ABSTRACT
Efficient cryopreservation of small numbers of human spermatozoa is essential in cases of severe male infertility, especially those requiring surgical sperm retrieval. Although vitrifying individual spermatozoa on sperm vitrification devices (SpermVD®) provided optimal cell retrieval upon warming, motility rates tended to be lower than with bulk-freezing. Post-warming motility is directly affected by cryoprotectant exposure; however, optimal cryoprotectant equilibration time is unknown. We evaluated several timeframes exposing individual spermatozoa to cryoprotectant before freezing and different cryoprotectants. A total of 2,925 spermatozoa from 20 patients ranging from normozoospermic to moderate oligoteratoasthenozoospermic were vitrified in small groups, on 60 SpermVD®, in 1 µl droplets of 1:1 v/v cryoprotectant/washing medium mixture. Each group was vitrified after 2–60 minutes equilibration time. Motility of each group was evaluated after warming. Leftover pellets were frozen in cryotubes in a mixture of 1:1 v/v cryoprotectant/washing medium after 10 minutes equilibration at room temperature and 10 minutes on liquid nitrogen vapors. Post-thaw motility correlated negatively with cryoprotectant exposure time. The highest post-warming motility rate (32.1%) was observed with 8-minutes equilibration. After 10 minutes, motility rate of vitrified sperm was lower than that of bulk-freezing (31.7% vs. 37.0%, p < 0.0001). Different cryoprotectants did not affect the results. Therefore, for vitrifying small numbers of spermatozoa, we suggest maximum equilibration time of 8-minutes to achieve maximum motility after warming.
Introduction
The concept of using liquid nitrogen to freeze human sperm began as early as 1943 (Hoagland and Pincus Citation1943). This practice has become routine in the assisted reproduction laboratory (Anger et al. Citation2003). Slow freezing is the prevalent method used by sperm banks worldwide. Sperm is suspended in a mixture of 1:1 v/v sperm washing medium and a cryoprotective agent for 10 minutes (Polge et al. Citation1949; McLaughlin et al. Citation1992) at room temperature and then gradually cooled to −192°C. This method improves survival and motility after thawing by reducing deleterious effects such as ice crystal formation, dehydration, increased salt concentration and thermal shock (Critser et al. Citation1987; Royere et al. Citation1996; Desrosiers et al. Citation2006). However, it requires a relatively large total liquid volume of 0.25 to 1 ml and is not appropriate when only a few spermatozoa are frozen, such as in cases of virtual azoospermia, cryptozoospermia or after testicular biopsy, due to inevitable loss of spermatozoa upon post-thaw sample processing.
Several methods for cryopreserving small numbers of spermatozoa have been introduced. These include empty zona pellucida (Cohen et al. Citation1997), cryoloops (Desai et al. Citation2004), ICSI pipettes (Gvakharia and Adamson Citation2001), spherical Volvox globator algae (Just et al. Citation2004), straws (Isachenko et al. Citation2005), cell sleepers (Endo et al. Citation2012; Coetzee et al. Citation2016) and agarose microspheres (Hatakeyama et al. Citation2017).
Recently, we reported a new method for cryopreservation of individual spermatozoa. The SpermVD® platform (Berkovitz et al. Citation2018) relies on rapid freezing of individual motile spermatozoa in a 1 µl droplet of cryoprotectant and washing medium mixture. However, since micro-manipulation of individual spermatozoa while transferring them onto the SpermVD® might be time-consuming, the first cells to be placed are exposed to cryoprotectant (equilibrated) for a longer period than are the last cells. Previous studies have shown that exposure to cryoprotectant for as little as 15 minutes during slow freezing has a detrimental effect on motility after warming (Critser et al. Citation1988). However, we were unable to find published information on optimal equilibration times regarding vitrification with low volume freezing. The current study investigated the optimal timeframe to equilibrate individual spermatozoa in a cryoprotectant solution before freezing and to evaluate the effects of different media on the results.
Results
The study included a cohort of 20 patients. The basic characteristics of semen samples prior to cryopreservation were as follows: Sperm concentration ranged from 3.5x106/ml to 220x106/ml with an average of 55.6x106/ml (SD = 59.5). Motility ranged from 20% to 75%, with an average of 51% (SD = 16). Total motile capacity ranged from 2 × 106 to 250 × 106 with an average of 59.1 (SD = 82). Survival rate ranged from 20% to 71% with an average of 37% (SD = 17) ().
Table 1. Basic characteristics of semen samples prior to cryopreservation.
