ABSTRACT
Multiple prevention therapy has gained importance for the prevention and treatment of sexually transmitted diseases, especially HIV/AIDS. Antiretroviral drugs encapsulated in nanoparticles have been developed for efficient delivery of the drugs to the vaginal surface. Lactoferrin nanoparticles (LFNPs) encapsulating anticancer or antiretroviral drugs are found to be promising agents to specifically deliver drugs at the target sites. Recent studies indicate that the bioavailability is higher for antiretroviral drugs delivered by LFNPs than when the drugs are administered alone. Although LFNP-mediated drug delivery via the oral or vaginal route for the treatment of HIV/AIDS is promising, the effect of such administrations is not well studied. Drug-loaded LFNPs when administered to rats by the vaginal route did not show any effect on the reproductive performance, fertility, and postnatal development. Oral administration of drug-loaded LFNPs caused a significant decrease in litter size, whereas the reproductive performance and postnatal development remained normal. In our model system, the results indicate that vaginal administration of drug-loaded LFNPs appears safer and can be projected for the delivery of antiretroviral agents via the vaginal route.
Abbreviations: LFNPs: lactoferrin nanoparticles; STIs: sexually transmitted diseases infections; NPs: nanoparticles; LF: lactoferrin; DL-LFNPs: drug loaded lactoferrin nanoparticles; MPT: multiple prevention techniques
Introduction
Globally, the incidence of sexually transmitted disease infections (STIs)-related morbidity and mortality is a major concern and there is always an urgent need to develop technologies by which drug delivery is specific and effective (Hill et al. Citation2007; Singh et al. Citation2010). To accomplish this, vaginal microbicide-based strategies are gaining importance (Liu et al. Citation2005; Thurman et al. Citation2011; Kizima et al. Citation2014), but with limitations. For example, the microbicide PRO 2000, BufferGel and Carraguard failed at different phases of clinical trials due to efficacy issues such as release at optimum concentrations, bioavailability, and side effects in the target organ (D’Cruz and Uckun Citation2004, Tao et al. Citation2008; Veazey Citation2008; Abdool Karim et al. Citation2011; Nutan Citation2011). Delivery of Anti-HIV drugs such as Tenofovir by way of intravaginal rings, gels, and tablets resulted in sustained release (Darroch and Frost Citation1999; El-Kamel et al. Citation2002; Bilensoy et al. Citation2006; Woolfson et al. Citation2006). In view of the side effects of these drugs, use of natural compounds has gained importance. One such compound is Curcumin, the active ingredient in the roots of the plant Curcuma longa. Side effects were not observed with curcumin because of its hydrophobic nature and low bioavailability (Hatcher et al. Citation2008). Although powerful drugs are available to treat many diseases, major obstacles in the treatment are the effective delivery of the drug to the target site and sustained release (Zhao et al. Citation2013). A recent development for the treatment of many infectious diseases including the sexually transmitted diseases is the specific delivery of drugs using protein-based nanoparticles (NPs).
NPs whose size is generally in the range of 10–100 nm can transport and release drugs (Mudshinge et al. Citation2011). Their use for biological and therapeutic purposes in the form of nano-carriers confers advantage in preventing drug degradation, increasing immune responses of the host, delivery of the drug to a specific target, enhanced bioavailability, delivery of drugs with poor cell membrane permeability, and also delivery of macromolecules such as peptides and nucleotides (Krishna et al. Citation2009; Mallipeddi and Rohan Citation2010). Biomaterial-based NPs using lipids and proteins have been developed and are currently being tested. Protein-based NPs have gained importance because of the ease of preparation in mild conditions, amphiphilic nature, biocompatibility, easy to modify for enhanced binding and their ability to entrap drugs due to charged groups on their surface (Gräfe and Hoffmann Citation2000, Wang and Uludag Citation2008; Mamo et al. Citation2010; Hu et al. Citation2011; Lohcharoenkal et al. Citation2014). Further, protein-based NPs have the ability to deliver the drugs in passive or active (ligand mediated) mode, thereby creating better choices. Protein-based NPs that are entrapped with curcumin were found to be effective in the treatment of AIDS (Gandapu et al. Citation2011; Hu et al. Citation2011; Wahome et al. Citation2012). Albumin, elastin, gelatin, transferrin, and lactoferrin (LF) are some of the proteins that have been used to generate NPs for drug delivery (Coester et al. Citation2000; Krishna et al. Citation2009; Bessa et al. Citation2010; Park Citation2012).
