ABSTRACT
The objective of this study was to identify proteins that are differentially expressed in the cystic wall tissues of ovarian endometriotic cysts, simple ovarian cysts, and in normal ovarian tissues. Specimens of ovarian endometriotic cyst wall tissue, simple ovarian cyst wall tissue, and normal ovarian tissue (six specimens per group) were collected from patients who received gynecologic surgery, respectively. Differentially expressed proteins related to the ovarian endometriotic cysts were screened by use of isobaric tags for relative and absolute quantitation (iTRAQ) combined with functional annotation and bioinformatics analyses. All differentially expressed proteins related to cysts were validated using immunohistochemistry methods in recurrent and non-recurrent ovarian endometriotic cyst. A total of 359 proteins were identified as up-regulated in ovarian endometriotic cyst groups when compared with both the normal ovary and simple ovarian cyst groups. The levels of 27 proteins were >two-fold higher in the ovarian endometriotic cyst group than that in the other two groups. Of note, the five most significantly upregulated proteins were Charcot-Leyden Crystal Galectin (CLC), Defensin, alpha 1 (DEFA1), S100 calcium-binding protein A9 (S100A9), S100 calcium-binding protein A8 (S100A8), and Ferritin Light Chain (FTL). Immunohistochemistry results showed that the changes of S100A9 and S100A8 were consistent with the results shown by iTRAQ. However, no similarity of CLC, DEFA1, and FTL proteins was found between iTRAQ and immunohistochemistry. The ratio of patients with abnormally high S100A9 and S100A8 expression in the recurrent ovarian endometriotic cyst group was significantly higher than that in the non-recurrent group (P < 0.05). Our data identify differentially expressed proteins S100A9 and S100A8, and suggest they may serve as novel molecular markers to predict postoperative recurrence of an ovarian endometriotic cysts.
Abbreviations: iTRAQ: isobaric tags for relative and absolute quantitation; HPRD: Human Protein Reference Database; GO: Gene Ontology; KEGG: Kyoto Encyclopedia of Genes and Genomes; EM: Endometriosis; COX-2: cyclooxyenase-2; NF-kB: nuclear factor kappa-B; PR-B: progesterone receptor type B.
Introduction
Endometriosis (EM) is one of the most common gynecological diseases associated with infertility in women of childbearing age, with an incidence of 10%~15%. In recent years, the incidence of EM has gradually increased and its recurrence rate after systematic treatment reaches up to 30.5% (Singh and Suen Citation2017; Dai et al. Citation2018; Klemmt and Starzinski-Powitz Citation2018). In order to reduce the recurrence rate of endometriosis, the corresponding risk factors were identified. Several studies have shown that a patient’s age, menstruation history, surgical history for endometriosis, a high intraoperative ASRM score, as well as a postoperative interruption of adjuvant drug therapy are all risk factors for EM recurrence (Carvalho Mde et al. Citation2015; Guo et al. Citation2016). However, surgical treatment can lead to a recurrence of ovarian endometriosis, and the best way to predict and control the potential recurrence remains to be further determined.
Previous studies indicated that overexpression of cyclooxyenase-2 (COX-2), accompanied by intraoperative focal adhesion, can predict the recurrence of an ovarian endometriotic cyst within 30 months after surgical treatment (Yuan et al. Citation2009). COX-2 is the target gene of nuclear factor kappaB (NF-κB), and the activation of the latter can reduce the immunological activity of progesterone receptor type B (PR-B). Therefore, both NF-κB and PR-B can be considered as important factors for predicting a recurrence of EM (Shen et al. Citation2009). The above evidence suggests that the recurrence of ovarian endometriotic cysts may be related to the overexpression of certain genes. However, such factors have not been used in clinical practice for the prognosis of cyst recurrence, probably due to their relatively low specificity. As for ovarian endometriotic cysts, several surgical approaches have been used to preserve the cystic wall (Alborzi et al. Citation2017); however, all recurrence rates associated with cysts are nearly 30%. The inconsistencies of recurrence under the same status of cystic wall preservation suggest that there are differences between the different cystic walls. In this regard, the pathological type of a cyst may be an important factor for the recurrence, and the cystic wall may be involved in regulation of the recurrence (Selcuk et al. Citation2016).
