ABSTRACT
Although reactive oxygen species in semen are associated with unfavorable results with respect to assisted reproductive technology, their effects based on the detailed stages of embryo development are unclear. We investigated the relationship between reactive oxygen species in semen and the oocyte fertilization rate, cleavage rate, and blastulation rate of intracytoplasmic sperm injections. This retrospective study enrolled 77 couples who underwent intracytoplasmic sperm injection and analyzed 887 eggs from 141 cycles of intracytoplasmic sperm injection. The reactive oxygen species level in semen was compared between the fertilized and nonfertilized groups, between the good-cleavage-embryo and non-developed-embryo groups, and between the good-quality-blastocyst and poor-quality-blastocyst groups. The cut-off level of reactive oxygen species was calculated to predict good-cleavage-embryo and good-quality-blastocyst development. The fertilization rate was 65.4%, and the mean reactive oxygen species levels were not significantly different between the fertilized and nonfertilized groups. The reactive oxygen species level was significantly higher in the non-developed-embryo group than in the good-cleavage-embryo group (P = 0.0026) and was significantly lower in the good-quality-blastocyst group than in the poor-quality-embryo group (P = 0.015). Cleavage embryos and blastocysts were divided into high- and low-reactive-oxygen-species groups using a cut-off value of 6601 and 4926 relative light units, as calculated from the receiver operating characteristic curve. The rates of good-cleavage embryos and good-quality blastocysts were lower in the high-reactive-oxygen-species group than in the low-reactive-oxygen-species group, which were both statistically significant. To conclude, reactive oxygen species in semen is considered to have an adverse effect on both the early and late stages of embryo development in intracytoplasmic sperm injection.
Abbreviations: GnRH, gonadotropin-releasing hormone; ICSI, intracytoplasmic sperm injection; IVF, in vitro fertilization; LPO, lipid peroxidation; NADPH, nicotinamide adenine dinucleotide phosphate; RLU, relative light units; ROC, receiver operating characteristic; ROS, reactive oxygen species
Introduction
Reactive oxygen species (ROS) in semen, first detected by Aitken and Clarkson (Citation1987) using chemiluminescence, are known to have adverse effects on semen quality and male fertility. A high ROS level causes DNA damage, lipid peroxidation (LPO), and induction of apoptosis in sperm cells (Agarwal et al. Citation2003), while a low ROS level is essential and advantageous for fertilization, including capacitation, hyperactivation, acrosome reaction, and sperm-oocyte fusion (de Lamirande et al. Citation1997; Du Plessis et al. Citation2015). Adverse effects of ROS on semen parameters and clinical outcomes have been reported; Takeshima et al. (Citation2017) demonstrated that logarithmically transformed ROS levels in unwashed semen are inversely correlated with semen parameters, such as sperm concentration, motility, mean head amplitude, and progressive motility. Yumura et al. (Citation2009) reported that the ROS level in semen was significantly higher in nonpregnant compared to pregnant couples with complaints of infertility. In the study, they also set a cut-off value of 4.35 mV/30 min/106 sperm to predict pregnancy. These findings indicate the adverse effects of ROS on semen quality, and on the outcome of fertility treatment.
As for assisted reproductive technology, the effects of ROS on semen have been evaluated in a few reports (Sukcharoen et al. Citation1996; Moilanen et al. Citation1998; Saleh et al. Citation2003; Zorn et al. Citation2003). However, these previous studies only evaluated the fertilization and pregnancy rates after in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI), but not in the detailed stages of embryo. Previous studies have reported that paternal factors, such as unrepaired DNA damage in sperm, are considered to have adverse effects on the late stages of embryo development in IVF or ICSI, which is termed the ‘late paternal effect’ (Tesarik et al. Citation2004). Since ROS in semen are reported to induce sperm DNA damage (Barroso et al. Citation2000; Irvine et al. Citation2000), there may be a significant correlation between ROS level and embryo development. Determination of the relationship between these two factors may help to predict embryo development based on the ROS level in semen, which can be easily measured in clinical practice. The objective of this study was to investigate the effects of ROS in semen on embryo development during different stages of ICSI among infertile couples.
