ABSTRACT
HOXA10 is an important regulator of embryo adhesion. Reduced expression may contribute to embryo implantation failure. The expression of Nur77 and HOXA10 has been shown to be reduced in the endometrium of patients with recurrent implantation failure. In this study, we found that Nur77 was directly bound to the HOXA10 promoter and promoted HOXA10 protein expression in a dose-dependent manner in Ishikawa cells. Furthermore, the promoting effect of Nur77 on embryo adhesion was significantly blocked when endogenous HOXA10 expression was knocked down in Ishikawa cells. Moreover, the Nur77 agonist 6-MP was also confirmed to promote embryo adhesion. This study is the first to show that Nur77 promotes embryo adhesion by transcriptionally regulating HOXA10 expression. Nur77 and 6-MP may provide a basis to develop a new treatment for patients who suffer from embryo adhesion dysfunction.
Abbreviations: HB-EGF: heparin-binding epidermal growth factor-like growth factor; HOXA10: homeobox A10; NGFI-B: nerve growth factor-induced gene B; RIF: recurrent implantation failure; RPL: recurrent pregnancy loss; 6-MP: 6-mercaptopurine; IGFBP-1: insulin-like growth factor binding protein-1; LIF: leukemia inhibitory factor; IL-1β: Interleukin - 1β; CRISPR/Cas9: Clustered Regularly Interspaced Short Palindromic Repeats; AF-1: activation function-1 domain; HIF-1α: hypoxia-inducible factor 1α; CREB: cAMP response element binding
Introduction
The coordination of both embryonic development and endometrial status is crucial for a successful pregnancy. The uterus is a hormone-regulated organ that has no embryo adhesion during most of the menstrual cycle. Endometrial receptivity refers to a hormone-limiting period in which the endometrium acquires a functional sex hormone-dependent state that allows the blastocyst to implant (Miravet-Valenciano et al. Citation2015). Embryo implantation begins with blastocyst attachment. Initial cell-to-cell contact mediates communication between trophoblast cells and apical plasma membranes of uterine epithelial cell. Uterine epithelial cells do not allow other cells to adhere to their apical surfaces, making trophoblast-uterine epithelial attachment a unique condition (Fitzgerald et al. Citation2008).
Several key factors, such as leukemia inhibitory factor (LIF) (Aghajanova Citation2010.), integrin β3 (ITGB3) (Achache and Revel Citation2006) and homeobox A10 (HOXA10) (Daftary et al. Citation2002), have been reported to participate in the regulation of the attachment process. HOXA10, which is a key transcriptional factor being considered to be a biomarker of endometrial receptivity, binds to the DNA sequence TAAT or TTAT on downstream target genes to regulate their transcriptional expression (Eun Kwon and Taylor Citation2004). For example, HOXA10 binds to a 41-bp TTAT-containing sequence on the ITGB3 promoter region to activate ITGB3 expression and promote embryo adhesion (Daftary et al. Citation2002). HOXA10 is dynamically expressed in the endometrial glands and stroma under the regulation of estrogen and progesterone throughout the menstrual cycle, reaching a peak in the middle and late stages of secretion (Taylor et al. Citation1998). In addition, HOXA10 is also regulated by several other factors, such as HB-EGF (Liu et al. Citation2007), VitD (Du et al. Citation2005), and IL-1β (Sarno et al. Citation2009). The expression level of HOXA10 is decreased in the endometrium of women with endometriosis (Zanatta et al. Citation2010), hydrosalpinx (Daftary and Taylor Citation2002), and polycystic ovary syndrome (Cermik et al. Citation2003), which may cause infertility. Furthermore, reduced expression of HOXA10 in the endometrial glandular epithelium has been clearly observed in recurrent implantation failure (RIF) patients (Yang et al. Citation2017). However, the mechanism of downregulated HOXA10 expression in implantation failure remains unclear.
The orphan nuclear receptor Nur77, a member of the steroid hormone NR4A superfamily of transcription factors, binds in a monomer/dimer form through a DNA binding domain (DBD) to an NBFI-B response element (NBRE: AAAGGTCA) or Nur-responsive element (NurREs: TGATATTTn6AAATGCCA) in the promoter region of the target gene to control their expression. This can be induced by a variety of physiological and pathological stimuli and participate in the regulation of cell proliferation, differentiation and apoptosis (Niu et al. Citation2014), leading to the occurrence of various diseases, such as tumors (Mohan et al. Citation2012), inflammation (McMorrow and Murphy Citation2011.), and atherosclerosis (Hamers et al. Citation2013). Our group previously found that the Nur77 expression is dramatically increased in human decidua tissue and peri-implantation mouse uteri and that Nur77 promotes the decidualization by increasing the dPRL and IGFBP-1 gene expression and secretion in human endometrial stromal cells (Jiang et al. Citation2016). Consistently, our previous study also found that Nur77 expression is significantly reduced not only in stromal cells but also in endometrial epithelial cells of RIF patients (Huang et al. Citation2017). These results indicated that Nur77 may be involved in regulating embryo implantation.