Adjuvant drug therapy is commonly required after surgery for an ovarian endometriotic cyst to prevent or delay its recurrence and increase the chance for a pregnancy. At present, the drugs most commonly used for this purpose in the clinic are gonadotropin-releasing hormone antagonists (GnRHa). However, due to the relatively high price and side effects, their continuous use may result in a waste of medical resources and as a sort of over-treatment, is unnecessary for non-recurrent patients. Therefore, the screen of specific markers in the prediction of disease recurrence can be important for guiding postoperative adjuvant therapy. The current methods used to predict recurrence mainly rely on the analysis of relevant high-risk factors. However, such risk factors are not easy to quantify. Some biological markers found in blood serum, such as cancer antigen 125 (CA125), cancer antigen 129 (CA199), and human epididymal secretory protein 4, are tumor-related markers that are often used to diagnose an ovarian endometriotic cyst (Socolov and Socolov et al. Citation2017). However, these markers are not highly specific, even for the diagnosis of tumors, and the value for predicting the recurrence of ovarian endometriotic cysts is limited, which unfortunately cannot meet the current clinical needs. Therefore, an individualized and highly specific biomarker should be identified for the prediction by using the cystic wall of an ovarian endometriotic cyst as an appropriate target. In this study, differentially expressed proteins were determined by using Isobaric Tags for Relative and Absolute Quantitation (iTRAQ) technique between wall tissues of ovarian endometriotic cysts and the wall tissues of simple ovarian cysts, and in normal ovarian tissues, which provides new leads for the molecular diagnosis of cyst recurrence.
Results
Screening for the differentially expressed proteins in ovarian endometriotic cysts
We compared the protein expressions in the ovarian endometriotic cyst and the control groups (normal ovarian tissues). In comparison to the control group, there were 1,437 differentially expressed proteins in the ovarian endometriotic cyst group, among which, the levels of 695 proteins were upregulated by 1.20~5.23-fold. Additionally, 742 proteins were significantly downregulated (0.14~0.83-fold) (). Compared to that in the simple ovarian cyst group, there were 1,471 differentially expressed proteins in the ovarian endometriotic cyst group, among which, 1,064 proteins were upregulated by 1.20 ~ 4.99-fold, while 407 proteins were significantly downregulated (0.11~0.83-fold expression) ().
Table 1. Expression of differentially expressed proteins in the ovarian endometriotic cyst group compared to the other two group.
Bioinformatics analysis
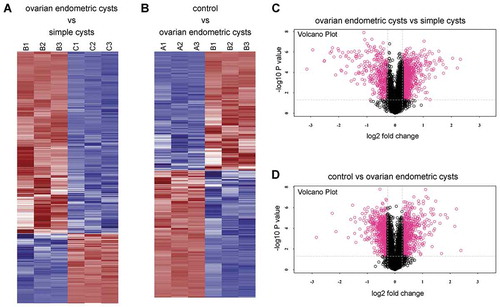
GO annotation analysis and metabolic pathway annotation analysis were conducted on the differentially expressed proteins to identify their associations with specific metabolic and signaling pathways. It indicated that in the comparisons between the ovarian endometriotic cyst group and the control group, ovarian endometriotic cyst group and the simple ovarian cyst group, a number of proteins were detected associated with regulation of biological process, cellular processes, catalytic activity, binding, organelle, etc. (,). KEGG pathways under different comparisons were also analyzed in present study. The top pathway enrichments in both comparisons include protein digestion and adsorption, EMC-receptor interaction. However, in the comparison between ovarian endometriotic cyst group and the simple ovarian cyst group, intestinal immune network for IgA production, NF-kappa B signaling pathways were obviously noted (,). In addition to the analysis of KEGG pathways, protein–protein interaction network analysis (PPI) was also determined (Figure S1), along with the protein clustering analysis and Volcano Plot (–).