LF, a member of the transferrin family, with a molecular mass of 80 KDa is water soluble, biodegradable and allows surface modification for drug interaction (Weber et al. Citation2000). It exhibits anti-HIV, antimicrobial, anticancer, anti-inflammatory, and antifungal activities and, thus, is clinically important (Actor et al., Citation2009; Ward et al. Citation2002; Legrand et al. Citation2005; Lönnerdal Citation2009) (Florisa et al. Citation2003; León-Sicairos et al. Citation2006, Golla et al. Citation2012). LF-based NPs (LFNPs) for drug delivery has been a better choice because of its ability to target cancer cells, since these cells are known to exhibit high levels of LF receptor. Because of its unique properties, it has been used as a ligand for many nano-carriers (Su et al. Citation2014; Lim et al. Citation2015). Using a patented technology, we generated very effective LFNPs to deliver anticancer and anti-HIV drugs (Krishna et al. Citation2009). We demonstrated that the efficacy, bioavailability, and safety of lactodoxonano, doxorubicin, carboplatin, zidovudine, 5-fluorouracil, and temozolomide were significantly improved when drugs were encapsulated and delivered using LFNPs in a variety of disease models (Golla et al. Citation2012, Citation2013b; Ahmed et al. Citation2014; Kumar et al. Citation2015; Bollimpelli et al. Citation2016; Kumari et al. Citation2017; Kumari and Kondapi Citation2017). Further, an oral formulation containing efavirenz-loaded LFNPs enhanced biodistribution and pharmacokinetic profile both in vitro and in vivo (Kumar et al. Citation2017a). Effective triple combination multiple prevention technology vaginal microbicide prepared by encapsulating efaverinz and curcumin in LFNPs was demonstrated to possess enhanced bioavailability, effective delivery, and low toxicity in a murine model (Lakshmi et al. Citation2016). LFNPs-encapsulated triple drug combination (Zidovudine, Efavirenz and Lamivudine) was found to have several advantages when compared to direct drug delivery and is projected to be an effective first-line nano-agent for HIV therapy (Kumar et al. Citation2017b). Although the efficacy of LF-encapsulated drugs has been tested, a comprehensive analyses on the systemic and reproductive toxicity are not yet reported. This is very important when attempting to use them as potential drug delivery agents in humans. In this study, we evaluated the reproductive toxicity in rats treated with Curcumin + Efavirenz-, Curcumin + Dapivirine- and Curcumin + Tenofovir loaded-LFNPs.
Results
We previously characterized LFNPs loaded with two drugs, namely, Curcumin and Efavirenz to develop treatment options for sexually transmitted diseases by employing multiple prevention techniques (MPT) (Lakshmi et al. Citation2016). Since DL-LFNPs are proposed to be used as a MPT strategy for sexually transmitted diseases, we analyzed their possible toxic effects of drug-loaded (Curcumin + Efavirenz or Curcumin + Dapivirine or Curcumin + Tenofovir) LFNPs on the reproductive system after administration by the oral and vaginal routes.
Effect of administration by intravaginal route
In this part of the study, we analyzed the effect of DL-LFNPs when delivered vaginally. Since the vaginal mucosa changes during menstrual cycle, it would be ideal to deliver the DL-LFNPs on the same menstrual cycle in all the animals. However, the goal of this study is to determine the effect of DL-LFNPs irrespective of the day of the menstrual cycle. No significant changes in body weight and organ weight were observed in female rats that received intravaginal administration of DL-LFNPs (). Administration of the drugs did not cause any damage to the vaginal architecture as evidence by histopathological examination (). Since the drugs have a tendency to diffuse from the site of application, their bioavailability in the vaginal lavage and blood was evaluated. In the vaginal lavage, 3 h post-administration, a significant decrease in the concentration of Curcumin, Efavirenz, Dapivirine and Tenofovir were observed and this decrease was evident in a time course manner (). In the blood, the drugs were detected 1 h after administration. The concentration of the drug detected in the blood significantly increased at 3 h time point followed by a time-dependent decrease ().
Table 1. Effect of intravaginal administration of LFNPs on body and organ weights (gm).
Table 2. Bioavailability of drugs (µg/ml) in the vaginal lavage of rats that received drug loaded LFNPs by intravaginal route.
Table 3. Bioavailability of drugs in the blood (ng/ml) of rats that received drug loaded LFNPs by intravaginal route.
Figure 1. Histopathology of vagina after vaginal administration of DL-LFNPs. Rats were administered 30 mg of DL-LFNPs via the vaginal route for 20 days. At the end of the treatment, vaginal tissue was collected, sectioned and stained with hematoxylin and eosin.
