice KC, Baumann MH. 2003. High-dose fenfluramine administration decreases serotonin transporter binding, but not serotonin transporter protein levels, in rat forebrain. Synap N Y N. 50:233–239.
- Russell LD, Ettlin RA, Sinha Hikim AP, Clegg ED. 1990. Histological and histopathological evaluation of the testis. Clearwater (FL, USA): Cache River Press.
- Sabour M, Khoradmehr A, Kalantar SM, Danafar AH, Omidi M, Halvaei I, Nabi A, Ghasemi-Esmailabad S, Talebi AR. 2017. Administration of high dose of methamphetamine has detrimental effects on sperm parameters and DNA integrity in mice. Int J Reprod Biomed Yazd Iran. 15:161–168.
- Sanders-Bush E, Bushing JA, Sulser F. 1972. LongTerm effects of p-cl-amphetamine on the tryptophan hydroxylase activity and on the levels of 5-hydroxytryptamine and 5-hydroxyindolacetic in brain. Eur J Pharmacol. 20:385–388.
- Sanders-Bush E, Sulser F. 1970. P-chloroamphetamine: in vivo investigations on the mechanism of action of the selective depletion of cerebral serotonin. J Pharmacol Exp Ther. 175:419–426.
- Seed J, Chapin RE, Clegg ED, Dostal LA, Foote RH, Hurtt ME, Klinefelter GR, Makris SL, Perreault SD, Schrader S, et al. 1996. Methods for assessing sperm motility, morphology, and counts in the rat, rabbit, and dog: a consensus report. ILSI risk science institute expert working group on sperm evaluation. Reprod Toxicol. 10:237–244.
- Sessa B. 2017. Why psychiatry needs 3,4-methylenedioxymethamphetamine: a child psychiatrist’s perspective. Neurother J Am Soc Exp Neurother. 14:741–749.
- Team RDC 2011. R: a language and environment for statistical computing [internet]. Vienna (Austria). http://www.R-project.org/ (Accessed on 18 October 2016).
- Tinajero JC, Fabbri A, Ciocca DR, Dufau ML. 1993. Serotonin secretion from rat Leydig cells. Endocrinology. 133:3026–3029.
- Tinajero JC, Fabbri A, Dufau ML. 1992. Regulation of corticotropin-releasing factor secretion from Leydig cells by serotonin. Endocrinology. 130:1780–1788.
- Tsai SC, Chen JJ, Chiao YC, Lu CC, Lin H, Yeh JY, Lo MJ, Kau MM, Wang SW, Wang PS. 1997. The role of cyclic AMP production, calcium channel activation and enzyme activities in the inhibition of testosterone secretion by amphetamine. Br J Pharmacol. 122:949–955.
- Walther DJ, Bader M. 2003. A unique central tryptophan hydroxylase isoform. Biochem Pharmacol. 66:1673–1680.
- Wang R-S, Yeh S, Tzeng C-R, Chang C. 2009. Androgen receptor roles in spermatogenesis and fertility: lessons from testicular cell-specific androgen receptor knockout mice. Endocr Rev. 30:119–132.
- Wright AS, Thomas LN, Douglas RC, Lazier CB, Rittmaster RS. 1996. Relative potency of testosterone and dihydrotestosterone in preventing atrophy and apoptosis in the prostate of the castrated rat. J Clin Invest. 98:2558–2563.