Figure 1. Gene ontology (GO) functional classification and enrichment analysis of differentiated expressed proteins. (A) The ovarian endometric cysts vs. simple cysts; (B) The control group vs. ovarian endometric cysts. The x-axis indicates the functional description of the GO classifications, the left y-axis represents the proportion of the number of proteins annotated to the GO database. All GO terms are grouped into three major categories: Red is for biological processes, purple is for cellular components and orange is for molecular function.
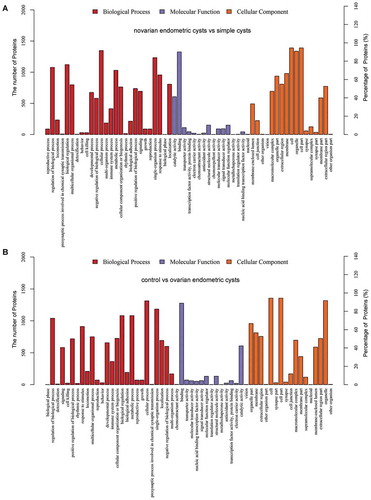
Figure 2. Kyoto Encyclopedia of Genes and Genomes (KEGG) classifications and pathway enrichment analysis of differentiated expressed proteins. (A) The ovarian endometric cysts vs. simple cysts; (B) The control group vs. ovarian endometric cysts. The x-axis indicates the functional description of the KEGG classifications, the left y-axis represents the percentage of the sequences annotated to the KEGG database. The yellow bar is for Difference Set and ble is for Reference Set.
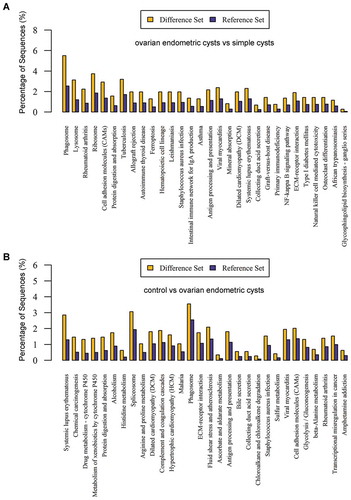
Figure 3. Heat map and Volcano Plot for protein clustering analysis. (A, C) The ovarian endometric cysts (B1, B2, B3) vs. the simple cysts group (C1, C2, C3); (B, D) the ovarian endometric cysts group (B1, B2, B3) vs. the control group (A1, A2, A3). The red indicates up regulation while blue denotes down regulation. The depth of color represents the degree of level change.
Cross-analysis of the screened differentially expressed proteins between groups
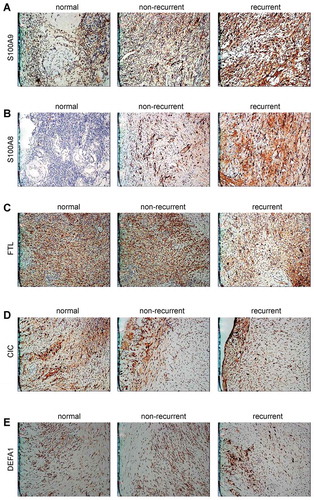
Our result showed that 27 of 359 proteins were differentially expressed with a >two-fold change in the ovarian endometriotic cyst group, among which, the levels of 5 proteins, Charcot-Leyden Crystal Galectin (CLC), Defensin, alpha 1 (DEFA1), S100 calcium-binding protein A9 (S100A9), S100 calcium-binding protein A8 (S100A8), and Ferritin Light Chain (FTL), were expressed more than 3.1-fold higher. Furthermore, another protein (A8K3Q9) showed a > 4.5-fold increase in expression, but its function remains unknown and to be further verified ().
Table 2. Proteins in the ovarian endometriotic cyst group that showed a > 2-fold increase.
Identification of differentially expressed proteins by immunohistochemistry
Immunohistochemistry results showed the changes for the differentially expressed proteins S100A9 and S100A8 were in line with the data obtained by iTRAQ detection. However, the CLC, FTL, and DEFA1 proteins showed no similar trends in variation by comparison. The positive rate for the S100A9 and S100A8 expression in the recurrent ovarian endometriotic cyst group was significantly higher than that in the non-recurrent group (P < 0.01); (, ). Follow-up results showed that there were 25 cases detected that corresponded to the post-operative recurrent and non-recurrent groups. The ratio of patients with high S100A9 and S100A8 expressions in the recurrent group was significantly higher than that in the non-recurrent group (P < 0.05). However, no differences were detected concerning the levels of FTL, CLC, and DEFA1 expressions in the different groups (P > 0.05) (, –).
