ABSTRACT
The oil overlay in microdrop culture systems prevents medium evaporation, helps to maintain appropriate pH and osmotic conditions and protects from microbial contamination. In the present study, we prospectively compared covering by Ovoil™, a paraffin oil, and LiteOil®, a mineral oil, on the in vitro development of human embryos and their suitability for transfer/freezing at day 3 and live birth rate. One hundred and one patients undergoing in vitro fertilization (IVF) treatment by intracytoplasmic sperm injection (ICSI) were enrolled in our study. After ICSI, 1237 oocytes were 1:1 randomly allocated into 2 groups according to the type of overlaying oil: Ovoil™ (616 oocytes) or LiteOil® (621 oocytes). Fertilization rate was assessed around 18 hours post-insemination (hpi) and embryos were checked for early cleavage at 25 hpi. Embryo morphology was recorded on days 2 and 3. A total of 437 (Ovoil™) and 438 day 3 embryos (LiteOil®) were analyzed. There were no differences between the two groups in terms of fertilization rate and occurrence of early cleavage. The proportion of top quality embryos (41.7% vs. 41.2%) and the final utilization rates (92.2% vs. 92.0%) were similar in Ovoil and LiteOil groups, respectively, at day 3. Live birth rate per transfer was essentially the same with Ovoil™ overlay (26.9%) when compared to LiteOil® (26.2%). Live birth rate in patients who simultaneously received embryos from both overlay types was 17.2%. Despite the different characteristics of these two oils regarding hydrocarbon saturation, packing and temperature storage, Ovoil™ and LiteOil® can be used in parallel in the same IVF protocol.
Abbreviations: ART: assisted reproductive technologies; hpi: hours post-insemination; hSA: human serum albumin; HTF: human tubal fluid; ICSI: intracytoplasmic sperm injection; IVF: in vitro fertilization; MII: metaphase II; MEA: mouse embryo assay; RT: room temperature.
Introduction
The culture system is a critical step in assisted reproductive technologies (ART), influencing embryonic development and the success of in vitro fertilization (IVF) programs. Mineral and paraffin oils are commonly used to overlay IVF media in order to maintain their stability throughout the embryonic culture. Overlay slows down evaporation, temperature and pH variations. Indeed, without any oil overlay, the medium osmolarity would quickly become too high and deleterious for embryo development (Wale and Gardner Citation2016). In addition, it plays a barrier role against contaminations by toxic compounds or microbes.
Several studies focused on a possible negative impact of overlaying oil on embryo development and pregnancy outcome. In fact, toxic elements present in crude oil such as unsaturated/aromatics hydrocarbons, short volatile carbon chains, peroxides and zinc (Erbach et al. Citation1995) and other undefined compounds could contaminate the oil used in IVF laboratories (Shimada et al. Citation2002; Gardner et al. Citation2005; Morbeck and Leonard Citation2012). The polycarbon lipid tail in mineral oil contains more unsaturated bonds than paraffin oil (Vitrolife, web site), the mineral product being therefore more sensitive to photooxidation and peroxidation (Elder Key and Bw Citation2015). High peroxidation in mineral culture overlay is reported to be detrimental to fertilization and embryo development because of toxic contamination and/or deterioration of oil quality (Otsuki et al. Citation2007). Because oil represents a component of embryo culture systems with the most potential variation in quality, a sensitive mouse embryo assay (MEA) was recently developed to test mineral oil peroxide toxicity. This test consists of extended culture to 144 hours rather than 96 hours as currently performed in standard MEA (Ainsworth et al. Citation2017). Some authors confirmed the oil embryo-toxicity in mouse (Miller et al. Citation1994; Otsuki et al. Citation2007), pig and human (Otsuki et al. Citation2009), is mostly caused by peroxide contamination which depends on heat, UV light exposure, extended storage, manufacture and batch number. High levels of peroxide affect fertilization, early cleavage and blastocyst growth.
The ultimate goal of all IVF laboratories is to optimize their culture system, in order to preserve embryonic viability and to lead to healthy liveborns (Behr and Wang Citation2004; Lane and Gardner Citation2007; Smith et al. Citation2012; Munck et al. Citation2015; Testori and Manager Citation2015). Despite the importance of an oil overlay in those systems, very few publications address this aspect and to our best knowledge, the Sifer study is the only one describing interested in human embryo development under several types of commercial oil (Sifer et al. Citation2009). Therefore, the aim of our study was to compare IVF and embryo utilization rates after culture under paraffin oil (Ovoil™, Vitrolife) and mineral oil (LiteOil®, LifeGlobal).