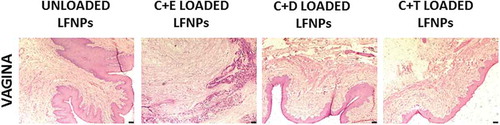
The effects of DL-LFNPs administered intravaginally on the reproductive performance and development of offspring were assessed by monitoring the pregnancy, litter size, litter weight, and signs of developmental abnormalities in the pups. Increase in body weight was observed in all the experimental groups as pregnancy progressed (). No changes in body weight were observed between the control (unloaded LFNPs) and treatment (DL-LFNPs) groups and also within the treatment groups. Pregnancy associated with vaginal swelling and bleeding was observed near the term in all treatment groups. There was no significant difference in the litter size and litter weight in pups born to rats treated with DL-LFNPs when compared with those pups born to rats treated with unloaded LFNPs (). Further, there was no significant difference in litter size and weight between the groups treated with DL-LFNPs. Developmental abnormalities (cleft in upper or lower lip, vestigial tail and malformations in limbs) were not observed in the pups born to rats treated with DL-LFNPs ().
Table 4. Effect of vaginal administration of drug-loaded LNPs on the progress of pregnancy and development of pups.
Effect of administration by oral route
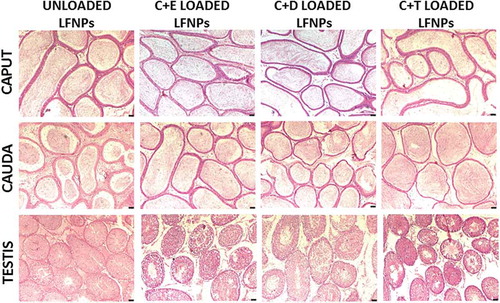
Oral administration of DL-LFNPs did not cause any change in the whole body and organ weights (). Further, histopathological analyses showed no signs of tissue damage in the epididymis (caput and cauda) and testes obtained from rats that received DL-LFNPs by the oral route (). To determine the effect of oral administration of DL-LFNPs on fertility, sperm count and fecundity were analyzed. Sperm count did not get altered in any of the groups tested (). The litter size and litter weight that resulted from mating of male rats (treated with DL-LFNPs) with untreated females did not alter significantly. Similarly, litter size was not significantly affected when female rats treated with C + E- or C + D-loaded LFNPs were mated with untreated males (). However, a significant decrease in litter size was observed when female rats treated with C + T-loaded LFNPs were mated with untreated males (). However, no change was observed in litter weight of pups obtained from female rats of different treatment groups ().
Table 5. Effect of oral administration of LFNPs on body and organ weights (gm).
Table 6. Effect of oral administration of LFNPs on sperm count and fertility.
Figure 2. Histopathology of reproductive tissues after oral administration of DL-LFNPs. Male wistar rats were administered 5 mg/Kg of DL-LFNPs for 3 weeks. Following treatment, caput, cauda and testis were collected, sectioned and stained with hematoxylin and eosin.
To analyze the effects of oral administration of DL-LFNPs during gestation and on the offspring, female rats that were proven to be pregnant were treated with 30 mg DL-LFNPs from tenth day of pregnancy till term. Within each group, the body weight of pregnant rats increased as pregnancy progressed (). No significant changes in the body weight were observed at all stages of pregnancy in the DL-LFNPs-treated group when compared with unloaded LFNPs group. Similarly, the body weight did not vary among the DL-LFNPs-treated groups (). Litter size significantly decreased in pregnant rats treated with C + E- or C + D-loaded LFNPs when compared to the rats treated with unloaded LFNPs. A decrease in litter size, though not significant, was also observed in C + T-loaded LFNP-treated groups (). No decrease in weight of pup was associated with the decreased litter size. Developmental abnormalities were not evident in all the pups born to pregnant rats treated with drug loaded or unloaded LFNPs ().
Table 7. Effect of oral administration of drug-loaded LNPs on the progress of pregnancy and development of pups.
Discussion
The potential of LFNPs as therapeutic agents has gained interest in the last two decades. Because of their high efficiency and specificity toward cancer cells, LFNPs have been used to treat glioma, melanoma edema, and hepatocellular carcinoma (Chen et al. Citation2011; Roseanu et al. Citation2010; Onishi et al. Citation2010; Golla et al. Citation2013a). We previously demonstrated the use of LFNPs for the delivery of anti-cancer and anti-HIV drugs in both in vitro and in vivo model systems (Golla et al. Citation2012, Citation2013a, Citation2013b; Kumar et al. Citation2015, Citation2017a, Citation2017b; Lakshmi et al. Citation2016; Kumari et al. Citation2017; Kumari and Kondapi Citation2017). Further, we also demonstrated the targeted delivery of DNA using LFNPs (Kumari and Kondapi Citation2018). In light of these potential applications of LFNPs as delivery agents in therapeutics, in the long run, they may be administered into the body for the delivery of drugs to treat many diseases. Hence, thorough analyses of the systemic effects with specific emphasis on the reproductive parameters were undertaken.