Table 3. The expression rates of differentially expressed proteins in found in the cyst wall tissues of the ovarian endometriotic cyst recurrent group, n (%).
Table 4. The expression rates of differentially expressed proteins in the ovarian endometriotic cyst recurrent and non-recurrent groups, n (%).
Discussion
Endometriosis mainly manifests as infertility, pelvic pain, and pelvic masses. The majority of pelvic masses are ovarian endometriosis that is commonly managed by surgical treatment. However, high postoperative recurrence rates remains to be solved in the clinic. Understanding the mechanism of endometriosis and biological markers may facilitate to predict its recurrence or guide the relevant therapy. It is proposed that the recurrence of ovarian endometriotic cysts may be related to the overexpression of several genes. Nevertheless, the functions of genes in the prediction of disease recurrence in clinical practice need further investigation.
There was no significant increase in the recurrence rate of ovarian endometriotic cysts that could be related to retention of the cystic wall tissues. It can be speculated that recurrence may be regulated by a number of certain specific genes, and the recurrence of the disease after subsequent adjuvant therapy is related to these genes. In view of the above clinical problems, it is important to screen for and identify the specific genes related to endometriosis recurrence. The current methods used in proteomics are convenient and able to provide highly reliable and reproducible qualitative and quantitative results. Furthermore, the target molecules of those proteins can be rapidly identified and analyzed for the biological functions. Therefore, proteomics has become a mainstream protein quantification technology used to search for and screen various biomarkers for diseases (Mokart et al. Citation2018).
In this study, the iTRAQ technique was used to detect proteins that were differentially expressed in the cystic wall tissues of ovarian endometriotic cysts, simple ovarian cysts, as well as in normal ovarian tissues. On account for the possible recurrence of cystic walls, it further demonstrated the role of the differentially expressed proteins in differentiation between recurrence and non-recurrence of cystic wall, which provides clinical value of the proteins. In total, there were 6,100 proteins, among which, 2,908 proteins were detected with more than 1.2-fold increase in expression or less than 0.83-fold of the level compared to that in the control group. A cross-analysis showed there were 359 differentially expressed proteins in ovarian endometriotic cysts, and 27 of those proteins were identified more than two-fold change in expression. Among those 27 proteins, 5 proteins (CLC, DEFA1, S100A9, S100A8, and FTL) were significantly upregulated by >3.1-fold. Those upregulated proteins were mainly found in the cytoskeleton or represented extracellular matrix components, adhesion molecules, signaling molecules, structural proteins, enzymes, immune-related, and tumor-related proteins. Finally, an immunochemistry study further verified the screened proteins S100A9 and S100A8 as previously determined by iTRAQ, indicating that specific proteins were indeed aberrantly expressed in ovarian endometriotic cysts. Considering the shortage of relevant literature concerning the proteomics of endometriosis, Williams KE performed a proteomic analysis of urine, peritoneal fluid, and the omental fat tissues of women with and without endometriosis (Williams et al. Citation2015). Those results indicated that the expression of matrix metalloproteinase-9 was significantly down-regulated in women without endometriosis, suggesting specific patterns of protein expression exist in various kinds of the organs and body fluids of patients with endometriosis. Furthermore, Piotr Marianowski performed a proteomic analysis of ectopic and eutopic endometrium and found that vimentin expression was significantly upregulated in ectopic endometrium (Marianowski et al. Citation2013). The results of our current study indicated that the up-regulated proteins were not only structural proteins, but also proteins that are involved in several biological functions. However, it is difficult to identify the factors that account for the inconsistency of these findings. Deviations in results may be related to the structure of the specimens being studied, how the specimens are selected, and the relative proportions of interstitium and glandular tissue in different specimens. Therefore, some investigators believe that any further proteomic analysis of endometriosis will require more stringent and unified standards for specimen collection. The present study also identified a new protein (A8K3Q9) that showed a 4.5-fold increase in expression in ovarian endometriotic cyst tissue. However, due to its unknown function, the protein could not be further verified, and requires further study.