Results
Fertilization and embryo quality
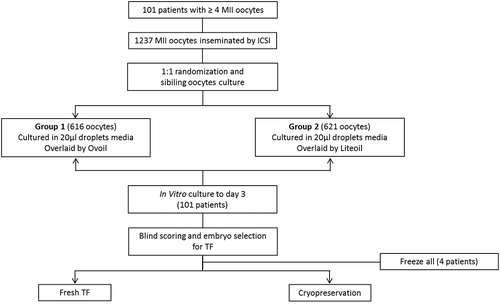
The present study included 1237 MII oocytes derived from 101 ICSI cycles (). They were cultured in Global medium overlaid either by Ovoil™ (616 oocytes, group1) or by LiteOil® (621 oocytes, group 2) ().
Table 1. Baseline group characteristics.
Fertilization and embryo development were investigated from day 1 to day 3 (). The percentage of normally fertilized oocytes (2PN) and early cleavage at 25 hpi were not significantly different: 75.5% versus 72.1% and 37.2% versus 40.1% with Ovoil™ and LiteOil®, respectively. Embryo development did not differ between group 1 and group 2 when compared at day 2 or day 3: respectively 65.2% versus 64.7% ≥4-cell stage (mean cell number: 4.2 vs. 4.1) and 70.9% versus 70.5% ≥6-cell stage (mean cell number: 8.3 vs. 8.5). Moreover, the two groups did not differ regarding the morphological quality at day 3 and utilization of the embryos.
Table 2. Embryo outcomes per group.
In term of frozen, transferred and discarded embryos, the rates were not significantly different in both groups. In fact, percentages of frozen and transferred embryos were 76.7% versus 71.9% and 15.6% versus 20.1%, respectively, in Ovoil versus LiteOil groups. Classification and scoring of transferred or frozen embryos in the two analyzed groups showed the same percentage of A- (41.7% group 1 vs. 41.2% group 2), B- (30.8% group 1 vs. 30.8% group 2) and C-quality (27.5% group 1 vs. 28.0% group 2) ().
Pregnancy rate
Overall pregnancy and live birth rates per transfer did not significantly differ between Ovoil and LiteOil groups (38.5% vs. 33.3% and 26.9% vs. 26.2%). The mixed group, which involved significantly older patients (p ≤ 0.001), ended with lower birth rate (17.2%) when compared to the two other groups ().
Table 3. Embryo transfers and pregnancy outcomes.
Discussion
Oil is widely used to overlay embryo culture media and can play a major role in IVF outcome. Paraffin and mineral oil are known to have different chemical properties. The polycarbon lipid tail in mineral oil contains more unsaturated bonds than paraffin oil (Vitrolife, web site), the mineral product being therefore more sensitive to photooxidation and peroxidation (Elder Key and Bw Citation2015). High peroxidation in mineral culture overlay is reported to be detrimental to fertilization and embryo development because of toxic contamination and/or deterioration of oil quality (Otsuki et al. Citation2007). The degree of peroxidation depends on heat and UV light exposure, storage conditions, manufacturer and batch number (Otsuki et al. Citation2007, Citation2009; Elder Key and Bw Citation2015). Some studies recommend to keep oil in a cool dark place to prevent it’s deterioration during long-term storage (Otsuki et al. Citation2009; Wale and Gardner Citation2016).
Despite careful quality control before release to customers, oil overlay can have different effects on embryo development. Indeed, Sifer et al. previously showed that the mean number of top quality human embryos at day 3 was statistically higher in their Ovoil group when compared to three other types of oil (Sifer et al. Citation2009). Investigation on bovine embryo confirms that oil type can influence embryo growth and utilization: development after 3 days in culture under sterile paraffin and washed light mineral oil is not affected but morula and blastocyst formation rates are significantly higher with paraffin (Tae et al. Citation2006).
In the present study including 1237 sibling oocytes, we prospectively compared effects of culture media covering by a 100% paraffin oil (Ovoil™) and a mineral oil (LiteOil®) on the in vitro development and utilization rates of day 3 human embryos. The two oils were stored according to respective manufacturer’s recommendations, in glass bottle at 4°C for the paraffin oil, and in dark plastic bottle at room temperature (RT) for the mineral oil.