Results
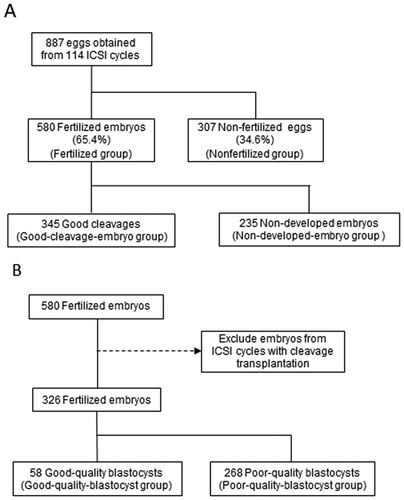
The patients’ characteristics and semen parameters are summarized in . Data are presented as mean ± standard deviation. Among 887 eggs, 580 fertilized embryos were obtained thorough ICSI, and the fertilization rate was 65.4%. Among the 580 fertilized embryos, 345 embryos were well-developed on day 3 (59.5%), which were divided into good-cleavage-embryo and non-developed-embryo groups (). Among 326 fertilized embryos in 107 cycles that did not include cleavage embryo transplantation, 58 good-quality blastocysts were acquired ().
Table 1. Patients’ characteristics and semen analyses (n = 77).
Figure 1. Experimental Design (A) Flowchart of how the eggs and embryos were divided into groups for between-group comparisons; the fertilized and nonfertilized groups and the good-cleavage-embryo and non-developed-embryo groups were compared. (B) Flowchart of how the fertilized embryos were divided to compare the good-quality-blastocyst and poor-quality-embryo groups.
The mean (± standard error) ROS levels of the fertilized and nonfertilized groups were 54,204 ± 7,454 and 52,018 ± 9,052 relative light units (RLU), respectively, which were not significantly different (P = 0.858) (, ). The ROS level was higher in the non-developed-embryo group than in the good-cleavage-embryo group, which was statistically significant (81,451 ± 15,234 and 35,645 ± 6,934 RLU, respectively; P = 0.0026) (, ). As calculated from the receiver operating characteristic (ROC) curve, the cut-off level of ROS was 6601 RLU (area under the curve = 0.539). Using this cut-off level of ROS, the embryos were divided into high- and low-ROS groups. The good-cleavage-embryo rate was lower in the high-ROS group (51.5%) than in the low-ROS group (63.8%), which was statistically significant (P = 0.004).
Table 2. Comparison of ROS levels in semen between the fertilized and nonfertilized groups.
Table 3. Comparison of ROS levels in semen between the good-cleavage-embryo and non-developed-embryo groups.
Figure 2. Comparison of reactive oxygen species (ROS) levels (A) Comparison of reactive oxygen species (ROS) levels between the fertilized and nonfertilized eggs. The mean (± standard error) ROS levels were not significantly different between the fertilized and nonfertilized groups (P = 0.858). (B) Comparison of ROS levels between the good-cleavage-embryo and non-developed-embryo groups. The ROS level was higher in the non-developed-embryo group than in the good-cleavage-embryo group, which was statistically significant (P = 0.0026). (C) Comparison of ROS levels between the good-quality-embryo and poor-quality-embryo groups. The ROS level was lower in the good-quality-blastocyst group than in the poor-quality-embryo group, which was statistically significant (P = 0.015).
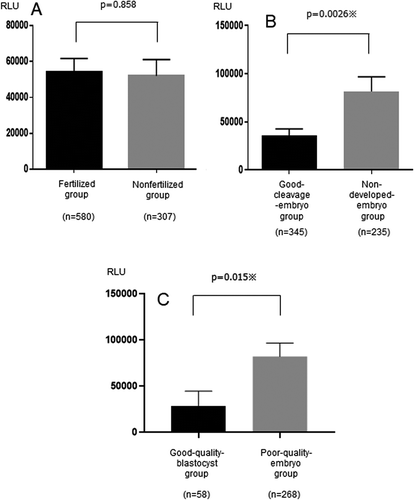
The ROS level was lower in the good-quality-blastocyst group than in the poor-quality-embryo group, which was statistically significant (27,916 ± 16,336 and 81,780 ± 14,498 RLU, respectively; P = 0.015) (, ). As calculated from the ROC curve, the cut-off level of ROS was 4,926 RLU (area under the curve = 0.608). The blastocysts were divided into high- and low-ROS groups using this cut-off value. Similar to the cleavage embryos, the good-quality-blastocyst rate was lower in the high-ROS group (11.2%) than in the low-ROS group (24.8%), which was statistically significant (P = 0.001).
Table 4. Comparison of ROS levels in semen between the good-quality-blastocyst and poor-quality-embryo groups.
