ABSTRACT
Endometriosis affects 6–10% of healthy women of reproductive age. Therefore, it is important to study the molecular mechanism by which endometriosis develops. This study examined whether aberrant expression of LINC01541 contributes to the pathogenesis of endometriosis. Human endometrial stromal cells (ESCs) were stimulated with 10 nmol/L of 17β-Estradiol (17β-E2) to simulate ectopic cells found in endometriosis. Next, the levels of proteins related to the epithelial-mesenchymal transition (EMT), cell invasion, and metastasis were investigated. The effects of LINCO1541 silencing and overexpression were also examined in ESCs. Cell proliferation and apoptosis were detected by cell counting kit-8 and flow cytometry assays, respectively. ESCs stimulated with 17β-E2 displayed increased levels of N-Cadherin and vimentin expression, but decreased levels of E-Cadherin expression. 17β-E2 promoted the migration and invasion of ESCs, and those affects were partially reversed by overexpression of LINC01541. Furthermore, silencing of LINC01541 attenuated apoptosis and promoted the EMT of ESCs, while overexpression of LINC01541 stimulated cell apoptosis, increased the levels of caspase 3 protein, and decreased the levels of B cell leukemia/lymphoma 2 protein. Overexpression of LINC01541 also decreased the expression of vascular endothelial growth factor A (VEGFA) by repressing the Wnt/β-catenin pathway. Our, results suggest that LINC01541 can inhibit the EMT process, metastasis of ESCs, and VEGFA expression by regulating the Wnt/β-catenin pathway, which may play an important role in the pathogenesis of endometriosis.
Abbreviations: ESCs: endometrial stromal cells; 17β-E2: 17β-Estradiol; EMT: epithelial-mesenchymal transition; CASP3: caspase 3; BCL2: B cell leukemia/lymphoma 2; VEGFA: vascular endothelial growth factor A; lncRNA: long non-coding RNA; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; RT-qPCR: reverse transcription-quantitative polymerase chain reaction
Introduction
Endometriosis is estimated to affect 6–10% of women of reproductive age by causing dysmenorrhea, pelvic pain, pelvic masses, infertility, and malignant-like cellular behavior (Giudice and Kao Citation2004; Ilangavan and Kalu Citation2009). This chronic and excruciating disease significantly impacts the quality of life of the affected patients and their partners (Simoens et al. Citation2012). Endometriosis is currently diagnosed by laparoscopy and mainly treated by surgery (Janssen et al. Citation2013; Dunselman et al. Citation2014); however, there are no reliable biomarkers or therapeutic targets that can be used for its diagnosis and treatment, respectively. As a common estrogen-dependent and refractory gynecological disorder, research in genetics and molecular biology may contribute to the development of new strategies for diagnosing and treating endometriosis.
Long non-coding RNAs (lncRNAs) are RNAs that contain >200 nucleotides but do not code for a protein (Kapranov et al. Citation2007). Numerous lncRNAs have been identified and are thought to play important roles in cell differentiation, proliferation, and apoptosis. LncRNAs are also believed to participate in many important regulatory processes, such as genomic imprinting, chromatin modification, transcription activation, transcriptional interference, and intra nuclear transport (Wang and Chang Citation2011; Wapinski and Chang Citation2011; Fatica and Bozzoni Citation2014). LncRNA H19 was reported to alter stromal cell growth via insulin-like growth factor signaling in the endometrium of women with endometriosis (Ghazal et al. Citation2015), and LINC00261 was shown to inhibit the growth and migration of human endometriosis cells (Sha et al. Citation2017). A genome-wide profiling study of lncRNA expression in ovarian endometriosis found that 948 lncRNA transcripts and 4,088 mRNA transcripts were dysregulated in samples of ectopic endometrial tissue when compared with paired samples of eutopic endometrial tissue (Sun et al. Citation2014). LncRNA LOC100505776, also known as LINC01541 (NR_038325.1), was identified as the most down-regulated lncRNA in ectopic endometrial tissue. However, little is known about the role of LINC01541 in endometriosis.
In 1927, Sampson proposed the retrograde menstruation theory, which suggests that viable endometrial tissue is refluxed through the Fallopian tubes into the peritoneal cavity during menstruation (John Citation1927); after which, ectopic endometrial tissue grows in the abdominal wall or around an ovary. Most endometriosis patients can be classified as having an estrogen-dependent disorder. Hormone therapy can sometimes induce postmenopausal endometriosis (Oxholm et al. Citation2007), and estrogen is now generally accepted as a major risk factor for endometriosis (Dassen et al. Citation2007). 17β-E2stradiol (17β-E2), a very potent estrogen, is composed of two parts: estrogen receptor alpha and estrogen receptor beta, both which are essential for the development and growth of endometrial tissues (Bulun et al. Citation2010). 17β-E2 was reported to exert an anti-apoptotic effect on endometrial cells obtained from patients with endometriosis (Andrade et al. Citation2013). However, further studies are needed to confirm the molecular mechanism by which 17β-E2 promotes endometriosis development.