Our data show that Ovoil™ and LiteOil® overlays result in similar embryo development and delivery rates. Oil plays an essential role in embryo culture and it is rather reassuring to have alternative options in the IVF laboratory. Our work can therefore help other IVF teams in the development of their protocols. Our results are consistent with Sifer et al. who obtained the same pregnancy rates with the four oils enrolled in their comparison. However, our study is more robust as we used sibling oocytes in order to strengthen the conclusion. We must concede that embryos allocated to the fresh transfer were more readily chosen among the LiteOil cohort (42 patients) than in the Ovoil group (26 patients). This seems to suggest a better developmental pattern for the embryos in the LiteOil system. This trend is not confirmed at the level of embryonic viability since we obtained the same pregnancy rate with both oils. It is worthy to note a higher miscarriage rate in patients who received a mix of embryos from the two culture systems (29 patients). It is probably due to their higher age and cycle rank.
Thus, we confirm the safety of these oils on a large cohort of human embryo until day 3 of development. Such a study could be helpful for other IVF laboratories to choose the appropriate oil to use.
Studies comparing oils from different companies in IVF are rather scare, especially sibling oocytes/embryos protocols as described in the present paper. In 2009, Sifer et al. (Citation2009) compared four different oil covering systems in different groups of patients rather than with a sibling oocyte protocol. Similar to our findings, reported day 2 embryonic morphologies were not different between their groups but, contrary to our data, they obtained more day 3 top embryos with Ovoil™ (Vitrolife) than with the three other systems (one being a mineral product from CryoBioSystem). They demonstrated that embryo quality could be affected by commercial oils used to overlay culture media.
It’s worthy to note that patients’ age range (22–44) and lack of peroxide level measurement as performed by Otsuki et al. (Citation2007) could represent some limitations of our study.
In conclusion, despite the different characteristics of the two tested oils regarding hydrocarbon saturation, packing and temperature storage, we showed that embryo quality and utilization rate were not impacted until day 3. Finally, oil should be used with caution by respecting storage recommendation of manufacturer and preventing long-term conservation even for unopened samples. More studies are required to confirm oil safety in IVF.
Materials and methods
Study design
This was a single-center prospective randomized study with sibling oocytes performed from August 2014 to July 2015. Our study enrolled 101 ICSI cycles realized in the Center for Assisted Medical Procreation at the University of Liège (CHR, Citadelle). Mean age of the female partner was 33 ± 6 years (range 22–44) and mean cycle rank was 2.0 ± 1.5 (). A minimum of four mature oocytes available for ICSI was required and patients could enter the study only once. Our prospective comparison was performed using two commercialized oils routinely employed in our laboratory before the start of the study. It doesn’t introduce any inconstancy in our IVF protocol. An informed consent for IVF treatment, approved by the Ethics Review Board of our University Hospital, has been provided by each patient.
Patient population and ovarian stimulation
Ovarian hyperstimulation protocols were mainly GnRH antagonist (93 patients), 8 patients received a GnRH agonist. Ovarian response to stimulation was monitored by hormonal blood tests and ultrasound assessment of follicular growth as previously described (Lédée et al. Citation2013). Oocytes retrieval was scheduled around 36 h after human chorionic gonadotrophin (hCG, Pregnyl®, Organon, Brussels, Belgium) injection, before ovulation.
The present study included partner (78 cycles) or donor (23 cycles) insemination with ejaculated or testicular spermatozoa, from either fresh or frozen samples.
Media and culture dishes
We used sequential media from LifeGlobal (USA): Human Tubal Fluid (HTF) for oocyte culture and Global medium after ICSI and for further cleavage till day 3 (LGGG). Both were supplemented with human serum albumin (hSA, 9988, Irvine Scientific, USA), respectively, 10% and 7.5%.
Culture dishes (60 mm easy grip, BDAA3004, Falcon) were prepared on the day before use and left in a 6% CO2/5% O2 incubator at 37°C in order to equilibrate pH and temperature. Two kinds of dishes were prepared for each patient:
(1) Group 1: 20 µl droplets overlaid with 6 ml Ovoil™ (10029, Vitrolife, Sweden) previously stored in transparent glass bottles at 4°C.