Discussion
One of the common maternal causes of fertilization failure in IVF is hardening of the zona pellucida. Moreover, oocyte activation failure is regarded to be the main cause of failure in ICSI (Vanden Meerschaut et al. Citation2014). Thus, paternal factors such as deficiency in oocyte activators (e.g., phospholipase C zeta) can lead to fertilization failure in ICSI (Tesarik et al. Citation2004). Less common paternal causes of failure in ICSI include a lack of motile spermatozoa, severe forms of teratozoospermia (globozoospermia), and technical problems with sperm injection (Yanagida Citation2004; Dam et al. Citation2007). Furthermore, paternal factors such as DNA damage in sperm are considered to have adverse effects on the late stages of embryo development. ROS in semen is known to induce sperm DNA fragmentation (Barroso et al. Citation2000; Irvine et al. Citation2000); hence, in this study, we investigated the effects of ROS on fertilization and embryo development.
ROS are categorized as free radicals (e.g., superoxide anions, hydroxyl radicals, peroxyl radicals) and nonradical species (e.g., hydrogen peroxide, hypochlorous acid) (Agarwal et al. Citation2003; Sanocka and Kurpisz Citation2004). Immature spermatozoa and seminal leukocytes are considered to be the sources of ROS in semen. Spermatozoal cytoplasm deposits contain large amounts of glucose-6-phosphate dehydrogenase enzyme, which produces nicotinamide adenine dinucleotide phosphate (NADPH) (Aitken and Clarkson Citation1987; Sharma et al. Citation2001; Sakkas et al. Citation2003; Koppers et al. Citation2010). NADPH generates ROS through an NADPH oxidase enzyme complex (Williams and Ford Citation2004). Owing to this mechanism, immature spermatozoa with large amounts of cytoplasm are considered to generate higher ROS levels. Furthermore, ROS generation from leukocytes is attributable to an inflammation or infection in the male genital tract, producing ROS through the myeloperoxidase system, which is an essential immune system for pathogens (Iwasaki and Gagnon Citation1992). High ROS levels in semen cause LPO of the sperm cells, resulting in the loss of fluidity and integrity, which are essential for the fusion of the sperm and oocyte (Aitken et al. Citation1993). Zabludovsky et al. (Citation1999) reported that LPO has adverse effects on sperm concentration and motility.
Unrepaired DNA damage in sperm has a negative effect on embryo development and implantation, as well as on pregnancy, which is termed the ‘late paternal effect’ (Tesarik et al. Citation2004). The late paternal effect occurs because embryo development is dependent on maternal factors at the time of fertilization, but switches to the embryo’s own genomes on days 2 and 3 (Tesařík et al. Citation1986). However, recent studies have shown that DNA damage in sperm also influences the early stages of embryo development (Simon et al. Citation2014; Neyer et al. Citation2015). In our study, a significantly higher ROS level in semen was confirmed in the non-developed-embryo and poor-quality-embryo groups; nonetheless, there was no significant difference in fertilization. These findings support those of earlier studies, indicating that a high ROS level in semen leads to damage during the late stages of embryo development, as well as the early stages of embryo development through DNA fragmentation. We did not measure the ROS level in prepared sperm (the condition of chosen sperm for ICSI); however, we did measure the ROS level in raw semen samples during the patient’s closest visit before ICSI. Measuring ROS levels in raw semen prior to ICSI may predict embryo development; we found significantly higher ROS levels in the non-developed-embryo and poor-quality embryo groups. Additionally, we found significant cut-off values of ROS in semen for good-cleavage (6,601 RLU) and good-quality blastocysts (4,926 RLU). This suggests that acquisition of a good-quality blastocyst requires a lower ROS level than acquisition of a good-cleavage embryo. These values may aid in predicting embryo development in ICSI by ROS level. Furthermore, the ROS levels did not significantly differ between the fertilized and nonfertilized groups in the present study, implying that maternal factors may act as a balance against sperm LPO and DNA fragmentation induced by ROS in semen at the time of fertilization.
In this study, we analyzed the fertilization, good-cleavage-embryo, and blastulation rates for each oocyte in order to assess the effects of ROS on embryo development. Several reports have described the negative effects of ROS in semen on the outcomes of IVF and ICSI and compared the outcomes according to the cycles of IVF or ICSI (Zorn et al. Citation2003; Das et al. Citation2008). In this manner of investigation, a ‘50% fertilization rate’ can mean 50 good embryos out of 100 per cycle, as well as one good embryo out of two. Thus, the fertilization rate should not be considered to be reflective of embryo development. Therefore, we analyzed embryo development by each oocyte, which is more accurate.