In this study we explored the role of LINC01541 in endometriosis. Endometrial stromal cells (ESCs) were stimulated with 17β-E2. The effects of LINC01541 silencing and overexpression were also explored in ESCs. Finally, we investigated the effects of LINC01541 on cell proliferation, cell metastasis, and VEGFA secretion.
Results
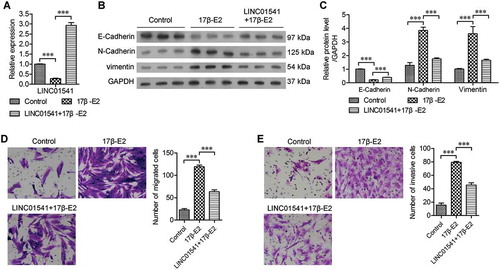
17β-E2 inhibited LINC01541 expression in ESCs
Because 17β-E2 plays a role in promoting endometriosis, we stimulated ESCs with 10 nmol/L of 17β-E2 for 48 h. Results of reverse transcription-quantitative polymerase chain reaction (RT-qPCR) studies showed that stimulation with 17β-E2 significantly inhibited LINC01541 expression (). To explore the function of LINC01541, we forced ESCs to overexpress LINC01541, and subsequently treated the cells with 17β-E2. Western blot studies revealed that 17β-E2 increased the levels of N-Cadherin and vimentin proteins, but decreased the levels of E-Cadherin (,). However, overexpression of LINC01541 significantly attenuated these effects of 17β-E2. Furthermore, cell migration and invasion assays showed that 17β-E2 significantly promoted metastasis and invasiveness of ESCs, and these effects could also be reversed by overexpression of LINC01541 (,). These results suggest that 17β-E2 inhibits LINC01541 expression and promotes the epithelial-mesenchymal transition (EMT) process in ESCs, and that overexpression of LINC01541 can attenuate the ability of 17β-E2 to promote ESC migration and invasion.
Figure 1. 17β-E2 promoted epithelial-mesenchymal transition and inhibited LINC01541 expression in ESCs. Normal ESCs and LINC01541-overexpressing ESCs were stimulated with 10 nmol/L of 17β-E2 for 48 h. (A) Down-regulated levels of LINC01541 expression in 17β-E2-treated ESCs were measured by RT-qPCR. (B, C) The levels of E-Cadherin, N-Cadherin, and vimentin proteins were detected by western blotting. Cell migration (D) and cell invasion (E) were promoted by 17β-E2 and inhibited by LINC01541 overexpression (Magnification, ×200). ***p < 0.001, vs. the control or 17β-E2 group.
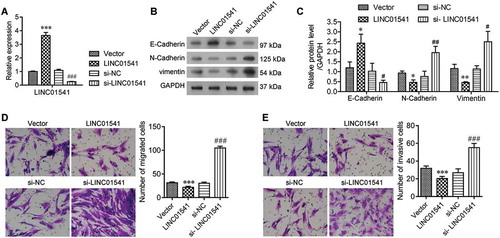
Silencing of LINC01541 promoted ESC migration and invasion
To further explore the function of LINC01541, we overexpressed and silenced LINC01541 in ESCs, respectively. Expression of LINC01541 was validated by RT-qPCR assays (). We observed that the levels of N-Cadherin and vimentin were significantly decreased in LINC01541 overexpressing ESCs, but were increased in LINC01541 silenced ESCs (). In contrast, the levels of E-Cadherin protein showed the opposite changes in response to LINC01541 overexpression and silencing, respectively. We also found that the migratory and invasive capabilities of ESCs were significantly reduced by overexpression of LINC01541, but significantly enhanced by silencing of LINC01541 (,). These results revealed that LINC01541 expression was correlated with the EMT process in ESCs and also the migratory and invasive capabilities of ESCs.
Figure 2. Silencing of LINC01541 promoted the migration and invasion of ESCs. ESCs were transfected with siRNA targeted toward LINC01541 or a recombinant LINC01541 overexpression vector, respectively. (A) LINC01541 expression levels were detected by RT-qPCR. (B, C) The levels of E-Cadherin, N-Cadherin, and vimentin proteins were detected by western blotting. Cell migration (D) and cell invasion (E) were promoted by silencing of LINC01541 and inhibited by LINC01541 overexpression (Magnification, ×200). *p < 0.05, **p < 0.01, ***p < 0.001, vs. vector group; #p < 0.05, ##p < 0.01, ###p < 0.001, vs. si-NC group.