S100 calcium-binding protein A8 (S100A8) and S100 calcium-binding protein A9 (S100A9) are both important members of the calcium-binding protein S100 family, and often bind to form heterodimers needed for various functions (Argyris et al. Citation2018). S100A8 and S100A9 function in regulating cell migration, promoting the production of proinflammatory factors, and as adhesion molecules (Cluzeau et al. Citation2017). They also mediate inflammatory reactions and modulate the amount and extent of a certain immune response. S100A8 is often included in tumor-related studies (Austermann et al. Citation2017; Wu et al. Citation2018). S100A8 and S100A9 are thought to play similar roles in the pathogenesis of endometriosis, despite the fact that no reports concerning their roles in endometriosis have been published. Adhesion, epithelial metaplasia, and immune factors exert critical function in the pathogenesis of endometriosis, and it can be speculated that S100A8 and S100A9 proteins may affect these factors to regulate the occurrence and recurrence of endometriosis (Moris et al. Citation2016). In addition, our results showed that the S100A8 and S100A9 proteins were expressed at different levels in normal ovarian tissues when compared to ovarian endometriotic cyst tissues, and were obviously up-regulated in ovarian endometriotic cysts. Consequently, S100A8 and S100A9 may be involved in the recurrence mechanism of ovarian endometriotic cysts, and represent new markers for the evaluation of disease severity, the effect of treatment, and disease recurrence.
There were no significant differences in the levels of Charcot-Leyden crystal (CLC) protein, ferritin light chain peptide, and alpha-defensin-1 (DEFA1) protein expression in the recurrent group when compared with either the non-recurrent group or normal ovarian tissue group. However, in view of the existing results, it can be concluded that these proteins are not significantly involved with the occurrence and development of endometriosis, and have limited clinical value for predicting EM recurrence.
There are several studies that aim to investigate the cause of recurrence in endometriosis. Mainly, the recurrent lesions might originate from residual lesions or alternatively from de novo cells coming through retrograde bleeding. Surgery, lymph node involvement, lymphovascular invasion, immunological factors are responsible for recurrence (Selcuk and Bozdag Citation2013). Previous finding showed that r-AFS stage and MTA1 overexpression were risk factors for the recurrence of endometriosis. MTA1 is closely associated with the occurrence and development of endometriosis and may be used as a new indicator to predict the progression of endometriosis (Zhang et al. Citation2018). The abnormal increasing level of ADP-ribosylation factor-like protein 4C (ARL4C) has been reported to be involved in progression processes of endometriosis-associated ovarian cancer (EAOC) (Wakinoue et al. Citation2019), suggesting differentially expressed proteins are associated with progression or recurrence of endometriosis and can be used as novel therapeutic targets. In this scenario, we try to screen potential targets at protein level though the tissue context in ovarian endometriotic cyst (most likely the unruptured follicles) and normal ovarian tissues (follicles with interstitial tissues) may vary. In clinical practice, there are significant differences in the incidence of disease recurrence among patients who have been operated for an ovarian endometriotic cyst. Furthermore, there are no indicators of the need for follow-up adjuvant drug therapy. In this study, the positive expression rates of S100A9 and S100A8 in the postoperative recurrent group were higher than those in the non-recurrent group, suggesting that these indicators may predict the possibility of the recurrence. Those high expression rates of certain proteins may also be valuable for the options of appropriate postoperative treatment. Despite our valuable findings in this study, it is possible that the bias on the result may exist owing to relatively small sample size in our study. Further prospective cohort studies and additional data mining are needed to confirm our current results to develop comprehensive predicting strategy based on extensive screening and identification of serum.