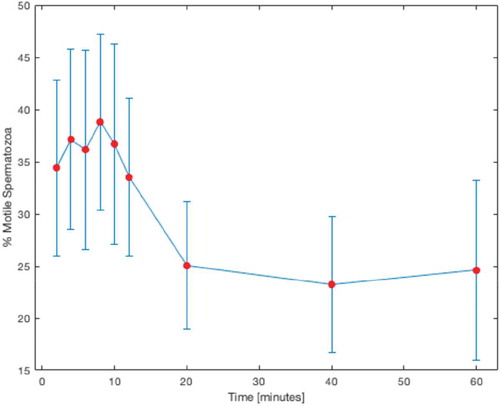
A total of 2,925 spermatozoa were frozen on 60 SpermVD® units, using 3 devices per patient and 3 different equilibration times per device (). Out of all vitrified spermatozoa, 2,785 (95%) were retrieved, of which 896 (32%) were motile. Motility rates increased with equilibration, with peak motility rate of 38.8% at 8 minutes (p = 0.03). From 10 minutes of equilibration and onward, the motility rates began to decrease (). shows the number of cryopreserved and retrieved spermatozoa and motility rates as a function of equilibration time.
Table 2. Equilibration durations for vitrification on SpermVD. Three SpermVD devices were frozen for each patient and each of the 3 sperm-containing droplets on a SpermVD was frozen after a specific equilibration time.
Table 3. Vitrification results as a function of equilibration time.
Using Spearman’s rank correlation coefficient, we found a negative correlation between cryoprotectant equilibration time and sperm motility after warming (p = 0.001) (). No correlation was found between initial sperm concentration and motility (p = 0.989) or to post-warming motility (p = 0.221).
Table 4. Correlation between initial parameters of sperm, equilibration time and post-warming motility.
When compared to two different media, no statistically significant difference was observed at short, medium and long exposure times ().
Table 5. Comparison of different cryopreservation media.
Discussion
This study exposed spermatozoa to cryoprotectant for varying durations and evaluated how this affected post-warming motility. Although the SpermVD® provides satisfactory results in terms of retrieval after warming, the optimal equilibration time for individual spermatozoa exposed to cryoprotectant has not been evaluated previously. As motility is a determining factor for sperm selection for ICSI and in cases of frozen sperm and as it is directly affected by exposure to cryoprotectant, we calculated the optimal equilibration time for individual spermatozoa.
A key factor in sperm cryotolerance is the large surface to volume ratio, which affects cytosol viscosity and glass transition, making them less susceptible to cryodamage (John Morris et al. Citation2012). However, without a cryoprotective agent, cold shock and ice crystals forming in the surrounding media may critically damage the cell membrane and organelles (Abdelhafez et al. Citation2009).
Although cryoprotectant is crucial for sperm survival during cryopreservation, it has detrimental effects of its own, which include causing extreme osmotic stress (Gao et al. Citation1995), generating reactive oxygen species (Yoon et al. Citation2016) and increasing lipid peroxidation (Alvarez et al., Citation1992).
Some studies reported that cryoprotectant exposure and post-thaw sperm motility were negatively correlated (Critser et al. Citation1988; Polcz et al. Citation1998; Ozkavukcu et al. Citation2008). However, cryoprotectant equilibration time was not consistent between these and other similar studies.
A recent study comparing vitrification versus conventional freezing showed an advantage for vitrification in terms of sperm morphology, although it was more damaging to motility, as compared to conventional freezing (Le et al. Citation2019). However, using our method, we achieved similar results for vitrification as compared to bulk freezing (36.7% vs. 37.0%, respectively, at 10 minutes). It should be taken into consideration, though, that bulk-freezing is not applicable for individual spermatozoa and currently, vitrification is the only option.
We found that equilibration duration and post-warming motility were negatively correlated. Interestingly, we observed a slight increase in post-warming motility at exposures of up to 8 minutes, while the decrease in motility began at 10 minutes and onward (). This slight timeframe shift compared to the equilibration time of 10 minutes with standard freezing protocols might occur because unlike in bulk freezing, where cryoprotectant is slowly added to a suspension of cells and has to diffuse toward the membranes, here each sperm comes into contact with the cryoprotectant immediately when it is placed on the SpermVD®, so equilibration seems to occur quicker than it does with slow freezing. This contributes both to the relative stability at lower equilibration times and to the rapid decrease in motility after warming at higher equilibration times, as both positive and negative effects of the cryoprotectant occur more quickly than with slow freezing.