Since LFNPs are proposed to be potent drug delivery particles, we analyzed their effects when administered through the oral and vaginal routes. Delivering antiretroviral agents using NPs is being actively studied (Gu et al. Citation2015; Machado et al. Citation2016; Srinivasan et al. Citation2016; Das Neves and Sarmento Citation2017; Su et al. Citation2017). We previously reported the vaginal delivery of triple combination MPT microbicide using LFNPs (Lakshmi et al. Citation2016). In this study, we observed that the availability of drugs loaded in the LFNPs was significantly increased followed by a steady decline in the vaginal lavage and blood in a time-dependent manner. This pattern of drug availability shows that LFNPs successfully delivered the drugs into the vaginal lavage, which in turn diffused into the blood stream. It is possible that the release of the drugs from the DL-LFNPs may significantly differ in the blood and vaginal lavage, which depends on the environment, dosage, and pharmacokinetics. On the same lines, the extent of drug release in vivo may not exactly match with the theoretical concentrations, i.e., when the LFNPs are allowed to release the drugs in an in vitro system. Application of DL-LFNPs did not cause any significant changes in the body or organ weight, reproductive performance and abnormalities in the offspring. These results suggest that vaginal delivery of anti-HIV drugs encapsulated in LFNPs does not cause any deleterious effects and thus could be explored as potential delivery agents.
We previously developed an oral formulation of Efavirenz-loaded LFNPs and assessed its ability to deliver the drug (Kumar et al. Citation2015, Citation2017a, Citation2017b). The development of gambogic acid-encapsulated LFNPs and their oral administration for the efficacy in hepatocellular carcinoma is reported (Zhang et al. Citation2013). Since, the impact on the reproductive performance of male and female rats after oral administration of DL-LFNPs is not yet reported, we assessed the same. Sperm count and fertility (litter size) were not altered in the male rats of all treatment groups suggesting that DL-LFNPs may not have any damaging effects on reproductive physiological processes such as spermatogenesis and fertilization. Absence of any tissue damage in the male and female reproductive tract tissues indicates that the DL-LFNPs do not exhibit any adverse effects in these organ systems. Abnormalities were not observed in pups born to pregnant rats that received DL-LFNPs indicating that the developmental processes are not affected. However, a decline in litter size was observed in female rats that received oral administration of C + T-loaded LFNPs. Similarly, litter size was decreased in all the treatment groups of pregnant rats. It is to be noted that litter size was not affected when male rats that received oral administration of DL-LFNPs were mated with regular females. These results indicate that oral administration of DL-LFNPs may affect reproductive physiology in the females. Usage of DL-LFNPs via the oral route needs extra caution and further assessment. It is to be noted that the Food and Drug Administration, USA, basing on a number of studies, advises that Efaverinz should not be used in first trimester of pregnancy, since it causes neural tube defects (for details, see http://www.accessdata.fda.gov/drugsatfda_docs/label/2016/020972s049-021360s038lbl.pdf), with no effects on fertility and reproduction in humans. Results presented in this study use rodent models and the decreased litter size could be due to the side effects of anti-HIV drugs used. The specific stage (production of ova or fertilization or implantation) at which the DL-LFNPs may interfere needs further investigation.
In conclusion, we report that Efavirenz or Dapivirine or Tenofovir in combination with Curcumin-loaded LFNPs when administered via the vaginal route effectively delivers the drugs and do not exhibit any side effects on the reproductive performance and pregnancy in the rat model system. DL-LFNPs when administered orally to pregnant rats affect only the litter size without any effect on the developmental process. However, further studies are required to determine whether their safe use in humans is possible so that they can be used as safe antiretroviral drug delivery agents.
Materials and methods
Preparation of drug loaded LFNPS
LF and olive oil were purchased from Symbiotic (USA) and Leonardo (Italy), respectively. Drug-loaded LFNPs (DL-LFNPs) that had a combination of Curcumin + Efavirenz or Curcumin + Dapivirine or Curcumin + Tenofovir were prepared using our patented technology (Krishna et al. Citation2009). LF was dissolved in 500 μl ice cold PBS. In a separate tube, the respective drug was dissolved in 100 μl of DMSO. Briefly, the drug and the protein were mixed in the ratio of 1:2 (w/w) on ice and 15 ml of olive oil was added with constant vortexing and sonication (Ultrasonic homogenizer 300 V/T, Biologics Inc., Manassas, Virginia, USA). Following sonication, the samples were placed in liquid nitrogen for 15 min and then incubated on ice for 4 h. DL-LFNPs formed were pelleted by centrifugation at 30,000 g for 20 min. The supernatant that contained oil was discarded and the pellet was extensively washed with ice cold diethyl ether and PBS. The pellet was suspended in PBS and used for further experiments. DL-LFNPs were previously characterized (physical properties and loading efficiency) extensively as described in Lakshmi et al. (Citation2016).