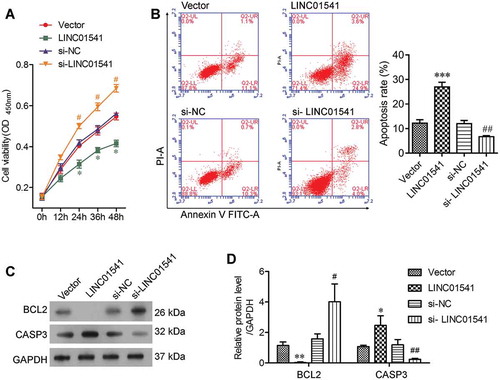
Overexpression of LINC01541 promoted apoptosis in ESCs
The effects of LINC01541 on the proliferation and apoptosis of ESCs were assessed by using the CCK8 assay and flow cytometry, respectively. Our results showed that overexpression of LINC01541 significantly reduced the viability of ESCs at 24 h after transfection, while silencing of LINC01541 significantly increased the percentage of viable ESCs (). In addition, the rates of apoptosis among ESCs that overexpressed LINC01514 were significantly higher than those in the vector control group, and silencing of LINC01541 induced a significant decrease in the numbers of apoptotic ESCs (). The role of LINC01541 in cell proliferation and apoptosis was also validated by detecting expression levels of the apoptosis related proteins, BCL2 and CASP3. An analysis of protein bands after electrophoresis showed that the levels of BCL2 protein were markedly decreased in the LINC01541 overexpressing group and markedly increased in the LINC01541 silencing group, when compared with their levels in the respective control groups (). We also observed that CASP3 protein levels were increased by overexpression of LINC01541 and decreased by silencing of LINC01541. These results demonstrated that the level of LINC01541 expression influenced the proliferation and apoptosis of ESCs.
Figure 3. Overexpression of LINC01541 promoted apoptosis in ESCs. ESCs were transfected with siRNA targeted toward LINC01541 or a recombinant LINC01541 overexpression vector, respectively. (A) Cell viability at 12, 24, 36, and 48 h after transfection was measured by the CCK8 assay. (B) Apoptosis of ESCs at 24 h after transfection was detected by flow cytometry. (C, D) The levels of BCL2 and CASP3 proteins were detected by blotting. *p < 0.05, **p < 0.01, ***p < 0.001, vs. vector group; #p < 0.05, ##p < 0.01 vs. si-NC group.
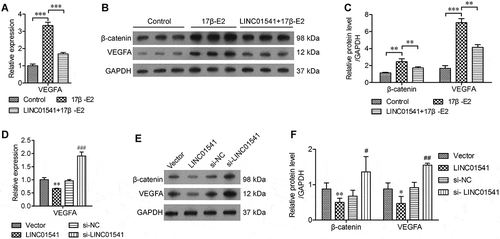
LINC01541 inhibited VEGFA expression and the Wnt/β-catenin pathway
Because a previous study reported that 17β-E2 promotes VEGFA expression via the Wnt/β-catenin pathway during the pathogenic process of endometriosis (Zhang et al. Citation2016), we examined VEGFA expression in ESCs that had been stimulated with 17β-E2 or transfected to overexpress LINC01541. Our results showed that VEGFA expression was markedly increased in the 17β-E2-stimulated ESCs and overexpression of LINC01541 could partially reverse this change (,). Moreover, the levels of β-catenin protein were also increased by 17β-E2 stimulation and decreased by siRNA targeting LINC01541 (). Furthermore, the levels of VEGFA mRNA were significantly increased after LINC01541 silencing (). Western blot studies also showed that the levels of VEGFA and β-catenin proteins in the LINC01541 silenced group were significantly higher than those in the negative control group (si-NC) (). These results suggest that LINC01541 can inhibit VEGFA expression in ESCs, possibly by repressing the Wnt/β-catenin pathway.
Figure 4. LINC01541 inhibited VEGFA expression and the Wnt/β-catenin signaling pathway. ESCs were stimulated with 10 nmol/L of 17β-E2 for 48 h. (A) The levels of VEGFA mRNA were detected by RT-qPCR. (B) The relative levels of β-catenin and VEGFA were detected by western blotting. **p < 0.01, ***p < 0.001, vs. the control or 17β-E2 group.(C) The levels of VEGFA mRNA in LINC01541 silenced and LINC01541 overexpressing ESCs. (D) The levels of β-catenin and VEGFA proteins in LINC01541 silenced and LINC01541 overexpressing ESCs. *p < 0.05, **p < 0.01, vs. vector group; #p < 0.05, ##p < 0.01, vs. si-NC group.