Interestingly, we found a Nur77 binding element (NBRE, AAAGGTCA, −3075/-3067) in the HOXA10 promoter region and hypothesized that Nur77 may facilitate embryo adhesion by transcriptionally regulating HOXA10 expression.
In the present study, we investigated whether Nur77 could bind to HOXA10 promoter and promote HOXA10 expression and facilitates embryo adhesion.
Results
Nur77 promotes the expression of HOXA10 in Ishikawa cells
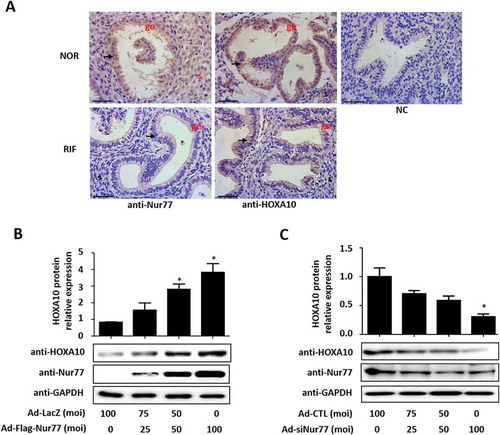
In the endometrium from all volunteers shown in , the Nur77 and HOXA10 expression levels were lower in human endometrial epithelial cells from the patients with RIF than in cells from the control patients (). Western blotting results showed that Nur77 promoted the expression of HOXA10 in a dose-dependent manner (). After silencing the expression of endogenous Nur77 in Ishikawa cells, the expression of the HOXA10 protein was significantly suppressed by more than 70% (). These results suggested that Nur77 could affect the expression of HOXA10 in endometrial epithelial cells.
Table 1. Demographic details of the participants in the study of endometrial Nur77 and HOXA10 expression.
Figure 1. Nur77 promotes HOXA10 expression in Ishikawa cells. (A) Immunohistochemical analysis was performed using Nur77 and HOXA10 antibodies. Endometrial tissue samples from female control patients (NOR) and female patients with RIF are shown at 400× magnification. The negative control (NC) is nonspecific rabbit serum. Scale bars, 50 μm. (B) Ishikawa cells were infected with Ad-LacZ or Ad-Flag-Nur77 at the indicated MOI for 48 h. HOXA10 protein expression was determined by Western blotting and quantitated by densitometric analysis. *p < 0.05 vs. the Ad-LacZ group. (C) Ishikawa cells were infected with Ad-CTL or Ad-siNur77 at the indicated MOI for 48 h, HOXA10 protein expression was determined by Western blotting and was quantitated by densitometric analysis. *p < 0.05 vs. the Ad-CTL group.
HOXA10 is a novel target gene for Nur77 in Ishikawa cells
Because the HOXA10 promoter region (−3075/-3067) contains a Nur77 binding element (NBRE, AAAGGTCA), we speculated that Nur77 transcriptionally regulates HOXA10 expression by binding to a specific binding site in the HOXA10 promoter region. First, we constructed a promoter region reporter gene plasmid containing the NBRE site (HOXA10-Luc) and a promoter reporter gene plasmid with an NBRE site deletion mutation (HOXA10-del-Luc) and then performed a luciferase reporter assay. As shown in ,B, Nur77 overexpression increased the luciferase activity of the HOXA10-Luc construct by more than 2-fold; however, the luciferase activity of HOXA10-del-Luc was unaffected. This result suggested that Nur77 could play a role in enhancing the activity of the HOXA10 promoter in Ishikawa cells.
Figure 2. HOXA10 is a direct target of Nur77. (A, B) Ishikawa cells were infected with the indicated adenoviruses for 24 h and then transfected with HOXA10-Luc or HOXA10-del-Luc (250 ng/well). After 24 h, luciferase assays were performed, and the data were plotted after normalization to Renilla luciferase activity. *p < 0.05 vs. the Ad-LacZ group. (C) ChIP-PCR amplification using primers against the human Nur77 promoter region (top). PCR products were separated by agarose gel electrophoresis. (D) ABCD assays were performed using biotinylated or nonbiotinylated (competitor) double-stranded HOXA10 wild-type (WT) and mutant (MUT) oligonucleotides with whole-cell extracts from Ishikawa cells.