Efficient, small-volume vitrification procedures are needed when dealing with very low numbers of spermatozoa. The SpermVD® was found to be effective when only a few spermatozoa need to be frozen, with 95% retrieval rate. The study results suggest that 8 minutes or less of equilibration achieves optimal motility post-warming when using the SpermVD® platform. Although there was no significant difference between motility rates between two different cryoprotectant media, additional research is needed to establish the optimal type and concentration of cryoprotectant to achieve better results.
Materials and methods
Study design
This prospective study was conducted in 2018 and included a cohort of 20 patients, ranging from normozoospermic to mild oligoasthenoteratozoospermic, who were referred for motile spermatozoa organelle microscopic examination (MSOME) after at least one failed IVF or IVF-ICSI cycle. After the procedure was completed, individual spermatozoa were removed from the sample and frozen on the SpermVD®. The remaining sample was frozen in a cryovial for comparison.
Sample preparation
The reagents used in the study included:
PureCeption Sperm Separation Media. A two-layered (upper layer of 80% v/v and lower layer of 80% v/v) colloidal suspension of silane-coated silica particles in HEPES-buffered human tubal fluid.
Quinn’s Sperm Washing Medium. A HEPES-buffered human tubal fluid containing human serum albumin, calcium, various energy sources and antioxidants.
Quinn’s advantage sperm freezing medium. A HEPES-buffered salt solution containing glycerol and sucrose as cryoprotectants (All Sage In-Vitro Fertilization, Inc., Trumbull, CT, USA).
The samples were loaded onto PureCeption Sperm Separation Media (Sage In-Vitro Fertilization, Inc.) after being left to liquefy at room temperature for 20 minutes. The samples were centrifuged for 20 minutes at 300 rpm. After removing the upper liquid, the pellet was resuspended in Quinn’s Sperm Washing Medium. It was centrifuged again for 5 min at 600 rpm. This procedure was performed twice, after which the majority of the liquid was removed. The pellet was diluted in washing medium to achieve a concentration of ~10x106 spermatozoa/ml. Next, 1 µl of the resuspended pellet was loaded into a 50 mm plate (World Precision Instruments Inc., Sarasota, FL, USA) containing a 4 µl droplet of polyvinyl pyrrolidone (PVP) 10% solution (Sage In-Vitro Fertilization, Inc.). A 0.6 µl droplet of Quinn’s sperm washing medium was added to the plate along with another PVP droplet for filling the microcapillary. The plate was covered with light paraffin oil (Sage In-Vitro Fertilization, Inc.) and placed under ×200 magnification. An inverted, phase contrast microscope (Eclipse Ti, Nikon Instruments Inc. Melville, NY, USA) equipped with DeltaPix software (DeltaPix, Smorum, Denmark) and an Invenio 3SII camera were used. The TransferMan NK2 micromanipulation system with a CellTram Oil pump (Eppendorf, Hamburg, Germany) was used to collect progressively motile spermatozoa from the PVP droplet into the washing medium collection droplet. The system was equipped with a non-angulated, glass microcapillary that had a 12-µm diameter tip (TPC, Thebarton, Australia).
Sperm vitrification
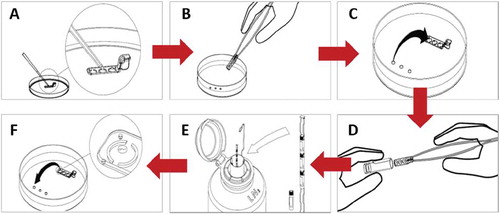
A 0.8–1 µl droplet of equal parts Quinn’s sperm washing medium and Quinn’s advantage sperm freezing medium (Sage In-Vitro Fertilization, Inc.) was placed in each well of the SpermVD® (Rafimed, Inc., Yavne, Israel) (), which was then submerged in oil on the plate containing the sperm (). Ten to 20 spermatozoa were transferred from the collection droplet to each droplet on the SpermVD® () and left to equilibrate for specific intervals. The time between transfers was calculated using a stopwatch and three intervals were frozen for each SpermVD®. Following this, the SpermVD® was removed from the plate and placed into a 3.6 ml cryovial (), which was immediately placed on an aluminum holder and submerged in liquid nitrogen ().
Figure 2. SpermVD® protocol. A. 1 µl of a cryoprotectant solution is placed on each well. B. The SpermVD® is placed under oil on a search plate. C. Spermatozoa are transferred from the collection droplets to the SpermVD®. D. The SpermVD® is inserted into a cryotube. E. The cryotube is placed in LN2. F. Warmed SpermVD® is placed on an ICSI dish and spermatozoa are transferred to washing medium droplets.