Discussion
Abnormal estrogen metabolism is a major risk factor for endometriosis. Endometriotic lesions produce higher levels of 17β-E2 than the eutopic endometrium of endometriosis patients and control subjects (Delvoux et al. Citation2009). Studies have revealed that 17β-E2 stimulation can promote the invasion and vascularization of endometriosis tissue (Wang et al. Citation2011), as well as pelvic inflammation and the growth of endometriosis (Khan et al. Citation2015). In our study, ESCs were stimulated with 10 nmol/L of 17β-E2 to simulate the internal environment of endometriosis. Results showed that the numbers of migrated cells and invasive cells increased after simulation, suggesting that 17β-E2 had significantly promoted the metastasis and invasiveness of ESCs. Moreover, the levels of N-Cadherin and vimentin proteins in the stimulated cells increased, while the levels of E-Cadherin decreased. As these proteins are considered to be biomarkers for EMT (Lamouille et al. Citation2014), our results suggest that 17β-E2 promotes the EMT process in ESCs. 17β-E2 has been reported to induce the transition of epithelial cells to mesenchymal cells (Huang et al. Citation2007; Zhao et al. Citation2012). As mentioned above, we found that 17β-E2 could promote both VEGFA and β-catenin expression, which is was also reported and validated by Zhang et al. in their study (Zhang et al. Citation2016).
17β-E2 simulation caused the abnormal expression of coding genes, as well as changes in the expression of non-coding RNAs. A previous study reported that H19 lncRNA mediates the 17β-E2-induced proliferation of breast cancer cells (Sun et al. Citation2015). In our study, we showed that 17β-E2 simulation significantly inhibited LINC01541 expression, and LINC01541 overexpression attenuated the 17β-E2-induced migration and invasion of ESCs. Moreover, silencing of LINC01541 promoted the EMT process and increased the migratory and invasive capabilities of ESCs, while overexpression of LINC01541 alone produced the reverse effect. Our results indirectly validated the change in LINC01541 expression reported in a previous study that involved a genome-wide profiling of lncRNA expression (Sun et al. Citation2014).
Our results showed that the Wnt/β-catenin signaling pathway plays an important role in the pathogenesis and development of endometriosis. In many types of tumor cells, inhibition of the Wnt/β-catenin signaling pathway has always simultaneously inhibited cellular metastasis and promoted apoptosis (Salomon et al. Citation2015; Wang and Li Citation2017). In addition, decreased rates of cell apoptosis have been associated with an enhancement of the EMT process (Shtivelman Citation1997; Robson et al. Citation2006). Both apoptosis and the EMT play fundamental roles in the pathogenesis of endometriosis. A recent study showed that human endometrial basal glandular epithelial cells express nuclear SOX9, a Wnt target gene, and contain a rare subpopulation of cells with nuclear β-catenin (Valentijn et al. Citation2013). Furthermore, another study showed that activation of the Wnt/β-catenin signaling pathway promotes the fibrosis found in endometriosis tissues (Matsuzaki and Darcha Citation2013). This signaling pathway is also thought to be involved in the process leading to granulosa cell atresia (Sanchez et al. Citation2014). These findings may explain why endometriosis affects approximately 25–50% of all women with infertility, and suggest the Wnt/β-catenin signaling pathway as a possible target for treating and/or preventing endometriosis (Matsuzaki et al. Citation2014). In this study, our results showed that overexpression of LINC01541 repressed β-catenin expression and the EMT process in 17β-E2-stimulated ESCs. Other studies have reported that activation of the Wnt/β-catenin signaling pathway promotes the EMT (Douchi et al. Citation2015; Ghahhari and Babashah Citation2015). Thus, we speculate that LINC01541 inhibits the EMT of ESCs by repressing the Wnt/β-catenin signaling pathway.