(2) Group 2: 20 µl droplets overlaid with 6 ml LiteOil® (AMLO, LifeGlobal, USA) previously stored in dark plastic bottles at RT.
The dishes were loaded with a maximum of 10 drops of media and each drop contained only one oocyte/embryo.
Gamete preparation
After pick up, cumulus oocyte complexes were incubated in HTF medium. Oocyte denudation was performed from 1 to 2 h after oocyte retrieval by quick exposure to a ready-to-use solution of hyaluronidase (LGHY-010, LifeGlobal, USA) and gentle mechanical dispersion through 170 and 140 µm pipettes (K-FPIP-1140 and K-FPIP-1170, Cook Medical, USA). Mature metaphase II oocytes (MII, n = 1237) were identified under stereo-microscope by the presence of the first polar body and enrolled in our study. Immature or dysmorphic oocytes were discarded.
Sperm was prepared by centrifugation (300 g) on a 100/70/40% density gradient for up to 20 min (Isolate, 99306, IrvineScientific, USA) and washed twice in sperm washing medium (AllGrad Wash, LifeGlobal, USA) at 500 g during 5 min. The final preparation was kept at RT until ICSI. Poorest samples were treated by simple washing.
Oocyte random distribution
A 1:1 randomization of oocytes was achieved after ICSI. Sibling oocytes from each patient were randomly allocated for culture in 20 µl drops of Global® medium in dishes overlayed by either Ovoil™ (group 1, n = 616) or LiteOil® (group 2, n = 621) ().
Fertilization and embryo outcome
Assessment of fertilization (presence of 2 pronuclei, 2PN) was performed at 18 hpi followed by the early cleavage stage evaluation at 25 hpi. Oocytes showing more than 2PN were discarded.
Day 2 and day 3 embryos were assessed using our routine examination protocol based on Istanbul consensus criteria (Balaban et al. Citation2011), considering blastomere number and symmetry, percentage of fragmentation and cytoplasmic appearance. Embryonic defects were defined by a ≥10% fragmentation or irregular blastomeres varying in size by a factor >2 or the presence of cyctoplasmic anomalies, as granulation, vacuoles or dark coloration. Briefly, morphological quality scores were attributed to each embryo as follows:
Score A: ≤1 defect
Score B: 2 defects
Score C: 3 defects.
Day 2 and day 3 embryos showing >30% fragmentation were included in the study as discarded. At day 3, stages under 6 cells were considered as developmental arrest and were also included in the study as discarded.
Freshly transferred embryos were chosen according to several criteria: (1) normal fertilization (2PN), (2) early cleavage at 25 hpi, (3) ongoing development between Days 2 and 3 and (4) day 3 morphology. Transfer was performed at day 3 with an embryo transfer catheter (K-JETS-7019, Cook, USA) in Global medium.
Supernumerary embryos were cryopreserved by vitrification using Irvine freeze kit (90133-SO, IrvineScientific, USA).
For 27 patients, some developmental data were missing (early cleavage or day 2 developmental stage) because the oocyte pickup took place on a Friday or a day before holiday.
Embryo transfer
Fresh embryo transfer was performed on day 3 in 97 cycles, freeze-all being necessary for 4 cycles because of ovarian hyperstimualtion syndrome.
Three groups of transfers can be distinguished according to the type of oil overlay:
The Ovoil group, including 26 patients who received embryos derived only from Ovoil overlay.
The LiteOil group, including 42 patients who received embryos derived only from LiteOil overlay.
The mixed group, including 29 patients simultaneously transferred with embryos coming from both Ovoil and LiteOil overlays.
Clinical pregnancy
Human Chorionic Gonadotropin serum level ≥100 IU/l 14 days after embryo transfer was considered as a positive pregnancy test.
Statistics
Wilcoxon signed-rank test using GraphPad Prism 5 Software was performed for paired comparison of outcome measures between the two types of oils, as recently used by Sfontouris et al. in a prospective randomized study with sibling oocytes (Sfontouris et al. Citation2017). Continuous variables were presented as means ± SD and categorical data were expressed as percentage and compared using the Chi-square test.
Disclosure of interest
The authors declare no conflicts of interest.
Additional information
Notes on contributors
Soraya Labied
Study design: SL, CJ, FW, SR, OG, FT, VG, LH, MN. Performed techniques in the IVF laboratory: SL, CJ, FW, SR, OG, FT. Data collection: SL, CJ, FW, SR. Statistical analysis: FW. Wrote the manuscript: SL, CJ. All authors approved revisions and the final paper.