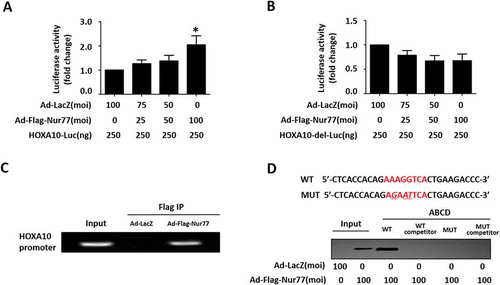
To further identify whether HOXA10 is a target gene for Nur77, we performed a ChIP-PCR assay. Using PCR amplification primers targeting HOXA10 predicted binding site sequences, the expected DNA fragments were amplified from immunoprecipitate of Ishikawa cells infected with Ad-Flag-Nur77, whereas the control group could not amplify the target band (). In addition, ABCD assays were performed using biotinylated double-stranded oligonucleotides corresponding to the wild-type (WT) (−3085/−3057 bp) and mutated (MUT) HOXA10 promoter sequences. The results showed that the Flag-tagged Nur77 proteins strongly bound to the WT probe but not to the MUT probe (). Overall, these data demonstrated that Nur77 transcriptionally promotes HOXA10 by directly binding to the conserved AAAGGTCA element in the promoter region.
Nur77 promotes embryo adhesion in vitro
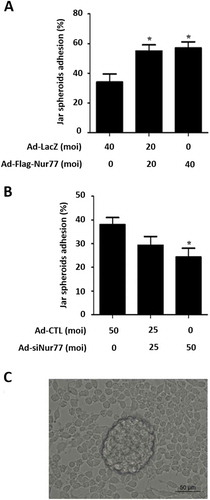
As shown in , overexpression of Nur77 in Ishikawa cells increased the ratio of Jar spheroid adhesion by 1.7-fold (57 ± 4.2% vs. 34 ± 5.5%, *p < 0.05, vs. Ad-LacZ). However, knockdown of endogenous Nur77 protein expression in Ishikawa cells obviously suppressed Jar spheroid attachment (24.3 ± 3.7% (Ad-siNur77) vs. 38 ± 3% (Ad-CTL, *p < 0.05, ). These results suggest that Nur77 contributes to embryo adhesion in vitro.
Figure 3. Nur77 facilitates embryo adhesion in vitro. (A) Ishikawa cells were infected with Ad-LacZ and Ad-Flag-Nur77 at MOI of 0, 20 or 40 for 48 h. (B) Ishikawa cells were infected with Ad-CTL and Ad-siNur77 at MOI of 0, 25 or 50. (C) A schematic of Jar spheroid attachment to the Ishikawa cell monolayer. The data represent the results of 3 independent experiments. ANOVA was used to compare the percentage of the attached spheroids in each treatment with that of the control. *p < 0.05 vs. the Ad-LacZ/CTL group.
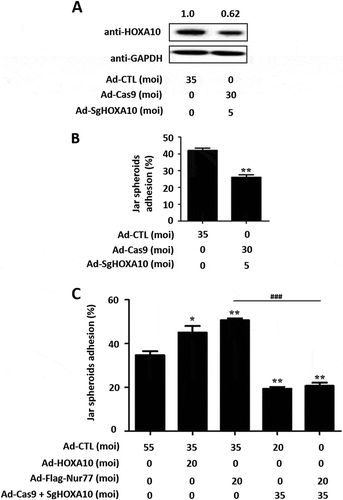
To further investigate whether HOXA10 mediates the process of Nur77-induced embryo adhesion, we used adenoviral CRISPR/Cas9 vectors for efficient gene inactivation. Western blotting showed that cotransduction of adenoviral Cas9 with HOXA10-targeting guide RNAs (SgHOXA10) effectively inhibited the endogenous expression of the HOXA10 protein by 40% in Ishikawa cells (). Subsequent Jar spheroid-attachment assays showed that the Jar spheroid-attachment rate decreased significantly to 26 ± 1.5% (vs. 42 ± 1.5%, **p < 0.01, vs. Ad-CTL) when HOXA10 expression was inhibited (). As shown in , Nur77-induced embryo adhesion was not observed after inhibition of endogenous HOXA10 expression, and the ratio of embryo adhesion was only 20.7 ± 1.5% (vs. 50.7 ± 0.88%, ###p < 0.001, vs. Ad- Flag-Nur77). These data indicated that HOXA10 mediates Nur77-induced embryo adhesion.
Figure 4. HOXA10 mediates Nur77 involvement in embryo adhesion. (A) Ishikawa cells were infected with Ad-Cas9 + Ad-SgHOXA10 at MOI of 0 or 35 for 48 h, and Western blotting detected the expression of the HOXA10 protein. (B) Ishikawa cells were infected with Ad-Cas9 + Ad-SgHOXA10 at MOI of 0 or 35 for 48 h, and a Jar spheroid attachment assay was conducted. **p < 0.01 vs. the Ad-CTL group. (C) Ishikawa cells were infected with Ad-Cas9 + Ad-SgHOXA10. After 24 h, the cells were treated with Ad-Flag-Nur77 as indicated for an additional 48 h. Several spheroids were transferred to confluent monolayers of Ishikawa cells. *p < 0.05 and **p < 0.01 compared with the Ad-CTL group at the same time point; ###p < 0.001 compared with the Ad-Flag-Nur77 group at the same time point.