Animals and treatment
Wistar rats used in this study were obtained from National Center for Laboratory Animals, NIN, Hyderabad. All the animal studies were approved by the Institutional Animal Ethics Committee of University of Hyderabad. In all the experimental set-ups, each group contained six animals (n = 6).
Intravaginal administration of DL-LFNPS and its effects
The objective of this part of the study was to determine the effect of DL-LFNPs when delivered vaginally irrespective of the day of the menstrual cycle. Female Wistar rats (90 days old; n = 6) were administered 30 mg DL-LFNPs via the vaginal route every day for 20 days. Control animals received unloaded LFNPs. The animals were monitored for general behavior and mortality. The treatment dose was standardized with pilot studies. Untreated controls were not included since our previous studies established that unloaded LFNPs did not cause any effects in rats.
Oral administration of DL-LFNPS and its effects
The effect of oral administration of DL-LFNPs was assessed as per the specifications of Limit test (Gad & Chengelis Citation1998). Briefly, 5 gm/Kg weight of DL-LFNPs were orally administered to the rats (n = 6 in each group) and kept under observation for general appearance and behavior for 3 weeks. Controls animals received unloaded LFNPs. Untreated controls were not included since our previous studies established that unloaded LFNPs did not cause any effects in rats.
Bioavailability
To determine the bioavailability of the drugs delivered by LFNPs in the vaginal lavage, a single dose of 30 mg of LFNPs containing a combination of Curcumin + Efavirenz or Curcumin + Dapivirine or Curcumin + Tenofovir are applied intravaginally. Untreated controls were not included since our previous studies established that unloaded LFNPs did not cause any effects in rats. Vaginal lavage was collected using cotton swabs at 1, 3, 6, 12, and 24 h after application. The cotton swabs were then placed in DMSO to recover the available drugs in the vaginal lavage. The concentration of the drugs was determined in UV-vis spectrophotometer at 430, 247, 260, and 290 nm for Curcumin, Efavirenz, Tenofovir, and Dapivirine respectively. Further, blood samples were collected at different time points (1 to 48 h) and sera were isolated. To 100 µl of each serum sample, 400 µl of DMSO was added and mixed thoroughly on a rocker for 30 min. To this 1 ml of 30%AgNO3 was added and mixed for 12 h to extract the drug from the sample. Following incubation, the sample was centrifuged at 12,000 rpm for 30 min. The absorbance of the supernatant was read at the above-mentioned wavelengths to determine the respective drugs.
Body and organ weight
At the end of treatment period, the body weights were noted. After sacrificing, the animals various organs were collected and their weights were noted determined.
Histopathology
Caput, corpus, cauda, testis, and vagina collected from rats subjected to oral or vaginal treatment with DL-LFNPs were fixed in Bouin’s fluid and dehydrated serially in 80, 90, and 100% ethanol followed by treatment with isopropanol before embedding in paraffin. Serial sections of the tissues were hydrated serially in 100, 90, 80, 70, and 50% ethanol and stained with Harris hematoxylin for 10 min and counter stained with 0.2% eosin Y. Sections were observed and photographs were taken on a Leica microscope.
Progress of pregnancy, fertility, and development of offspring
The effect of DL-LFNPs on these parameters was evaluated in three experimental set-ups.
Male and female rats were subjected to Limit test (treatment with 5 mg/kg body weight) of LFNPs containing a combination of Curcumin and Efavirenz or Curcumin and Dapivirine or Curcumin and Tenofovir for 3 weeks. Following treatment, they were allowed to mate with rats that did not receive any treatment.
Female rats were intravaginally administered 30 mg of LFNPs containing a combination of Curcumin and Efavirenz or Curcumin and Dapivirine or Curcumin and Tenofovir. After administration for 6 h, the rats were allowed to mate with rats that did not receive any treatment.
In another set of rats 30 mg of DL-LFNPs were orally administered to female rats from the 10th day of their pregnancy until term. Body weight and vaginal swelling, redness and bleeding were monitored throughout pregnancy was noted. The resulting offspring was observed for litter size, litter weight, and any developmental abnormalities (cleft in the upper or lower lip, vestigial tail, and limb defects). In all the experimental set-ups, rats treated with unloaded LFNPs served as controls.
Statistical analyses
Statistical analyses were performed using one-way ANOVA and Holm-Sidak test available in Sigma Plot software (SPSS Inc., Chicago, IL, USA). Values shown are Mean ± S.D.
Acknowledgments
The authors thank the Department of Biotechnology, Indian Council of Medical Research and DST-Nanomission of Government of India for financially supporting this study in the form of research funding. We thank the facilities extended by UGC-SAP, UGC-CAS, DBT-CREBB, and FIST programs at School of Life Sciences, University of Hyderabad. GB received a fellowship from University Grants Commission and Council for Scientific and Industrial Research, Government of India.