Bulk sample freezing
The leftover pellet was resuspended in Quinn’s advantage sperm washing medium to achieve a 0.5 ml volume. The same amount of Quinn’s freezing medium was then added to the suspension, which was left to equilibrate for 10 minutes at room temperature, in accordance with the manufacturer’s protocol. This mixture was transferred into a labeled 1.8 ml cryovial and placed above liquid nitrogen vapor for another 10 minutes, after which it was submerged into liquid nitrogen.
SpermVD® warming
Leaving sufficient room for the SpermVD®, a 4 µl droplet of 10% PVP for filling the microcapillary and several 0.6 µl droplets of Quinn’s sperm washing medium were placed on a sterile 50 mm dish. The dish was covered with light paraffin oil. The cryovials containing the SpermVD® were removed from the liquid nitrogen, opened at room temperature and left until the oil covering the droplets liquified completely. The SpermVD® was then placed under oil on the dish and examined under ×200 magnification with an inverted phase contrast microscope. Spermatozoa found in the SpermVD® droplets were transferred to the collection droplets (). Motility was determined based on careful observation of the spermatozoa in the collection droplet immediately after transfer. To compensate for Brownian motion, motility was defined as any separate movement of the sperm head or tail. The percentage of motile spermatozoa was defined as the motility recovery rate. Cases where spermatozoa were not found were considered lost spermatozoa. This procedure was repeated for each SpermVD® separately.
We chose to determine viability of retrieved sperm based on motility because it is the main criterion used by embryologists performing ICSI, when choosing which cell to inject. We previously found that motile sperm resulted in a 50% higher fertilization rate, as compared to immotile cells (Berkovitz et al. Citation2018).
Bulk sample thawing
The cryovial was removed from the liquid nitrogen and left at room temperature until the contents were completely liquefied. The sample was then transferred to a centrifuge tube, an equal volume of washing medium was added. The tube was centrifuged for 5 min at 600 rpm, after which the upper liquid was removed. The pellet was examined using a sperm counting chamber (High Tech Solutions, New Delhi, India). The percentage of motile sperm was recorded and survival rate was defined as the percentage of motile spermatozoa after thawing, as compared to the original motility percentage.
Comparison to additional media
To check the compatibility of the method with different media, the same procedure was performed using a mixture of 0.7/1 v/v GM501 SpermStore as cryoprotectant and GM501 SpermAir as washing medium (Gynemed, Lensahn, Germany). Three exposure times were compared.
Statistical analysis
Data are described as mean, standard deviation, minimum and maximum for continuous variables and as percentage of total for nominal parameters. The data were evaluated for normal distribution using Kolmogorov-Smirnov and Shapiro-Wilk tests. Spearman’s correlation coefficient was used to assess the relation between initial concentration and motility and of equilibration time on post-warming motility. To evaluate the optimal exposure time, we conducted t-test between exposure times. P values <0.05 were considered significant. SPSS-20 was used to analyze the data.
Ethics approval
The local Institutional Review Board approved the study (#00119-16-ASMC). All patients provided written informed consent.
Author contributions
Planned and performed the experiments and drafted the manuscript: MB; performed the experiments: DI, VF, OR, SA; reviewed the manuscript and analyzed the data: NM; planned the experiments, interpreted the data and reviewed the manuscript: AB. All authors have read and approved the final version of the manuscript and agree with the order of presentation of the authors.
Disclosure statement
The authors have no competing financial interests to declare.
References
- Abdelhafez F, Bedaiwy M, El-Nashar SA, Sabanegh E, Desai N. 2009. Techniques for cryopreservation of individual or small numbers of human spermatozoa: A systematic review. Hum Reprod. 15:153–164.
- Alvarez JG, Storey BT. 1992. Evidence for increased lipid peroxidative damage and loss of superoxide dismutase activity as a mode of sublethal cryodamage to human sperm during cryopreservation. J Androl. 13:232–241.
- Anger JT, Gilbert BR, Goldstein M. 2003. Cryopreservation of sperm: indications, methods and results. J Urol. 170:1079–1084.
- Berkovitz A, Miller N, Silberman M, Belenky M, Itsykson P. 2018. A novel solution for freezing small numbers of spermatozoa using a sperm vitrification device. Hum Reprod. 33:1975–1983.
- Coetzee K, Ozgur K, Berkkanoglu M, Bulut H, Isikli A. 2016. No reliable single sperm cryopreservation in cell sleepers for azoospermia management. Andrologia. 48:203–210.