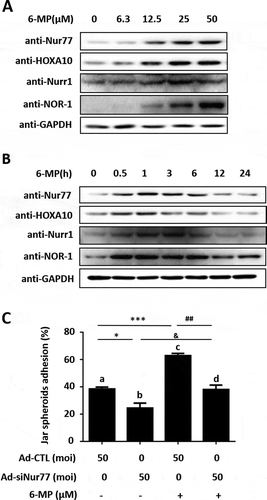
The Nur77 agonist 6-MP promotes HOXA10 expression and embryo adhesion
Previous studies have shown that 6-MP, which is a Nur77-specific agonist, promotes Nur77 expression and activates Nur77 AF-1 region to enhance the transcriptional activity of Nur77 (Huang et al. Citation2016). We have demonstrated that Nur77 promoted embryo adhesion through transcriptionally regulating HOXA10. Next, we clarified whether 6-MP has an agonistic effect on Nur77-induced embryo adhesion. Western blotting results showed that the Nur77 and HOXA10 protein expression were both promoted by 6-MP treatment in Ishikawa cells and the expression of Nur77 and HOXA10 were the highest in Ishikawa cells after treating the cells with 25 μM 6-MP for 1 h (,B). Furthermore, 6-MP induced the expression of Nurr1 and NOR-1 proteins. 6-MP significantly promoted Jar spheroid attachment to Ishikawa cells (62.7 ± 1.8% (lane c) vs. 38.3 ± 1.4% (lane a), ***p < 0.001). When the expression of endogenous Nur77 was knocked down in Ishikawa cells, embryo adhesion was inhibited, and 6-MP reversed this inhibitory effect (24.3 ± 3.7% (lane b) vs. 38 ± 3.2% (lane d), & p < 0.05, ). These results indicated that 6-MP significantly promotes embryo adhesion.
Figure 5. 6-MP promotes HOXA10 expression and embryo adhesion. (A) Ishikawa cells were stimulated by 6-MP at the indicated concentrations and then subjected to Western blotting. (B) Ishikawa cells were stimulated with 25 μM 6-MP at the indicated timepoints and then subjected to Western blotting. (C) Ishikawa cells were infected with Ad-CTL and Ad-siNur77 at MOI of 0 or 50 for 48 h, and then stimulated with 25 μM 6-MP for 1 h. Several spheroids were transferred to confluent monolayers of Ishikawa cells. *p < 0.05, *** p < 0.001 compared with lane a; ##p < 0.01 compared with lane c; and &p < 0.05 compared with lane b (repeated measures data ANOVA).
Discussion
Although assisted reproduction has developed rapidly, the rate of successful pregnancy remains very low. Patients with implantation failure account for approximately 40% of the IVF cycles, which is the main reason for the low assisted reproduction pregnancy rate (Zohni et al. Citation2016). Embryo implantation involves the process of an embryo recognizing the maternal endometrium, being contained within the maternal endometrium and interacting with the maternal endometrium. Embryo implantation begins with the localization and adhesion of the blastula to the endometrium.
Previous studies have shown that Nur77 plays an important role in embryo implantation by promoting decidualization (Jiang et al. Citation2016). Nur77 is one of orphan nuclear receptor NR4A family, which also includes Nurr1 and NOR-1 (Rodríguez-Calvo et al. Citation2017). This NR4A family members consist of three major domains: The N-terminal AF-1 domain (transcription activation domain), the DNA binding domain (DBD), and the C-terminal ligand domain (LBD). The DBD homology >90%, the LBD homology is centered, AF-1 transcriptional activation domain conservation is low, the homology between Nur77 and Nurr1 is 27%, and the homology between Nur77 and NOR-1 is 21%. All of them can exert transcriptional activation by binding to specific sites close in the promoter region of the target gene (Kurakula et al. Citation2014). Similar to Nur77, Nurr1 could play a similar role promoting aldosterone expression by transcriptionally activating Cyp11b2 (Bassett et al. Citation2004). NOR-1 could take the place of Nur77 and transcriptionally promote Cyp21 expression in adrenal cortical tumor cells (Fernandez et al. Citation2000). This suggested compensation occurs among the three members of the NR4A family. Nur77−/- mice do not show significant abnormalities in body size, growth, development, or behavior (Crawford et al. Citation1995). However, Nurr1−/- mice die shortly after birth due to severe abnormalities in dopamine neurons (Zetterstrom et al. Citation1997). These observations show that the specific functions of NR4A family members cannot be completely interchanged. Jiang et al. showed that the expression levels of Nur77, Nurr1 and NOR-1 were all elevated in human uterine decidua tissues and mouse uterine tissues during the peri-implantation period, and Nur77 elevation was more obvious (Jiang et al. Citation2016). These results suggest that NR4A is involved in decidualization regulation during peri-implantation. Nur77−/- mice ovulate normally, and the expression of Nurr1 and Nor-1 occur as a compensatory increase in Nur77−/- mouse uteruses (Jiang et al. Citation2016). This result indicated that the decline in fertility is mainly associated with loss of Nur77 expression in Nur77−/- mouse, due to the loss of Nur77 expression leading to decidualization disorder. In his study, we found that Nur77 promotes embryo adhesion in vitro. However, whether Nurr1 and Nor-1 could facilitate embryo adhesion requires further study.