References
- Ainsworth AJ, Fredrickson JR, Morbeck DE. 2017. Improved detection of mineral oil toxicity using an extended mouse embryo assay. J Assist Reprod Genet. 34:391–397.
- Balaban B, Brison D, Calderón G, Catt J, Conaghan J, Cowan L, Ebner T, Gardner D, Hardarson T, Lundin K, et al. 2011. Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Reprod Biomed Online. 22:632–646.
- Behr B, Wang H. 2004. Effects of culture conditions on IVF outcome. J Obstet Gynecol Reprod Biol. 115:72–76.
- Elder Key MVDB, Bw. 2015. Troubleshooting and problem-solving in the IVF laboratory. Cambridge: Press CU, editor; p. 35–37.
- Erbach GT, Bhatnagar P, Baltz JM, Biggers JD. 1995. Zinc is a possible toxic contaminant of silicone oil in microdrop cultures of preimplantation mouse embryos. Hum Reprod. 10:3248–3254.
- Gardner DK, Reed L, Linck D, Sheehan C, Lane M. 2005. Quality control in human in vitro fertilization. Semin Reprod Med. 23:319–324.
- Lane M, Gardner DK. 2007. Embryo culture medium : which is the best? Hum Fertil Embryol Auth. 21:83–100.
- Lédée N, Gridelet V, Ravet S, Jouan C, Gaspard O, Wenders F, Thonon F, Hincourt N, Dubois M, Foidart JM, et al. 2013. Impact of follicular G-CSF quantification on subsequent embryo transfer decisions: A proof of concept study. Hum Reprod. 28:406–413.
- Miller KF, Goldberh JM, Collins RL. 1994. Covering embryo cultures with mineral oil alters embryo growth by acting as a sink for an embryotoxic substance. J Assist Reprod Genet. 11:9–12.
- Morbeck DE, Leonard PH. 2012. Culture systems: mineral oil overlay. Methods Mol Biol. 912:325–331.
- Munck ND, Mateizel I, Verheyen G. 2015. Reduced blastocyst formation in reduced culture volume. J Assist Reprod Genet. 32:1365–1370.
- Otsuki J, Nagai Y, Chiba K. 2007. Peroxidation of mineral oil used in droplet culture is detrimental to fertilization and embryo development. Fertil Steril. 88:741–743.
- Otsuki J, Ph D, Nagai Y, Chiba K, Ph D. 2009. Damage of embryo development caused by peroxidized mineral oil and its association with albumin in culture. Fertil Steril. 91:1745–1749.
- Sfontouris IA, Kolibianakis EM, Lainas GT, Venetis CA, Petsas GK, Tarlatzis BC, Lainas TG. 2017. Blastocyst utilization rates after continuous culture in two commercial single-step media: a prospective randomized study with sibling oocytes. J Assist Reprod Genet. 34:1377–1383.
- Shimada M, Kawano N, Terada T. 2002. Delay of nuclear maturation and reduction in developmental competence of pig oocytes after mineral oil overlay of in vitro maturation media. Reproduction. 124:557–564.
- Sifer C, Pont J, Porcher R, Martin-Pont B, Benzacken B, Wolf J. 2009. A prospective randomized study to compare four different mineral oils used to culture human embryos in IVF/ICSI treatments. Eur J Obstet Gynecol Reprod Biol. 147:52–56.
- Smith GD, Takayama S, Swain JE. 2012. Minireview rethinking in vitro embryo culture : new developments in culture platforms and potential to improve assisted reproductive technologies 1. Biol Reprod. 86:1–10.
- Tae JC, Kim EY, Lee WD, Park SP, Lim JH. 2006. Sterile filtered paraffin oil supports in vitro developmental competence in bovine embryos comparable to co-culture. J Assist Reprod Genet. 23:121–127.
- Testori S, Manager CP. 2015. Embryo culture media update. Hum Fertil Embryol. Auth; p. 1–13. www.hfea.gov.uk
- Vitrolife. https://www.vitrolife.com/en/products/ivf-media--oil/ovoil---culture-oil/.
- Wale PL, Gardner DK. 2016. The effects of chemical and physical factors on mammalian embryo culture and their importance for the practice of assisted human reproduction. Hum Reprod Update. 22:2–22.