Materials and methods
Specimens for iTRAQ detection
The endometriotic cyst group included six patients aged of 22–43. These patients had a regular menstrual cycle and received surgical treatment for the first time (clinical stage of ASRM I – III). Inclusion criteria: Preoperative ultrasonography and intraoperative exploration indicated that the diameter at least one side of an ovary was enlarged more than 5 cm. All six patients had a confirmed history of dysmenorrhea and infertility, and received intraoperative cystectomy. Patients with cervical carcinoma were required to remove their ovaries, and a postoperative pathology examination showed no evidence of ovarian metastasis or complications of adenomyosis. The patients in all three groups had no history of hormone therapy, use of an intrauterine contraceptive device, genital tract deformity, malignancy or other type of chronic disease during 6 months prior to tissue sampling. All the patients in each group received their pathological diagnosis after surgery. The tissue sampling protocol was approved by the Hospital’s Ethics Committee, and all patients enrolled in this study had written informed consents after a detailed explanation.
Specimens for immunohistochemistry analysis
All specimens were paraffin embedded. The recurrent ovarian endometriotic cyst group included 25 patients with a mean age of 31.5 ± 4.2. The non-recurrent ovarian endometrial cyst group included 25 patients with a mean age of 30.5 ± 4.8. The normal ovary group included 25 patients with a mean age of 33.7 ± 4.9. Postoperative pathology results for the patients with cervical carcinoma showed no evidence of ovarian metastasis. Each patient diagnosed with an ovarian endometriotic cyst had a regular menstrual cycle, and all patients received their first surgical treatment for ASRM I – III. Inclusion criteria: Results of preoperative ultrasonography and an intraoperative exploration indicated that at least one side of the ovary was enlarged by >5 cm in diameter. All patients had a history of dysmenorrhea and infertility. Cystectomy was conducted intraoperatively. No patient in the 3 groups had received hormone therapy, used an intrauterine contraceptive device, or had a genital tract deformity, malignant tumor or other chronic disease within 6 months prior to tissue sampling. Cystectomy was carried out intraoperatively. A GnRH-a was injected on the first day after menstruation for a total of 6 times (once every 28 days). All patients were followed up for at least 2 years by telephone and outpatient service, and no patient had a successful conception during that time period. The pathological number and recurrence condition of each patient were recorded at the same time. The following criteria were used to indicate recurrence of an endometriotic ovarian cyst: (1) clinical symptoms had returned to their preoperative levels or higher; (2) transvaginal color Doppler ultrasound indicated an ovarian endometriotic cyst and excluded a physiological cyst. There was no significant difference in the mean age of the three groups.
Reagents and instruments
A high-performance liquid chromatography system, Q Exactive high-resolution mass spectrometer, and TMTsixplex™ Isobaric Label Reagent Set Kit were purchased from Thermo Fisher Scientific (Waltham, MA, USA). A Strata-X C18 column was purchased from Phenomenex (Torrance, CA, USA), and the analytical column was purchased from SCIEX (Framingham, MA, USA). CLC rabbit anti-human polyclonal antibody (No. 25225-1-AP), S100A9 rabbit anti-human polyclonal antibody (No. 26992-1-AP), DEFA1 rabbit anti-human polyclonal antibody (No. 18057-1-AP), S100A8 rabbit anti-human polyclonal antibody (No. ZA-0709), FTL rabbit anti-human polyclonal antibody (No. 10727-1-AP), a concentrated DAB Kit, and an immunohistochemistry staining kit were purchased from Wuhan Proteintech Group, Inc. (China). S100A8 rabbit anti-human polyclonal antibody (No. ab92331) was purchased from Abcam Biological Technology Co., Ltd. (Cambridge, MA, USA).
Protein isolation
Tissue specimens (about 10 g each) were obtained under aseptic conditions and incubated on ice for 5 min. The specimens were then rinsed with PBS and stored in liquid nitrogen for future use. After being homogenized and centrifuged, the supernatant of the sample was collected. Protein concentrations were determined by using the Bradford method. The samples were diluted as required, and the absorbance of each sample at 595 nm was determined with a microplate reader. The protein content in each sample was calculated using a standard curve.