In addition, we hope to achieve clinical translation of Nur77 to provide a new direction for the clinical treatment of embryo implantation failure. In most cases, the expression of the Nur77 protein is the major mechanism regulating its transcriptional activity without a ligand (Paulsen et al. Citation1995). Hyperoside, which is Nur77 agonist, rapidly induces Nur77 expression in vascular smooth muscle cells (VSMCs) via the MEK1/2/CREB pathway, which in turn increases the transcriptional activity of Nur77 to prevent atherosclerosis and vascular restenosis by inhibiting VSMC proliferation and neovascular intima formation (Huo et al. Citation2014). 6-MP enhances transcriptional activity of HIF-1α resulting in new vessel formation via the induction of Nur77 expression and suggesting a potential application of this drug in therapies for human HIF-1-associated vascular diseases (Yoo et al. Citation2007). In our study, 6-MP was also shown to activate the expression and transcriptional activity of Nur77, leading to increased embryo adhesion. However, we found that hyperoside had no obvious effect on embryo adhesion but 6-MP significantly increased the embryo adhesion rate. It is possible that 6-MP is an activator of the NR4A subfamily members (Wansa et al. Citation2003). As shown in ,B, the expression of the Nur77 protein was found to be induced by 6-MP treatment. Treatment with 6-MP also induced the expression of Nurr1 and Nor-1 proteins, which are other members of the NR4A subfamily, in Ishikawa cells. However, hyperoside only activates Nur77 expression and its transcriptional activity (Huo et al. Citation2014). Therefore, the obvious promotion of embryo adhesion by 6-MP may be due to the simultaneous activation of NR4A subfamily in Ishikawa cells. In the future, we aim to further explore the role of Nurr1 and Nor-1 in embryo adhesion.
In summary, this is the first study to identify that the orphan nuclear receptor Nur77 promotes embryo adhesion through transcriptionally upregulating HOXA10 expression in vitro. These findings suggest that Nur77 and its agonist 6-MP are expected to provide a new treatment basis for patients who suffer from implantation failure caused by embryo adhesion dysfunction in assisted reproduction. However, the limitation of this study is that we preformed all experiments in Ishikawa cells as previous studies did (Yan et al. Citation2018), since there are still many difficulties in culturing primary endometrial epithelial cells even though we isolated them successfully. In the future, we will focus on the isolation and culture of primary uterine epithelial cells in our further research.
Materials and methods
Patients and sample collection
Endometrial biopsies for this study were obtained from female patients attending the Center for Reproductive Medicine of Nanjing Drum Tower Hospital. All samples were collected with the informed consent of the patients, and approval from the ethics committee was obtained for this study. Secretory endometria (between days 19 and 23 of the regular menstrual cycle) were obtained from six female control patients and six female patients with RIF after in vitro fertilization and embryo transfer (IVF-ET). Patients who had experienced at least one normal pregnancy and/or delivery were recruited as normal women. RIF was defined as failure to achieve pregnancy following a minimum of three fresh or frozen cycles, during which at least four good-quality embryos were transferred to the uterus (Yang et al. Citation2017).
Cell culture
Ishikawa cell, a well-differentiated human endometrial adenocarcinoma cell line who bears functional steroid receptors and possess apical adhesiveness, were maintained in Dulbecco’s modified Eagle’s medium (DMEM)/F12 (Corning, NY, USA) supplemented with 10% (v/v) fetal bovine serum (FBS, Corning) and 1% penicillin/streptomycin (HyClone, Thermo Scientific, South Logan, UT, USA). Jar cells were cultured in Roswell Park Memorial Institute (RPMI) 1640 media (HyClone) containing 10% FBS and 1% penicillin/streptomycin. The cells were cultured in a humidified atmosphere containing 5% CO2 at 37 °C. The cells were used at the second through fifth passages for this study.
Construction of adenovirus
The adenovirus harboring the full-length Nur77 gene (Ad-Flag-Nur77) and human Nur77 siRNA oligonucleotides (Ad-siNur77) were previously constructed (Jiang et al. Citation2011). Cas9 and SgHOXA10 adenoviruses were purchased from Abm (Richmond, BC, Canada). The virus was amplified in HEK293A cells and purified using CsCl banding. Titering was measured by Adeno-X Rapid Titer Kit (BD Biosciences Clontech, Palo Alto, CA, USA) according to the manufacturer’s instructions.