Disclosure statement
The authors report no conflict of interest.
Additional information
Notes on contributors
Lavanya Madugulla
Conceived and designed the experiments: SY, AKK; Performed the experiments: LM, ARR; Analyzed the data: SY, AKK; Contributed reagents/materials/analysis tools: SY, AKK; Wrote the manuscript: SY, AKK. All authors approve the final paper.
References
- Abdool Karim SS, Richardson BA, Ramjee G, Hoffman IF, Chirenje ZM, Taha T, Kapina M, Maslankowski L, Coletti A, Profy A, et al.; Team, H.I.V.P.T.N.S. 2011. Safety and effectiveness of BufferGel and 0.5% PRO2000 gel for the prevention of HIV infection in women. AIDS 25:957–966.
- Actor JK, Hwang S-A, Kruzel ML. 2009. Lactoferrin as a natural immune modulator. Curr Pharm Des. 15:1956–1973.
- Ahmed F, Ali MJ, Kondapi AK. 2014. Carboplatin loaded protein nanoparticles exhibit improve anti-proliferative activity in retinoblastoma cells. Int J Biol Macromol. 70:572–582.
- Bessa PC, Machado R, Nürnberger S, Dopler D, Banerjee A, Cunha AM, Rodríguez-Cabello JC, Redl H, Van Griensven M, Reis RL, et al. 2010. Thermoresponsive self-assembled elastin-based nanoparticles for delivery of BMPs. J Control Release. 142:312–318.
- Bilensoy E, Rouf MA, Vural I, Sen M, Hincal AA. 2006. Mucoadhesive, thermosensitive, prolonged-release vaginal gel for clotrimazole: beta-cyclodextrincomplex. AAPS PharmSciTech. 7:E38.
- Bollimpelli VS, Kumar P, Kumari S, Kondapi AK. 2016. Neuroprotective effect of curcumin-loaded lactoferrin nano particles against rotenone induced neurotoxicity. Neurochem Int. 95:37–45.
- Chen H, Qin Y, Zhang Q, Jiang W, Tang L, Liu J, He Q. 2011. Lactoferrin modified doxorubicin-loaded procationic liposomes for the treatment of gliomas. Eur J Pharm Sci. 44:164–173.
- Coester CJ, Langer K, Van Briesen H, Kreuter J. 2000. Gelatin nanoparticles by two step desolvation–a new preparation method, surface modifications and cell uptake. J Microencapsul. 17:187–193.
- D’Cruz OJ, Uckun FM. 2004. Clinical development of microbicides for the prevention of HIV infection. Curr Pharm Des. 10:315–336.
- Darroch JE, Frost JJ. 1999. Women’s interest in vaginal microbicides. Fam Plann Perspect. 31:16–23.
- Das Neves J, Sarmento B. 2017. Antiretroviral drug-loaded nanoparticles-in-films: a new option for developing vaginal microbicides? Expert Opin Drug Deliv. 14:449–452.
- El-Kamel A, Sokar M, Naggar V, Al Gamal S. 2002. Chitosan and sodium alginate-based bioadhesive vaginal tablets. AAPS PharmSci. 4:E44.
- Florisa R, Recio I, Berkhout B, Visser S. 2003. Antibacterial and antiviral effects of milk proteins and derivatives thereof. Curr Pharm Des. 9:1257–1275.
- Gad S, Chengelis CP. 1998. Acute toxiclogy testing: Perspective and horizons. 2nd Ed. San Diego (CA): Academic Press; p. 404–466.
- Gandapu U, Chaitanya RK, Kishore G, Reddy RC, Kondapi AK, Antopolsky M. 2011. Curcumin-loaded apotransferrin nanoparticles provide efficient cellular uptake and effectively inhibit HIV-1 replication in vitro. PLoS One. 6:e23388.
- Golla K, Bhaskar C, Ahmed F, Kondapi AK. 2013a. A target-specific oral formulation of doxorubicin-protein nanoparticles: efficacy and safety in hepatocellular cancer. J Cancer. 4:644–652.
- Golla K, Cherukuvada B, Ahmed F, Kondapi AK, Sarkar D. 2012. Efficacy, safety and anticancer activity of protein nanoparticle-based delivery of doxorubicin through intravenous administration in rats. PLoS One. 7:e51960.
- Golla K, Reddy PS, Bhaskar C, Kondapi AK. 2013b. Biocompatibility, absorption and safety of protein nanoparticle-based delivery of doxorubicin through oral administration in rats. Drug Deliv. 20:156–167.
- Gräfe KA, Hoffmann H. 2000. Development and validation of an indirect enzyme-linked immunosorbent assay (ELISA) for the nonsteroidal anti-inflammatory drug S-ibuprofen. Pharmazie. 55:286–292.