SDS-PAGE
A 20 µg aliquot of total protein from each sample was added to a fivefold volume of buffer solution. Next, the diluted protein sample was boiled in a water bath and the proteins were separated on a 12% SDS-PAGE gel. The gel was then stained with Coomassie Brilliant Blue.
iTRAQ labeling
Trypsin was added to 30 μL of protein solution and the peptide fragments were quantified with a Nano Drop 2000 spectrophotometer. Next, 100 μg of peptide fragments were treated with reagents in an iTRAQ labeling kit (AB SCIEX, Framingham, MA, USA) according to the manufacturer’s instructions. The labeled peptide fragments were re-dissolved and eluted, and separated on an analytical column (Thermo Fisher Scientific, Acclaim PepMap RSLC, 50 μm x 15 cm, Nano Viper, P/N164943).
Mass spectrometry
Each peptide segment obtained by liquid-phase separation was re-dissolved and eluted. The supernatant fraction was analyzed with a Q-Exactive mass spectrometer (Waltham, MA, USA). Furthermore, a qualitative analysis was performed by searching specific parameters in the Mascot 2.5 database. For a quantitative analysis, Proteome Discoverer 2.1 software was used to identify the reported ion peak intensity of each peptide segment for further quantification and normalization. Based on protein abundance, differentially expressed proteins were defined as those that were expressed at >120% or <80% of their normal levels. P-values < 0.05 were considered to be statistically significant.
Bioinformatics analysis and comparison
Bioinformatics analysis of selected differentially expressed proteins: The differentially expressed proteins were identified and their functions were annotated simultaneously. Proteins identified by mass spectrometry were further analyzed in a Human Protein Reference Database (HPRD) analysis, Gene Ontology (GO) annotation and enrichment analysis, and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis. A protein clustering analysis and protein–protein interaction network analysis (PPI) were also performed. When the mass spectroscopy analysis identified a large number of differentially expressed proteins, the standard difference multiplier was improved to narrow the expression range of the target proteins. Differentially expressed proteins that were strongly related to disease recurrence were selected after a pathway enrichment analysis. Differentially expressed proteins that showed the same variation trends were searched during the above network analysis to further narrow the screening range. A cross-analysis was performed on the differentially expressed proteins in the different groups, as identified by iTRAQ technology. There were two different data comparisons: the ovarian endometriotic cyst group vs. the normal ovary group, and the ovarian endometriotic cyst group vs. the simple ovarian cyst group. Differentially expressed proteins that showed a significant up-regulation and only existed in the cystic wall tissues of ovarian endometriotic cysts were used to verify the clinical tissue samples. The t-test was used to compare the mean values in different groups, and the χ2 test was used for analyzing categorical data. A P-value < 0.05 was considered statistically significant.
Immunohistochemical verification
The positive expression of proteins manifested as brownish yellow granules in the cytoplasm, cell membrane or nucleus. The S100A8, FTL, S100A9, DEFA1 and CLC proteins were detected. A semi-quantitative analysis was performed by combining the staining intensity and proportion of positive cells observed at a high magnification. Ten visual fields per section were randomly observed at high magnification.
Authors’ contributions
Contributed to the conception and design of the study: GHZ, JK; performed the experiments: GHZ, XHF, FJL, HYH, LH; analyzed the data: GHZ; contributed materials and facilities: JK, XHF, KW; wrote the manuscript: GHZ. All authors approved any revisions and the final paper.
Supplemental Material
Download Zip (17.7 MB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Alborzi S, Hosseini-Nohadani A, Poordast T, Shomali Z. 2017. Surgical outcomes of laparoscopic endometriosis surgery: a 6 year experience. Curr Med Res Opin. 33(12):2229–2234.
- Argyris PP, Slama ZM, Ross KF, Khammanivong A, Herzberg MC. 2018. Calprotectin and the initiation and progression of head and neck cancer. J Dent Res. 97(6):674–682.
- Austermann J, Zenker S, Roth J. 2017. S100-alarmins: potential therapeutic targets for arthritis. Expert Opin Ther Targets. 21(7):739–751.
- Carvalho Mde S, Pereira AM, Martins JA, Lopes RC. 2015. Predictive factors for recurrence of ovarian endometrioma after laparoscopic excision. Rev Bras Ginecol E Obstet. 37(2):77–81.
- Cluzeau T, McGraw KL, Irvine B, Masala E, Ades L, Basiorka AA, Maciejewski J, Auberger P, Wei S, Fenaux P, et al. 2017. Pro-inflammatory proteins S100A9 and tumor necrosis factor-alpha suppress erythropoietin elaboration in myelodysplastic syndromes. Haematologica. 102(12):2015–2020.