Western blotting
Proteins were extracted as previously described (Jiang et al. Citation2016). Immunoblotting was performed with primary antibodies against Nur77 (1:500 dilution, Cell Signaling Technology, Danvers, MA, USA), HOXA10 (1:1000 dilution, Santa Cruz, CA, USA), GAPDH (1:10000 dilution, Bioworld Technology, St Louis Park, MN, USA), NOR-1 (1:500 dilution, Bioworld), Nurr1 (1:500 dilution, Bioworld), followed by donkey anti-goat (1:5000 dilution, Santa Cruz) or goat anti-rabbit (1:10000 dilution, Bioworld) horseradish peroxidase (HRP)-conjugated secondary antibodies. The signal was detected with an ECL kit (Millipore, Billerica, MA, USA).
Jar spheroid-attachment assay
A blastocyst-like spheroid implantation model was employed broadly because of its accuracy and effectiveness (Montazeri et al. Citation2016). Jar cells (1 × 106 cells per 6 ml RPMI medium in 60 mm dishes) were agitated at 37 °C in 5% CO2 on a horizontal shaker at 80–90 rpm for 24 h to form Jar spheroids. Fifty spheroids were gently diverted into each well containing a confluent monolayer of Ishikawa cells. After the coculture was maintained for 1 h at 37 °C, the plate was placed on a horizontal shaker (80–90 rpm for 3 min) to detach loosely bound or unbound spheroids from the monolayer. The cells were washed with PBS twice to remove nonadherent spheroids. The number of attached spheroids was counted under a microscope. The results are presented as a fold change relative to control-treated cells.
Transfection and luciferase reporter assays
The wide-type human HOXA10 promoter sequence (−3198 to −2708) was amplified by polymerase chain reaction (PCR) from human genomic DNA using the following primers: 5ʹ-GCATGGTACCGCTGCCATACATGAGCTTAC-3ʹ (forward) and 5ʹ-GCGCCTCGAGAATGCATATCTGTATATTCC-3ʹ (reverse). In addition, we also designed the following primers for the HOXA10 promoter deletion mutant (lacking AAAGGTCA): 5ʹ-TCTTCAGCTGTGGTGAGCCA-3ʹ and 5ʹ-ACCACAGCTGAAGACCCAAG-3ʹ. The PCR products were cloned into pGL3 promoter luciferase reporter plasmids (Promega, Madison, WI, USA) at the XhoI and KpnI sites. The identity of inserts was confirmed by sequencing. Preconfluent (75–80%) Ishikawa cells in 24-well plates were infected with Ad-Flag-Nur77 and then transfected with 250 ng of HOXA10 reporter plasmid and 5 ng of Renilla luciferase plasmid for 48 h using MaxPEI transfection reagent (TG1002, Tigergene Technologies, PA, USA). Luciferase activity of cell lysates was determined by a Dual-Luciferase Assay System (Promega) and measured by a Centro XS3 LB 960 luminescence counter (Berthold Technologies, KG, Germany), as specified (Jiang et al. Citation2016).
Chromatin immunoprecipitation (ChIP)-PCR assay
Ishikawa cells (75–80% confluence) were infected with Ad-LacZ and Ad-Flag-Nur77 (multiplicity of infection (MOI) = 100) for 48 h and then used for ChIP as previously described (Jiang et al. Citation2016). The purified DNA fragments were pulled down by Flag-Beads (Sigma, St. Louis, MO, USA) and used as a template for PCR amplification. The specific primers used to amplify HOXA10 promoter DNA fragments containing a Nur77 binding sequence were 5′-CCAACATCCCGTTTGGTTTA-3′ and 5′-CCAGCCGAAGAGTAGAGGAG-3′.
Avidin-biotin conjugate DNA precipitation (ABCD) assay
The following double-stranded oligonucleotides were used for the ABCD assay. The 3′-end of the sense strand was biotinylated, and a mutation was introduced (AAAGGTCA to AGAATTCA) to remove the specific binding site for Nur77. The following primers were designed: human HOXA10 wild-type: 5′-CTCACCACAGAAAGGTCACTGAAGACCC-biotin-3′; human HOXA10 wild-type reverse: 5′-GGGTCTTCAGTGAATTCTCTGTGGTGAG-3′; and human HOXA10 mutant: 5′-CTCACCACAGAGAATTCACTGAAGACCC-biotin-3′. Ishikawa cells were infected with Ad-LacZ and Ad-Flag-Nur77 (MOI = 100) for 48 h and then used for ABCD as previously described (Jiang et al. Citation2016). The proteins pulled down by double-stranded DNA were eluted, separated by SDS-PAGE, and then probed with an anti-Flag-HRP antibody (1:5000 dilution, Sigma).
Immunohistochemistry
Endometrial tissues were fixed in 10% neutral-buffered formalin overnight, and then embedded in paraffin. Tissue sections were immunostained with primary antibodies against HOXA10 (1:300 dilution; Abcam, Cambridge, CA, USA), and Nur77 (1:300 dilution; Abcam Cambridge, CA, USA) overnight at 4°C. Subsequently, the sections were incubated with a goat anti-rabbit secondary antibody at 37°C for 20 min. Finally, the sections were stained with 3, 3′-diaminobenzidine and counterstained with hematoxylin. Control sections were run concurrently with the experimental sections using nonspecific rabbit IgG, and they were similarly pretreated. Nonspecific staining was not detected in the controls.