This study had some limitations. First, we focused on fertilization and embryo development in this study and did not hence evaluate the pregnancy rate, which includes several maternal factors (e.g., uterine factors). Second, although a previous study reported that ROS were positive in 111 out of 243 patients with idiopathic infertility (45.6%) and 105 out of 206 patients with varicocele (51.0%) (Yumura et al. Citation2017), we did not measure the ROS level in each background of male infertility. Third, the total antioxidant capacity in semen, which has been reported to be useful for evaluating the oxidant stress status (Sharma et al. Citation1999), was not estimated in this study. The balance between ROS and total antioxidant capacity is worth investigating, and our data imply that ROS has an adverse effect on days 3 and 5 of embryo development. Fourth, not analyzing embryo development by cycles of ICSI, but rather by each oocyte, is not a standard method of assessment. However, we consider it to be a novel and more accurate way to assess embryo development. In order to determine the adverse effect of ROS, not only ROS level measurement but also further testing of sperm DNA fragmentation or chromatin integrity should be conducted in future research.
In conclusion, ROS in semen adversely affected the circumstances of embryo development in ICSI with respect to the development of both good cleavage and good blastocysts, rather than fertilization. As expected, ROS in semen were associated with both early and late paternal effects. These findings may aid in predicting the development of good cleavage and blastocysts from the ROS level in semen.
Materials and methods
Population
A total of 77 infertile couples who underwent ICSI at the Reproduction Centre of Yokohama City University Medical Centre between March 2013 and December 2016 were enrolled. All husbands visited the urology department and underwent measurement of ROS levels in semen at least once. Subjects were included based on the wife’s age (≤43 years) and the presence of at least one metaphase II oocyte for ICSI. A total of 887 eggs acquired from 141 cycles of ICSI procedures were analyzed. Eggs were first divided into two groups – namely, the fertilized group (embryos fertilized through ICSI) and the nonfertilized group (nonfertilized eggs) (Supplemental Figure 1A, B). Among the fertilized embryos, those on day 3 were subsequently divided into the good-cleavage-embryo group (successfully developed embryos) and the non-developed-embryo group (unsuccessfully developed embryos). Good cleavage was defined as an embryo with more than 7 grade 3 cells according to the Veeck classification on day 3 (Supplemental Figure 1C, D).
Among the fertilized embryos, blastocysts were also divided into good-quality and poor-quality blastocysts. Good-quality blastocysts were defined as blastocysts >3BB according to the Gardner classification on day 5 (Supplemental Figure 1E, F). To avoid selection bias when evaluating blastocyst development, we excluded the ICSI cycles with cleavage transplantation on day 3 (Supplemental Figure 1B).
ROS assessment
For the measurement of ROS levels, the unprocessed semen sample at the time closest to oocyte retrieval was used. The ROS levels in semen samples were measured using Monolight 3010™ Luminometer (BD Biosciences Pharmingen, Ltd., San Diego, CA, USA). Before and after adding 40 μL of 100 mmol/L luminol to 500 μL of the raw semen sample, the subtraction of the integrated chemiluminescence was measured between 0 and 200 seconds (Supplemental Figure 2). The chemiluminescence value was expressed in RLU.
The ROS levels in semen were compared between the fertilized and nonfertilized groups, between the good-cleavage-embryo and non-developed-embryo groups, and between the good-quality-blastocyst and poor-quality-blastocyst groups. The cut-off value of ROS was calculated from the ROC curve to predict good-cleavage-embryo and good-quality-blastocyst development.
ICSI procedures
Female partners underwent ovarian stimulation using either a short or long protocol of gonadotropin-releasing hormone (GnRH) agonist, or GnRH antagonist, which was chosen depending on her age and serum anti-Müllerian hormone level. Human chorionic gonadotropin (10,000 IU) was administered until the follicles were 18–20 mm in diameter. Oocytes were retrieved with transvaginal ultrasound assistance at 36 hours after human chorionic gonadotropin injection. After retrieval, oocytes were pre-incubated for 4 hours prior to sperm injection (37.0℃, 6.0% CO2, 5.0% O2). Cumulus was removed from the oocytes just before ICSI using hyaluronidase (ICSI Cumulase®; CooperSurgical Inc., Trumbull, CT, USA).