- Gu J, Yang S, Ho EA. 2015. Biodegradable film for the targeted delivery of siRNA-loaded nanoparticles to vaginal immune cells. Mol Pharm. 12:2889–2903.
- Hatcher H, Planalp R, Cho J, Torti FM, Torti SV. 2008. Curcumin: from ancient medicine to current clinical trials. Cell Mol Life Sci. 65:1631–1652.
- Hill K, Thomas K, Abouzahr C, Walker N, Say L, Inoue M, Suzuki E; Maternal Mortality Working Group. 2007. Estimates of maternal mortality worldwide between 1990 and 2005: an assessment of available data. Lancet 370:1311–1319.
- Hu K, Shi Y, Jiang W, Han J, Huang S, Jiang X. 2011. Lactoferrin conjugated PEG-PLGA nanoparticles for brain delivery: preparation, characterization and efficacy in Parkinson’s disease. Int J Pharm. 415:273–283.
- Kizima L, Rodríguez A, Kenney J, Derby N, Mizenina O, Menon R, Seidor S, Zhang S, Levendosky K, Jean-Pierre N, et al. 2014. A potent combination microbicide that targets SHIV-RT, HSV-2 and HPV. PLoS One. 9:e94547.
- Krishna ADS, Mandraju RK, Kishore G, Kondapi AK, Antopolsky M. 2009. An efficient targeted drug delivery through apotransferrin loaded nanoparticles. PLoS One. 4:e7240.
- Kumar P, Lakshmi YS, Bhaskar C, Golla K, Kondapi AK, Dezzutti CS. 2015. Improved safety, bioavailability and pharmacokinetics of zidovudine through lactoferrin nanoparticles during oral administration in rats. PLoS One. 10:e0140399.
- Kumar P, Lakshmi YS, Kondapi AK. 2017a. An oral formulation of efavirenz-loaded lactoferrin nanoparticles with improved biodistribution and pharmacokinetic profile. HIV Med. 18:452–462.
- Kumar P, Lakshmi YS, Kondapi AK. 2017b. Triple drug combination of zidovudine, efavirenz and lamivudine loaded lactoferrin nanoparticles: an effective nano first-line regimen for HIV therapy. Pharm Res. 34:257–268.
- Kumari S, Ahsan SM, Kumar JM, Kondapi AK, Rao NM. 2017. Overcoming blood brain barrier with a dual purpose temozolomide loaded lactoferrin nanoparticles for combating glioma (SERP-17-12433). Sci Rep. 7:6602.
- Kumari S, Kondapi AK. 2017. Lactoferrin nanoparticle mediated targeted delivery of 5-fluorouracil for enhanced therapeutic efficacy. Int J Biol Macromol. 95:232–237.
- Kumari S, Kondapi AK. 2018. Receptor-mediated targeted delivery of DNA using lactoferrin nanoparticles. Int J Biol Macromol. 108:401–407.
- Lakshmi YS, Kumar P, Kishore G, Bhaskar C, Kondapi AK. 2016. Triple combination MPT vaginal microbicide using curcumin and efavirenz loaded lactoferrin nanoparticles. Sci Rep. 6:25479.
- Legrand D, Elass E, Carpentier M, Mazurier J. 2005. Lactoferrin: a modulator of immune and inflammatory responses. Cell Mol Life Sci. 62:2549–2559.
- León-Sicairos N, Reyes-López M, Ordaz-Pichardo C, De La Garza M. 2006. Microbicidal action of lactoferrin and lactoferricin and their synergistic effect with metronidazole in entamoeba histolytica. Biochem Cell Biol. 84:327–336.
- Lim LY, Koh PY, Somani S, Al Robaian M, Karim R, Yean YL, Mitchell J, Tate RJ, Edrada-Ebel R, Blatchford DR, et al. 2015. Tumor regression following intravenous administration of lactoferrin- and lactoferricin-bearing dendriplexes. Nanomedicine. 11:1445–1454.
- Liu S, Lu H, Neurath AR, Jiang S. 2005. Combination of candidate microbicides cellulose acetate 1,2-benzenedicarboxylate and UC781 has synergistic and complementary effects against human immunodeficiency virus type 1 infection. Antimicrob Agents Chemother. 49:1830–1836.
- Lohcharoenkal W, Wang L, Chen YC, Rojanasakul Y. 2014. Protein nanoparticles as drug delivery carriers for cancer therapy. Biomed Res Int. 2014:180549.
- Lönnerdal B. 2009. Nutritional roles of lactoferrin. Curr Opin Clin Nutr Metab Care. 12:293–297.
- Machado A, Cunha-Reis C, Araújo F, Nunes R, Seabra V, Ferreira D, Das Neves J, Sarmento B. 2016. Development and in vivo safety assessment of tenofovir-loaded nanoparticles-in-film as a novel vaginal microbicide delivery system. Acta Biomater. 44:332–340.