- Cohen J, Garrisi GJ, Congedo-Ferrara TA, Kieck KA, Schimmel TW, Scott RT. 1997. Cryopreservation of single human spermatozoa. Hum Reprod. 12:994.
- Critser JK, Huse-Benda AR, Aaker DV, Arneson BW, Ball GD. 1987. Cryopreservation of human spermatozoa. I. Effects of holding procedure and seeding on motility, fertilizability, and acrosome reaction. Fertil Steril. 47:656–663.
- Critser JK, Huse-Benda AR, Aaker DV, Arneson BW, Ball GD. 1988. Cryopreservation of human spermatozoa. III. The effect of cryoprotectants on motility. Fertil Steril. 505:314–320.
- Desai NN, Blackmon H, Goldfarb J. 2004. Single sperm cryopreservation on cryoloops: an alternative to hamster zona for freezing individual spermatozoa. Reprod Biomed Online. 9:47–53.
- Desrosiers P, Légaré C, Leclerc P, Sullivan R. 2006. Membranous and structural damage that occur during cryopreservation of human sperm may be time-related events. Fertil Steril. 85:1744–1752.
- Endo Y, Fujii Y, Shintani K, Seo M, Motoyama H, Funahashi H. 2012. Simple vitrification for small numbers of human spermatozoa. Reprod Biomed Online. 24:301–307.
- Gao DY, Liu C, McGann LE, Watson PF, Kleinhans FW, Mazur P, Critser JK. 1995. Prevention of osmotic injury to human spermatozoa during addition and removal of glycerol. Hum Reprod. 10:1109–1122.
- Gvakharia M, Adamson GD. 2001. A method of successful cryopreservation of small numbers of human spermatozoa. Fertil Steril. 76:S101.
- Hatakeyama S, Tokuoka S, Abe H, Araki Y, Araki Y. 2017. Cryopreservation of very low numbers of spermatozoa from male patients undergoing infertility treatment using agarose capsules. Hum Cell. 30:201–208.
- Hoagland H, Pincus G. 1943. Revival of mammalian sperm after immersion in liquid nitrogen. J Gen Physiol. 25:337–344.
- Isachenko V, Isachenko E, Montag M, Zaeva V, Krivokharchenko I, Nawroth F, Dessole S, Katkov II, van der Ven H. 2005. Clean technique for cryoprotectant-free vitrification of human spermatozoa. Reprod Biomed Online. 10:350–354.
- John Morris G, Acton E, Murray BJ, Fonseca F. 2012. Freezing injury: the special case of the sperm cell. Cryobiology. 64:71–80.
- Just A, Gruber I, Wöber M, Lahodny J, Obruca A, Strohmer H. 2004. Novel method for the cryopreservation of testicular sperm and ejaculated spermatozoa from patients with severe oligospermia: a pilot study. Fertil Steril. 82:445–447.
- Le MT, Nguyen TTT, Nguyen TT, Nguyen VT, Nguyen TTA, Nguyen VQH, Cao NT. 2019. Cryopreservation of human spermatozoa by vitrification versus conventional rapid freezing: effects on motility, viability, morphology and cellular defects. Eur J Obstet Gynecol Reprod Biol. 234:14–20.
- McLaughlin EA, Ford WC, Hull MG. 1992. The contribution of the toxicity of a glycerol-egg yolk-citrate cryopreservative to the decline in human sperm motility during cryopreservation. J Reprod Fertil. 95:749–754.
- Ozkavukcu S, Erdemli E, Isik A, Otzuna D, Karahuseyinoglu S. 2008. Effects of cryopreservation on sperm parameters and ultrastructural morphology of human spermatozoa. J Assist Reprod Genet. 25:403–411.
- Polcz TE, Stronk J, Xiong C, Jones EE, Olive DL, Huszar G. 1998. Optimal utilization of cryopreserved human semen for assisted reproduction: recovery and maintenance of sperm motility and viability. J Assist Reprod Genet. 15:504–512.
- Polge C, Smith AU, Parkes AS. 1949. Revival of spermatozoa after vitrification and dehydration at low temperatures. Nature. 164:666.
- Royere D, Barthelemy C, Hamamah S, Lansac J. 1996. Cryopreservation of spermatozoa: a 1996 review. Hum Reprod Update. 2:553–559.
- Yoon SJ, Rahman MS, Kwon WS, Park YJ, Pang MG. 2016. Addition of cryoprotectant significantly alters the epididymal sperm proteome. PLoS One. 11:e0152690.