- Dai Y, Li X, Shi J, Leng J. 2018. A review of the risk factors, genetics and treatment of endometriosis in Chinese women: a comparative update. Reprod Health. 15(1):82.
- Guo H, Shen A, Xu S, Yang J. 2016. Analysis of relevant factors for recurrence of ovarian endometriosis after conservative laparoscopic surgery. J Cent South Univ Med Sci. 41(4):405–410.
- Klemmt PAB, Starzinski-Powitz A. 2018. Molecular and cellular pathogenesis of endometriosis. Curr Women’s Health Rev. 14(2):106–116.
- Marianowski P, Szymusik I, Malejczyk J, Hibner M, Wielgos M. 2013. Proteomic analysis of eutopic and ectopic endometriotic tissues based on isobaric peptide tags for relative and absolute quantification (iTRAQ) method. Neuro Endocrinol Lett. 34(7):717–721.
- Mokart D, Saillard C, Zemmour C, Bisbal M, Sannini A, Chow-Chine L, Brun JP, Faucher M, Boher JM, Toiron Y, et al. 2018. Early prognostic factors in septic shock cancer patients: a prospective study with a proteomic approach. Acta Anaesthesiol Scand. 62(4):493–503.
- Moris D, Spartalis E, Angelou A, Margonis GA, Papalambros A, Petrou A, Athanasiou A, Schizas D, Dimitroulis D, Felekouras E. 2016. The value of calprotectin S100A8/A9 complex as a biomarker in colorectal cancer: A systematic review. J BUON. 21(4):859–866.
- Selcuk I, Bozdag G. 2013. Recurrence of endometriosis; risk factors, mechanisms and biomarkers; review of the literature. J Tur Ger Gynecol Assoc. 14(2):98–103.
- Selcuk S, Cam C, Koc N, Kucukbas M, Ozkaya E, Eser A, Karateke A. 2016. Evaluation of risk factors for the recurrence of ovarian endometriomas. Eur J Obstet Gynecol Reprod Biol. 203:56–60.
- Shen F, Liu X, Geng JG, Guo SW. 2009. Increased immunoreactivity to SLIT/ROBO1 in ovarian endometriomas: a likely constituent biomarker for recurrence. Am J Pathol. 175(2):479–488.
- Singh SS, Suen MW. 2017. Surgery for endometriosis: beyond medical therapies. Fertil Steril. 107(3):549–554.
- Socolov R, Socolov D, Sindilar A, Pavaleanu I. 2017. An update on the biological markers of endometriosis. Minerva Ginecol. 69(5):462–467.
- Wakinoue S, Chano T, Amano T, Isono T, Kimura F, Kushima R, Murakami T. 2019. ADP-ribosylation factor-like 4C predicts worse prognosis in endometriosis-associated ovarian cancers. Cancer Biomarkers. 24(2):223–229.
- Williams KE, Miroshnychenko O, Johansen EB, Niles RK, Sundaram R, Kannan K, Albertolle M, Zhou Y, Prasad N, Drake PM, et al. 2015. Urine, peritoneal fluid and omental fat proteomes of reproductive age women: endometriosis-related changes and associations with endocrine disrupting chemicals. J Proteomics. 113:194–205.
- Wu DM, Wang S, Shen M, Wang YJ, Zhang B, Wu ZQ, Lu J, Zheng YL. 2018. S100A9 gene silencing inhibits the release of pro-inflammatory cytokines by blocking the IL-17 signalling pathway in mice with acute pancreatitis. J Cell Mol Med. 22(4):2378–2389.
- Yuan L, Shen F, Lu Y, Liu X, Guo SW. 2009. Cyclooxygenase-2 overexpression in ovarian endometriomas is associated with higher risk of recurrence. Fertil Steril. 91(4 Suppl):1303–1306.
- Zhang J, Wang H, Meng Q, Chen J, Wang J, Huang S. 2018. Expression of MTA1 in endometriosis and its relationship to the recurrence. Medicine. 97(35):e12115.