Our results showed that LINC01541 inhibits VEGFA expression in ESCs. It is generally known that VEGF concentrations are elevated in the peritoneal fluid and blood serum of women with endometriosis (Mclaren et al. Citation1996; Gagné et al. Citation2003). Studies have also shown that polymorphism of the VEGF gene is a risk factor for endometriosis (Zhao et al. Citation2008; Szczepańska et al. Citation2015), and that high levels of VEGF expression enhance the EMT in tumors (Luo et al. Citation2016). Therefore, it can be presumed that LINC01541 suppresses the growth of ectopic tissue and the EMT in endometriosis by inhibiting VEGFA expression. In addition, we found that overexpression of LINC01541 increased the levels of CASP3 protein and decreased the levels of BCL2 protein, which induced apoptosis in ESCs. An aberrant down-regulation of LINC01541 may inhibit the apoptosis process. Impaired spontaneous apoptosis in endometrial tissue contributes to the etiology and/or pathogenesis of endometriosis (Gebel et al. Citation1998). However, the molecular mechanism by which LINC01541 influences CASP3 and BCL2 expression remains unknown, and requires further study.
In summary, we found that silencing of LINC01541 enhanced the invasion ability of ESCs by promoting the EMT process. Overexpression of LINC01541 induced apoptosis and inhibited VEGFA expression in ESCs. These findings suggest that LINC01541 plays an important role in the pathogenesis of endometriosis.
Materials and methods
Cell culture
ESCs were purchased from the Procell Life Science & Technology Co., Ltd. (Wuhan, China) and cultured in Dulbecco’s modified Eagle’s medium (Hyclone, Logan, Utah, USA) supplemented with 10% heat-inactivated fetal bovine serum (Gibco-BRL, Gaithersburg, MD, USA), 100 U/mL of penicillin, and 100 U/mL of streptomycin (Beyotime, Shanghai, China) The ESCs were grown in a 5% CO2 atmosphere at 37°C.
Cell stimulation and transfection
As 17β-E2 plays an important role in promoting the development of endometriosis (Khan et al. Citation2015), we used 17β-E2 to stimulate ESCs. 17β-E2 was purchased from Sigma-Aldrich (St. Louis, MO, USA) and diluted to a concentration of 10 nmol/L for use. Cells were harvested after being stimulated with 17β-E2 for 48 h.
Small interfering RNA targeting LINC01541 (si-LINC01541) and the corresponding negative control (si-NC) were purchased from GenePharma (Suzhou, China). The sequence used for si-LINC01541 was 5ʹ-AUU CAA UUG UUU UUA AUC CAU TT -3ʹ and the sequence used for si-NC was 5ʹ-UUC UCC GAA CGU GUC ACG UTT -3ʹ. Additionally, the full-length sequence of LINC01541 was cloned from ESCs via the polymerase chain reaction (PCR) and inserted into the expression plasmid, pCDNA3.1. ESCs were transfected with the above siRNAs or recombinant overexpression vector by using a Lipofectamine 2000 Kit (Invitrogen, Carlsbad, CA, USA).
Assessment of cell viability
ESCs were transfected for 0, 12, 36, or 48 h, and then collected and transferred into 96-well flat-bottomed microplates (5 × 103 cells/well). ESC viability was measured according to instructions provided by the CCK8 Kit manufacturer (Dojindo, Kumamoto-ken, Japan). Briefly, the ESCs were incubated with 100 μL of CCK8 solution for 4 h at 37°C; after which, the optical density of each well at 490 nm (OD 490 nm) was recorded by a Microplate Reader (Thermo Scientific, Waltham, MA, USA).
Flow cytometry
After transfection for 24 h, ESCs were harvested, treated with reagents in an Annexin V/PI apoptosis kit (Multi Sciences, Hangzhou, China), and their apoptosis was detected by flow cytometry. In brief, ESCs (5 × 105 cells) were placed into six well culture plates and incubated with Annexin V/PI solution for 15 min in the dark. They were then analyzed for apoptosis with a BD FACS Calibur™ flow cytometer (BD Biosciences, Franklin, NJ, USA).
Cell migration and invasion assays
Cell migration assays were performed using a Transwell system (Corning Corp., Corning, NY, USA). After treatment for 48 h, ESCs were washed and cultured with serum-free medium for 12 h. Next, a 100 µL aliquot of the above single cell suspension (2 × 104 cells) was placed into each upper chamber of a Transwell plate. The lower chamber was filled with 700 µL of medium containing 10% FBS. After 48 h of incubation in a 5% CO2 atmosphere at 37°C, the cells on the lower side of the filter were fixed in 3.8% formaldehyde for 20 min, and then stained with 0.1% crystal violet solution. Six randomly selected microscopic fields were photographed with a CX41 microscope (Olympus, Tokyo, Japan), and the number of cells in each field was counted by using Image-Pro Plus software (Media Cybernetics, Rockville, MD, USA). Cell invasion assays were performed according to the same procedure, except that a Matrigel insert was placed between each upper and lower Transwell chamber.