Spermatozoa were prepared for ICSI with the two-layer concentration gradient method using SpermGrad®, SpermRinse® (Vitrolife, Göteborg, Sweden) and centrifugation at 500 g for 20 min. After washing (centrifugation at 162 g for 10 min), the swim-up method was used. A metaphase II oocyte was aspirated, and one selected spermatozoon was injected into the ooplasm. After confirmation of fertilization, the embryo was cultured for 3 days in Sydney IVF Cleavage Medium® (Cook Medical, Bloomington, IN, USA). Subsequently, the cleavage embryo was cultured to a blastocyst in IVF Blastocyst Medium® (Cook Medical, Bloomington, IN, USA).
Statistical analyses
Statistical analyses were performed using the Statistical Package for the Social Sciences version 21 (IBM Corp., Armonk, NY, USA). Unpaired t-tests were used to compare each group. For all tests, P-values ≤0.05 were considered statistically significant.
Ethical approval
The study was approved by the Yokohama City University Review Board. Written informed consent was obtained from each couple prior to study initiation. All procedures were conducted in accordance with the World Medical Association’s Code of Ethics (1964 Declaration of Helsinki, as revised in 2003, and the 1975 Declaration of Tokyo, as revised in 2006).
Authors’ contributions
Wrote the manuscript: SK; Conceived and designed the experiments: SK, TT, KT, YY; Performed the experiments: SK, TT, KT, KU, KY, HS; Analyzed the data: SK, KT, TK; Contributed reagents/materials/analysis tools: KT, HU, MM, YY.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Supplemental Material
Download Zip (462.6 KB)Acknowledgments
We are grateful to Mizuki Yamamoto (embryologist) for her helpful discussions on ICSI procedures.
Data availability statement
The data that support the findings of this study are available upon request from the corresponding author, TT.
Disclosure statement
The authors report no conflict of interest.
Supplementary material
Supplemental data for this article can be accessed here.
References
- Agarwal A, Saleh RA, Bedaiwy MA. 2003. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil Steril. 79(4):829–843.
- Aitken RJ, Clarkson JS. 1987. Cellular basis of defective sperm function and its association with the genesis of reactive oxygen species by human spermatozoa. J Reprod Fertil. 81(2):459–469.
- Aitken RJ, Harkiss D, Buckingham D. 1993. Relationship between iron-catalysed lipid peroxidation potential and human sperm function. J Reprod Fertil. 98(1):257–565.
- Barroso G, Morshedi M, Oehninger S. 2000. Analysis of DNA fragmentation, plasma membrane translocation of phosphatidylserine and oxidative stress in human spermatozoa. Hum Reprod. 15(6):1338–1344.
- Dam AH, Feenstra I, Westphal JR, Ramos L, van Golde RJ, Kremer JA. 2007. Globozoospermia revisited. Hum Reprod Update. 13(1):63–75.
- Das S, Chattopadhyay R, Jana SK, Babu KN, Chakraborty C, Chakravarty B, Chaudhury K. 2008. Cut-off value of reactive oxygen species for predicting semen quality and fertilization outcome. Syst Biol Reprod Med. 54(1):47–54.
- de Lamirande E, Jiang H, Zini A, Kodama H, Gagnon C. 1997. Reactive oxygen species and sperm physiology. Rev Reprod. 2(1):48–54.
- Du Plessis SS, Agarwal A, Halabi J, Tvrda E. 2015. Contemporary evidence on the physiological role of reactive oxygen species in human sperm function. J Assist Reprod Genet. 32(4):509–520.
- Irvine DS, Twigg JP, Gordon EL. 2000. DNA integrity in human spermatozoa: relationships with semen quality. J Androl. 21(1):33–44.
- Iwasaki A, Gagnon C. 1992. Formation of reactive oxygen species in spermatozoa of infertile patients. Fertil Steril. 57(2):409–416.
- Koppers AJ, Garg ML, Aitken RJ. 2010. Stimulation of mitochondrial reactive oxygen species production by unesterified, unsaturated fatty acids in defective human spermatozoa. Free Radic Biol Med. 48(1):112–119.
- Moilanen JM, Carpén O, Hovatta O. 1998. Flow cytometric light scattering analysis, acrosome reaction, reactive oxygen species production and leukocyte contamination of semen preparation in prediction of fertilization rate in vitro. Hum Reprod. 13(9):2568–2574.