- Mallipeddi R, Rohan LC. 2010. Progress in antiretroviral drug delivery using nanotechnology. Int J Nanomedicine. 5:533–547.
- Mamo T, Moseman EA, Kolishetti N, Salvador-Morales C, Shi J, Kuritzkes DR, Langer R, Von Andrian U, Farokhzad OC. 2010. Emerging nanotechnology approaches for HIV/AIDS treatment and prevention. Nanomedicine (Lond). 5:269–285.
- Mudshinge SR, Deore AB, Patil S, Bhalgat CM. 2011. Nanoparticles: emerging carriers for drug delivery. Saudi Pharm J. 19:129–141.
- Nutan. 2011. Microbicides: a new hope for HIV prevention. Indian J Med Res. 134:939–949.
- Onishi H, Koyama K, Sakata O, Machida Y. 2010. Preparation of chitosan/alginate/calcium complex microparticles loaded with lactoferrin and their efficacy on carrageenan-induced edema in rats. Drug Dev Ind Pharm. 36:879–884.
- Park K. 2012. Albumin: a versatile carrier for drug delivery. J Control Release. 157:3.
- Roseanu A, Florian PE, Moisei M, Sima LE, Evans RW, Trif M. 2010. Liposomalization of lactoferrin enhanced its anti-tumoral effects on melanoma cells. Biometals. 23:485–492.
- Singh S, Sedgh G, Hussain R. 2010. Unintended pregnancy: worldwide levels, trends, and outcomes. Stud Fam Plann. 41:241–250.
- Srinivasan P, Zhang J, Martin A, Kelley K, Mcnicholl JM, Buckheit RW Jr., Smith JM, Ham AS. 2016. Safety and pharmacokinetics of quick-dissolving polymeric vaginal films delivering the antiretroviral IQP-0528 for preexposure prophylaxis. Antimicrob Agents Chemother. 60:4140–4150.
- Su JT, Teller RS, Srinivasan P, Zhang J, Martin A, Sung S, Smith JM, Kiser PF. 2017. A dose ranging pharmacokinetic evaluation of IQP-0528 released from intravaginal rings in non-human primates. Pharm Res. 34:2163–2171.
- Su Z, Xing L, Chen Y, Xu Y, Yang F, Zhang C, Ping Q, Xiao Y. 2014. Lactoferrin-modified poly(ethylene glycol)-grafted BSA nanoparticles as a dual-targeting carrier for treating brain gliomas. Mol Pharm. 11:1823–1834.
- Tao W, Richards C, Hamer D. 2008. Enhancement of HIV infection by cellulose sulfate. AIDS Res Hum Retroviruses. 24:925–929.
- Thurman AR, Clark MR, Doncel GF. 2011. Multipurpose prevention technologies: biomedical tools to prevent HIV-1, HSV-2, and unintended pregnancies. Infect Dis Obstet Gynecol. 2011:1–10.
- Veazey RS. 2008. Microbicide safety/efficacy studies in animals: macaques and small animal models. Curr Opin HIV AIDS. 3:567–573.
- Wahome N, Pfeiffer T, Ambiel I, Yang Y, Keppler OT, Bosch V, Burkhard P. 2012. Conformation-specific display of 4E10 and 2F5 epitopes on self-assembling protein nanoparticles as a potential HIV vaccine. Chem Biol Drug Des. 80:349–357.
- Wang G, Uludag H. 2008. Recent developments in nanoparticle-based drug delivery and targeting systems with emphasis on protein-based nanoparticles. Expert Opin Drug Deliv. 5:499–515.
- Ward PP, Uribe-Luna S, Conneely OM. 2002. Lactoferrin and host defense. Biochem Cell Biol. 80:95–102.
- Weber C, Coester C, Kreuter J, Langer K. 2000. Desolvation process and surface characterisation of protein nanoparticles. Int J Pharm. 194:91–102.
- Woolfson AD, Malcolm RK, Morrow RJ, Toner CF, Mccullagh SD. 2006. Intravaginal ring delivery of the reverse transcriptase inhibitor TMC 120 as an HIV microbicide. Int J Pharm. 325:82–89.
- Zhang Z-H, Wang X-P, Ayman WY, Munyendo WLL, Lv H-X, Zhou J-P. 2013. Studies on lactoferrin nanoparticles of gambogic acid for oral delivery. Drug Deliv. 20:86–93.
- Zhao Z, Meng H, Wang N, Donovan MJ, Fu T, You M, Chen Z, Zhang X, Tan W. 2013. A controlled-release nanocarrier with extracellular pH value driven tumor targeting and translocation for drug delivery. Angew Chem Int Ed Engl. 52:7487–7491.