Statistical analysis
All experiments were performed at least three times. Student’s t-test was used for comparisons between two groups. Statistical analysis was conducted by ANOVA followed by the Student-Newman-Keuls test for experiments involving more than two groups. Differences were considered statistically significant at p < 0.05.
Ethics approval and consent to participate
Ethical approval for collection of human endometrial tissue was supported by the Construction and Management of the Nanjing Multi-Center Biobank Project. No. 2013-081-01. Registered 10 Dec. 2013.
Authors’ contributions
Responsible for the conception and design of the study: QZ, LHS; Responsible for acquisition of data: JTS, XDZ, MZ, YJ, GJY, ZLW, LHS, QZ; Performed the data analysis and drafted the manuscript: JTS, MZ, QZ. Revised and commented the draft: LHS, QZ. All authors read and approved the final version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Achache H, Revel A. 2006. Endometrial receptivity markers, the journey to successful embryo implantation. Hum Reprod Update. 12(6):731–746.
- Aghajanova L. 2010. Update on the role of leukemia inhibitory factor in assisted reproduction. Curr Opin Obstet Gynecol. 22(3):213–219.
- Bassett MH, Suzuki T, Sasano H, White PC, Rainey WE. 2004. The orphan nuclear receptors NURR1 and NGFIB regulate adrenal aldosterone production. Mol Endocrinol. 18(2):279–290.
- Cermik D, Selam B, Taylor HS. 2003. Regulation of HOXA-10 expression by testosterone in vitro and in the endometrium of patients with polycystic ovary syndrome. J Clin Endocrinol Metab. 88(1):238–243.
- Crawford PA, Sadovsky Y, Woodson K, Lee SL, Milbrandt J. 1995. Adrenocortical function and regulation of the steroid 21-hydroxylase gene in NGFI-B-deficient mice. Mol Cell Biol. 15(8):4331–4336.
- Daftary GS, Taylor HS. 2002. Hydrosalpinx fluid diminishes endometrial cell HOXA10 expression. Fertil Steril. 78(3):577–580.
- Daftary GS, Troy PJ, Bagot CN, Young SL, Taylor HS. 2002. Direct regulation of beta3-integrin subunit gene expression by HOXA10 in endometrial cells. Mol Endocrinol. 16(3):571–579.
- Du H, Daftary GS, Lalwani SI, Taylor HS. 2005. Direct regulation of HOXA10 by 1, 25-(OH)2D3 in human myelomonocytic cells and human endometrial stromal cells. Mol Endocrinol. 19(9):2222–2233.
- Eun Kwon H, Taylor HS. 2004. The role of HOX genes in human implantation. Ann N Y Acad Sci. 1034:1–18.
- Fernandez PM, Brunel F, Jimenez MA, Saez JM, Cereghini S, Zakin MM. 2000. Nuclear receptors Nor1 and NGFI-B/Nur77 play similar, albeit distinct, roles in the hypothalamo-pituitary-adrenal axis. Endocrinology. 141(7):2392–2400.
- Fitzgerald JS, Poehlmann TG, Schleussner E, Markert UR. 2008. Trophoblast invasion: the role of intracellular cytokine signalling via signal transducer and activator of transcription 3 (STAT3). Hum Reprod Update. 14(4):335–344.
- Hamers AA, Hanna RN, Nowyhed H, Hedrick CC, de Vries CJ. 2013. NR4A nuclear receptors in immunity and atherosclerosis. Curr Opin Lipidol. 24(5):381–385.
- Huang C, Jiang Y, Zhou J, Yan Q, Jiang R, Cheng X, Xing J, Ding L, Sun J, Yan G, et al. 2017. Increased Kruppel-like factor 12 in recurrent implantation failure impairs endometrial decidualization by repressing Nur77 expression. Reprod Biol Endocrinol. 15(1):25.
- Huang HY, Chang HF, Tsai MJ, Chen JS, Wang MJ. 2016. 6-Mercaptopurine attenuates tumor necrosis factor-α production in microglia through Nur77-mediated transrepression and PI3K/Akt/mTOR signaling-mediated translational regulation. J Neuroinflammation. 13(1):78.
- Huo Y, Yi B, Chen M, Wang N, Chen P, Guo C, Sun J. 2014. Induction of Nur77 by hyperoside inhibits vascular smooth muscle cell proliferation and neointimal formation. Biochem Pharmacol. 92(4):590–598.
- Jiang Y, Hu Y, Zhao J, Zhen X, Yan G, Sun H. 2011. The orphan nuclear receptor Nur77 regulates decidual prolactin expression in human endometrial stromal cells. Biochem Biophys Res Commun. 404(2):628–633.