Reverse transcription-quantitative polymerase chain reaction
Total RNA was extracted from cells with Trizol reagent (Takara Biotechnology Co., Ltd., Dalian, China), and 1 μg of total RNA was reverse-transcribed to cDNA by using a BestarTM PCR RT kit (DBI, Ludwigshafen, Germany). The RT-qPCR was performed by using BestarTM qPCR Master Mix (DBI) in an ABI Real time PCR system (Applied Biosystems, Waltham, MA, USA). All gene-specific primers were synthesized by Sangon Biotech Co., Ltd. (Shanghai, China). The forward and reverse primer sequences were as follows: LINC01541 forward, 5ʹ-TGT GTG GCT GCA TTC TGA AAT-3ʹ, and reverse, 5ʹ-AGG AAA GCA GAT AGG TGG CAT-3ʹ; VEGFA forward, 5ʹ-AGG GCA GAA TCA TCA CGA AGT-3ʹ, and reverse, 5ʹ-AGG GTC TCG ATT GGA TGG CA-3ʹ; GAPDH forward, 5ʹ-TGT TCG TCA TGG GTG TGA AC-3ʹ, and reverse, 5ʹ-ATG GCA TGG ACT GTG GTC AT-3ʹ. The RT-qPCR reactions were performed at the following conditions: 95°C for 10 min, followed by 40 cycles of 95°C for 10 s and 60°C for 30 s. The relative abundance of LINC01541 or VEGFA was determined by normalizing to GAPDH by using the 2−ΔΔCt method.
Western blotting
The total cellular proteins were extracted with a lysis buffer that contained 50 mM Tris (pH 7.6), 150 mM NaCl, 1% TritonX-100, 1% deoxycholate, 0.1% SDS, 1 mM PMSF, and 0.2% aprotinin (Sigma), and the total protein concentration in each extract was determined by using a BCA Protein Assay kit (Beyotime). Next, a 20 μg sample of total protein from each extract was separated by SDS-PAGE, and the protein bands were transferred onto a polyvinylidene fluoride membrane, which was subsequently blocked with 5% bovine serum albumin at room temperature for 1 h. After being blocked, the membrane was incubated with anti-E-Cadherin (Abcam, Cambridge, MA, USA, diluted 1:2000), anti-N-Cadherin (Abcam, diluted 1:2000), anti-vimentin (Abcam, diluted 1:2000), anti-β-catenin (Abcam, diluted 1:2000), anti-VEGFA (Abcam, diluted 1:1000), anti-BCL2 (Abcam, diluted 1:1000), or anti-CASP3 (Abcam, diluted 1:1000) at 4°C overnight. GAPDH (Abcam, diluted 1:5000) was used as an internal control. After incubation, the blots were washed with TBST and incubated with a horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA, diluted 1:2000) at room temperature for 1 h. Finally, the protein bands were visualized with a Pierce ECL Plus Western Blotting Substrate (Thermo Scientific).
Statistical analysis
All experiments were performed in triplicate. Data were analyzed by Student’s t-test as performed using Prism 7.0 software (GraphPad Software, La Jolla, CA, USA). Results represent the mean ± standard deviation (SD).
Notes on contributors
Contributed to the conception and design of the study: HM, YPW, HX ; Performed the experiments: HM, YPW, YY, SJH, HSL; Analyzed the data: HM, YL, XPL; Contributed materials and facilities: XFC, HJS, CZL; Wrote the manuscript: HM, HX. All authors approved any revisions and the final paper.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Andrade S, Azevedo AC, Monasterio I, Paredes-Gamero E, Gonçalves G, Bonetti T, Albertoni G, Schor E, Barreto J, Luiza Oliva M. 2013. 17β-Estradiol and steady-state concentrations of H2O2: antiapoptotic effect in endometrial cells from patients with endometriosis. Free Radic Biol Med. 60(10):63–72.
- Bulun SE, Cheng YH, Pavone ME, Xue Q, Attar E, Trukhacheva E, Tokunaga H, Utsunomiya H, Yin P, Luo X. 2010. Estrogen receptor-beta, estrogen receptor-alpha, and progesterone resistance in endometriosis. Semin Reprod Med. 28(1):36–43.
- Dassen H, Punyadeera C, Kamps R, Delvoux B, Van Langendonckt A, Donnez J, Husen B, Thole H, Dunselman G, Groothuis P. 2007. Estrogen metabolizing enzymes in endometrium and endometriosis. Hum Reprod. 22(12):3148–3158.
- Delvoux B, Groothuis P, D’Hooghe T, Kyama C, Dunselman G, Romano A. 2009. Increased production of 17beta-estradiol in endometriosis lesions is the result of impaired metabolism. J Clin Endocrinol Metab. 94(3):876–883.