- Neyer A, Zintz M, Stecher A, Bach M, Wirleitner B, Zech NH, Vanderzwalmen P. 2015. The impact of paternal factors on cleavage stage and blastocyst development analyzed by time-lapse imaging – a retrospective observational study. J Assist Reprod Genet. 32(11):1607–1614.
- Sakkas D, Seli E, Bizzaro D, Tarozzi N, Manicardi GC. 2003. Abnormal spermatozoa in the ejaculate: abortive apoptosis and faulty nuclear remodelling during spermatogenesis. Reprod Biomed Online. 7(4):428–432.
- Saleh RA, Agarwal A, Nada EA, El-Tonsy MH, Sharma RK, Meyer A, Thomas AJ Jr. 2003. Negative effects of increased sperm DNA damage in relation to seminal oxidative stress in men with idiopathic and male factor infertility. Fertil Steril. 79:1597–1605.
- Sanocka D, Kurpisz M. 2004. Reactive oxygen species and sperm cells. Reprod Biol Endocrinol. 2(1):12.
- Sharma RK, Pasqualotto FF, Nelson DR, Agarwal A. 2001. Relationship between seminal white blood cell counts and oxidative stress in men treated at an infertility clinic. J Androl. 22(4):575–583.
- Sharma RK, Pasqualotto FF, Nelson DR, Thomas AJ Jr, Agarwal A. 1999. The reactive oxygen species-total antioxidant capacity score is a new measure of oxidative stress to predict male infertility. Hum Reprod. 14(11):2801–2807.
- Simon L, Murphy K, Shamsi MB, Liu L, Emery B, Aston KI, Carrell DT. 2014. Paternal influence of sperm DNA integrity on early embryonic development. Hum Reprod. 29(11):2402–2412.
- Sukcharoen N, Keith J, Irvine DS, Aitken RJ. 1996. Prediction of the in-vitro fertilization (IVF) potential of human spermatozoa using sperm function tests: the effect of the delay between testing and IVF. Hum Reprod. 11(5):1030–1034.
- Takeshima T, Yumura Y, Yasuda K, Sanjo H, Kuroda S, Yamanaka H, Iwasaki A. 2017. Inverse correlation between reactive oxygen species in unwashed semen and sperm motion parameters as measured by a computer-assisted semen analyzer. Asian J Androl. 19(3):350–354.
- Tesarik J, Greco E, Mendoza C. 2004. Late, but not early, paternal effect on human embryo development is related to sperm DNA fragmentation. Hum Reprod. 19(3):611–615.
- Tesařík J, Kopečný V, Plachot M, Mandelbaum J. 1986. Activation of nucleolar and extranucleolar RNA synthesis and changes in the ribosomal content of human embryos developing in vitro. J Reprod Fertil. 78(2):463–470.
- Vanden Meerschaut F, Nikiforaki D, Heindryckx B, De Sutter P. 2014. Assisted oocyte activation following ICSI fertilization failure. Reprod Biomed Online. 28(5):560–571.
- Williams AC, Ford WC. 2004. Functional significance of the pentose phosphate pathway and glutathione reductase in the antioxidant defenses of human sperm. Biol Reprod. 71(4):1309–1316.
- Yanagida K. 2004. Complete fertilization failure in ICSI. Hum Cell. 17(4):187–193.
- Yumura Y, Iwasaki A, Saito K, Ogawa T, Hirokawa M. 2009. Effect of reactive oxygen species in semen on the pregnancy of infertile couples. Int J Urol. 16(2):202–207.
- Yumura Y, Takeshima T, Kawahara T, Sanjo H, Kuroda SN, Asai T, Mori K, Kondou T, Uemura H, Iwasaki A. 2017. Reactive oxygen species measured in the unprocessed semen samples of 715 infertile patients. Reprod Med Biol. 16(4):354–363.
- Zabludovsky N, Eltes F, Geva E, Berkovitz E, Amit A, Barak Y, Bartoov B. 1999. Relationship between human sperm lipid peroxidation, comprehensive quality parameters and IVF outcome. Andrologia. 31(2):91–98.
- Zorn B, Vidmar G, Meden-Vrtovec H. 2003. Seminal reactive oxygen species as predictors of fertilization, embryo quality and pregnancy rates after conventional in vitro fertilization and intracytoplasmic sperm injection. Int J Androl. 26(5):279–285.