- Jiang Y, Jiang R, Cheng X, Zhang Q, Hu Y, Zhang H, Cao Y, Zhang M, Wang J, Ding L, et al. 2016. Decreased expression of NR4A nuclear receptors in adenomyosis impairs endometrial decidualization. Mol Hum Reprod. 22(9):655–668.
- Kurakula K, Koenis DS, van Tiel CM, de Vries CJ. 2014. NR4A nuclear receptors are orphans but not lonesome. Biochim Biophys Acta. 1843(11):2543–2555.
- Liu X, Zhu G, Zhong G. 2007. Regulatory effect of estrogen, progestin and HB-EGF on the expression of HOXA10 gene in Ishikawa cells. J Huazhong Univ Sci Technolog Med Sci. 27(4):464–467.
- McMorrow JP, Murphy EP. 2011. Inflammation: a role for NR4A orphan nuclear receptors? Biochem Soc Trans. 39(2):688–693.
- Miravet-Valenciano JA, Rincon-Bertolin A, Vilella F, Simon C. 2015. Understanding and improving endometrial receptivity. Curr Opin Obstet Gynecol. 27(3):187–192.
- Mohan HM, Aherne CM, Rogers AC, Baird AW, Winter DC, Murphy EP. 2012. Molecular pathways: the role of NR4A orphan nuclear receptors in cancer. Clin Cancer Res. 18(12):3223–3228.
- Montazeri M, Sanchez-Lopez JA, Caballero I, Maslehat LN, Elliott S, Fazeli A. 2016. Interleukin-1 receptor antagonist mediates toll-like receptor 3-induced inhibition of trophoblast adhesion to endometrial cells in vitro. Hum Reprod. 31(9):2098–2107.
- Niu G, Lu L, Gan J, Zhang D, Liu J, Huang G. 2014. Dual roles of orphan nuclear receptor TR3/Nur77/NGFI-B in mediating cell survival and apoptosis. Int Rev Cell Mol Biol. 313:219–258.
- Paulsen RF, Granas K, Johnsen H, Rolseth V, Sterri S. 1995. Three related brain nuclear receptors, NGFI-B, Nurr1, and NOR-1, as transcriptional activators. J Mol Neurosci. 6(4):249–255.
- Rodríguez-Calvo R, Tajes M, Vázquez-Carrera M. 2017. The NR4A subfamily of nuclear receptors: potential new therapeutic targets for the treatment of inflammatory diseases. Expert Opin Ther Targets. 21(3):291–304.
- Sarno J, Schatz F, Huang SJ, Lockwood C, Taylor HS. 2009. Thrombin and interleukin-1beta decrease HOX gene expression in human first trimester decidual cells: implications for pregnancy loss. Mol Hum Reprod. 15(7):451–457.
- Taylor HS, Arici A, Olive D, Igarashi P. 1998. HOXA10 is expressed in response to sex steroids at the time of implantation in the human endometrium. J Clin Invest. 101:1379–1384.
- Wansa KD, Harris JM, Yan G, Ordentlich P, Muscat GE. 2003. The AF-1 domain of the orphan nuclear receptor Nor-1 mediates trans-activation, coactivator recruitment, and activation by the purine anti-metabolote 6-mercaptopurine. J Biol Chem. 278(27):24776–24790.
- Yan Q, Huang C, Jiang Y, Shan H, Jiang R, Wang J, Liu J, Ding L, Yan G, Sun H. 2018. Calpain7 impairs embryo implantation by downregulating β3-integrin expression via degradation of HOXA10. Cell Death Dis. 9(3):291.
- Yang Y, Chen X, Saravelos SH, Liu Y, Huang J, Zhang J, Li TC. 2017. HOXA-10 and E-cadherin expression in the endometrium of women with recurrent implantation failure and recurrent miscarriage. Fertil Steril. 107(1):136–143.e2.
- Yoo YG, Na TY, Yang WK, Kim HJ, Lee IK, Kong G, Chung JH, Lee MO. 2007. 6-Mercaptopurine, an activator of Nur77, enhances transcriptional activity of HIF-1alpha resulting in new vessel formation. Oncogene. 26(26):3823–3834.
- Zanatta A, Rocha AM, Carvalho FM, Pereira RM, Taylor HS, Motta EL, Baracat EC, Serafini PC. 2010. The role of the Hoxa10/HOXA10 gene in the etiology of endometriosis and its related infertility: a review. J Assist Reprod Genet. 27(12):701–710.
- Zetterstrom RH, Solomin L, Jansson L, Hoffer BJ, Olson L, Perlmann T. 1997. Dopamine neuron agenesis in Nurr1-deficient mice. Science. 276:248–250.
- Zohni KM, Gat I, Librach C. 2016. Recurrent implantation failure: a comprehensive review. Minerva Ginecol. 68(6):653–667.