- Douchi D, Ohtsuka H, Ariake K, Masuda K, Kawasaki S, Kawaguchi K, Fukase K, Oikawa M, Motoi F, Naitoh T. 2015. Silencing of LRRFIP1 reverses the epithelial–mesenchymal transition via inhibition of the Wnt/β-catenin signaling pathway. Cancer Lett. 365(1):132–140.
- Dunselman GAJ, Vermeulen N, Becker C, Calhazjorge C, D’Hooghe T, De Bie B, Heikinheimo O, Horne AW, Kiesel L, Nap A. 2014. ESHRE guideline: management of women with endometriosis. Hum Reprod. 29(3):400–412.
- Fatica A, Bozzoni I. 2014. Long non-coding RNAs: new players in cell differentiation and development. Nat Rev Genet. 15(1):7–21.
- Gagné D, Pagé M, Robitaille G, Hugo P, Gosselin D. 2003. Levels of vascular endothelial growth factor (VEGF) in serum of patients with endometriosis. Hum Reprod. 18(8):1674–1680.
- Gebel HM, Braun DP, Tambur A, Frame D, Rana N, Dmowski WP. 1998. Spontaneous apoptosis of endometrial tissue is impaired in women with endometriosis. Fertil Steril. 69(6):1042–1047.
- Ghahhari N, Babashah S. 2015. Interplay between microRNAs and WNT/β-catenin signalling pathway regulates epithelial-mesenchymal transition in cancer. Eur J Cancer. 51(12):1638–1649.
- Ghazal S, Mckinnon B, Zhou J, Mueller M, Men Y, Yang L, Mueller M, Flannery C, Huang Y, Taylor HS. 2015. H19 lncRNA alters stromal cell growth via IGF signaling in the endometrium of women with endometriosis. EMBO Mol Med. 7(8):996–1003.
- Giudice LC, Kao LC. 2004. Endometriosis. Lancet. 364(9447):1789–1799.
- Huang Y, Fernandez SV, Goodwin S, Russo PA, Russo IH, Sutter TR, Russo J. 2007. Epithelial to mesenchymal transition in human breast epithelial cells transformed by 17beta-estradiol. Cancer Res. 67(23):11147–11157.
- Ilangavan K, Kalu E. 2009. High prevalence of endometriosis in infertile women with normal ovulation and normospermic partners. Fertil Steril. 92(1):68–74.
- Janssen EB, Rijkers AC, Hoppenbrouwers K, Meuleman C, D’Hooghe TM. 2013. Prevalence of endometriosis diagnosed by laparoscopy in adolescents with dysmenorrhea or chronic pelvic pain: a systematic review. Hum Reprod Update. 19(5):570–582.
- John AS. 1927. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Clin Obstet Gynecol. 14(4):93–94.
- Kapranov P, Cheng J, Dike S, Nix D, Duttagupta R, Willingham A, Stadler P, Hertel J, Hackermüller J, Hofacker I, et al. 2007. RNA maps reveal new RNA classes and a possible function for pervasive transcription. Science. 316(5830):1484–1488.
- Khan KN, Kitajima M, Inoue T, Fujishita A, Nakashima M, Masuzaki H. 2015. 17β-estradiol and lipopolysaccharide additively promote pelvic inflammation and growth of endometriosis. Reprod Sci. 22(5):585–594.
- Lamouille S, Xu J, Derynck R. 2014. Molecular mechanisms of epithelial–mesenchymal transition. Nat Rev Mol Cell Biol. 15(3):178–196.
- Luo M, Hou L, Li J, Shao S, Huang S, Meng D, Liu L, Feng L, Xia P, Qin T. 2016. VEGF/NRP-1axis promotes progression of breast cancer via enhancement of epithelial-mesenchymal transition and activation of NF-κB and β-catenin. Cancer Lett. 373(1):1–11.
- Matsuzaki S, Botchorishvili R, Pouly JL, Canis M. 2014. Targeting the Wnt/β-catenin pathway in endometriosis: a potentially effective approach for treatment and prevention. Mol Cell Ther. 2(1):36.
- Matsuzaki S, Darcha C. 2013. Involvement of the Wnt/β-catenin signaling pathway in the cellular and molecular mechanisms of fibrosis in endometriosis. PLoS ONE. 8(10):e76808.
- Mclaren J, Prentice A, Charnock-Jones DS, Smith SK. 1996. Vascular endothelial growth factor (VEGF) concentrations are elevated in peritoneal fluid of women with endometriosis. Hum Reprod. 11(1):220–223.
- Oxholm D, Knudsen UB, Kryger-Baggesen N, Ravn P. 2007. Postmenopausal endometriosis. Acta Obstet Gynecol Scand. 86(10):1158–1164.
- Robson EJ, Khaled WT, Abell K, Watson CJ. 2006. Epithelial-to-mesenchymal transition confers resistance to apoptosis in three murine mammary epithelial cell lines. Differentiation. 74(5):254–264.
- Salomon A, Keramidas M, Maisin C, Thomas M. 2015. Loss of β-catenin in adrenocortical cancer cells causes growth inhibition and reversal of epithelial-to-mesenchymal transition. Oncotarget. 6(13):11421–11433.
- Sanchez AM, Viganò P, Quattrone F, Pagliardini L, Papaleo E, Candiani M, Paninabordignon P. 2014. The WNT/β-catenin signaling pathway and expression of survival promoting genes in luteinized granulosa cells: endometriosis as a paradigm for a dysregulated apoptosis pathway. Fertil Steril. 101(6):1688–1696.
- Sha L, Huang L, Luo X, Bao J, Gao L, Pan Q, Guo M, Zheng F, Wang H. 2017. Long non-coding RNA LINC00261 inhibits cell growth and migration in endometriosis. J Obstet Gynaecol Res. 43(10):1563–1569.
- Shtivelman E. 1997. A link between metastasis and resistance to apoptosis of variant small cell lung carcinoma. Oncogene. 14(18):2167–2173.
- Simoens S, Dunselman G, Dirksen C, Hummelshoj L, Bokor A, Brandes I, Brodszky V, Canis M, Colombo GL, Deleire T. 2012. The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Hum Reprod. 27(5):1292–1299.
- Sun H, Wang G, Peng Y, Zeng Y, Zhu QN, Li TL, Cai JQ, Zhou HH, Zhu YS. 2015. H19 lncRNA mediates 17beta-estradiol-induced cell proliferation in MCF-7 breast cancer cells. Oncol Rep. 33(6):3045–3052.
- Sun PR, Jia SZ, Lin H, Leng JH, Lang JH. 2014. Genome-wide profiling of long noncoding ribonucleic acid expression patterns in ovarian endometriosis by microarray. Fertil Steril. 101(4):1038–1046.e1037.
- Szczepańska M, Mostowska A, Wirstlein P, Skrzypczak J, Jagodziłski PP. 2015. Involvement of vascular endothelial growth factor −460 C/T, +405 G/C and +936 C/T polymorphisms in the development of endometriosis. Biomed Rep. 3(2):220–224.
- Valentijn AJ, Palial K, Allamee H, Tempest N, Drury J, Von ZT, Saretzki G, Murray P, Gargett CE, Hapangama DK. 2013. SSEA-1 isolates human endometrial basal glandular epithelial cells: phenotypic and functional characterization and implications in the pathogenesis of endometriosis. Hum Reprod. 28(10):2695–2708.
- Wang BM, Li N. 2017. Effect of the Wnt/β-catenin signaling pathway on apoptosis, migration and invasion of transplanted hepatocellular carcinoma cells after transcatheter arterial chemoembolization in rats. J Cell Biochem. 119(5):4050–4060.
- Wang D, Liu Y, Han J, Zai D, Ji M, Cheng W, Xu L, Yang L, He M, Ni J. 2011. Puerarin suppresses invasion and vascularization of endometriosis tissue stimulated by 17β-estradiol. PLoS ONE. 6(9):e25011.
- Wang KC, Chang HY. 2011. Molecular mechanisms of long noncoding RNAs. Mol Cell. 43(6):904–914.
- Wapinski O, Chang HY. 2011. Long noncoding RNAs and human disease. Trends Cell Biol. 21(6):354–361.
- Zhang L, Xiong W, Yao X, Liu H, Liu Y. 2016. 17 β-Estradiol promotes vascular endothelial growth factor expression via the Wnt/β-catenin pathway during the pathogenesis of endometriosis. Mol Hum Reprod. 22(7):526–535.
- Zhao G, Nie Y, Lv M, He L, Wang T, Hou Y. 2012. ERÎ2-mediated estradiol enhances epithelial mesenchymal transition of lung adenocarcinoma through increasing transcription of midkine. Mol Endocrinol. 26(8):1304–1315.
- Zhao ZZ, Nyholt DR, Thomas S, Treloar SA, Montgomery GW. 2008. Polymorphisms in the vascular endothelial growth factor gene and the risk of familial endometriosis. Mol Hum Reprod. 14(9):531–538.
