ABSTRACT
The aim of the study was to investigate the micro-structures of the spermatic cord using histological examination with three-dimensional (3D) reconstruction of the serial tissue sections of the cord for clinical application in microscopic varicocelectomy. Human spermatic cord specimens obtained from 13 adult male cadavers were used to prepare serial transverse sections. The sections were stained to allow observation of the spermatic cord microstructures. The 3D reconstruction was performed with digitized serial sections by Mimics software. The microscopic varicocelectomy was performed based on the anatomical results of 3D reconstruction of the spermatic cord. The results showed the number of small spermatic veins, large spermatic veins, arteries, lymphatics or nerves were not markedly different between the subinguinal and inguinal regions or between the right and left sperm cord. The number of medium spermatic veins in the subinguinal region was obviously higher than at the inguinal level. The internal spermatic vessels and the vas deferens together with other associated vessels within the cremaster were separately enclosed by two thin and translucent sheaths, the internal spermatic fascia and the vas deferens fascia. We conclude that internal spermatic vessels and the vas deferens together with the associated neurovascular vessels are wrapped by two distinct sheaths separating them from the surrounding tissues. Microscopic varicocelectomy based on the anatomical results of 3D reconstruction of the spermatic cord is feasible.
Abbreviations
3D: three-dimensional; ISF: internal spermatic fascia; ESF: external spermatic fascia; MHIV: High inguinal microsurgical varicocelectomy; MSIV: subinguinal microsurgical varicocelectomy; CAAD: computer-assisted anatomic dissection; HE: hematoxylin-eosin
Introduction
Currently, microsurgical varicocelectomy is the mainstay procedure for the treatment of varicocele and refractory orchialgia – the two major conditions that affect male fertility (Avila-Vergara et al. Citation2001; Masarani and Cox Citation2003; Mirilas and Mentessidou Citation2012). Most andrologists that perform a subinguinal microscopic varicocelectomy suggest subinguinal microscopic varicocelectomy is better than other surgical procedures. Because only a few high-quality clinical trials have been published, other surgical procedures are still used for varicocele (Bryniarski et al. Citation2017). Therefore, the standard procedure of the microsurgical varicocelectomy needs to be confirmed. The anatomic relationship between the vas deferens and the internal spermatic fascia is not clearly defined. Most clinical studies on varicocelectomy suggest that the deferens should be separated first to open the internal spermatic fascia (ISF) after opening the external spermatic fascia (ESF) and the cremaster muscle. According to such studies, the deferens is located posterior to the ISF (Baazeem and Zini Citation2009; Beck et al. Citation1992; Zini et al. Citation2006; Zini Citation2007). On the contrary, other studies suggest that ISF should be opened first to reveal the deferens because both the deferens and internal spermatic vessels are enclosed by the IFS (Mirilas et al. Citation2008; Mirilas Citation2011; Mirilas and Mentessidou Citation2012). There is no practice guideline on the specific steps to be followed during the procedure. In addition, there is no conventional surgical approach for varicocelectomy. High inguinal microsurgical varicocelectomy (MHIV) and subinguinal microsurgical varicocelectomy (MSIV) are the two commonly used procedures. The study by Hopps et al. showed that the subinguinal approach increased the difficulty of the procedure and the risk of artery injury although it reduced the pain and shortened the recovery time (Hopps et al. Citation2003). However, the study by Tuccar et al. demonstrated that the subinguinal approach did not increase the procedure difficulty because the number of internal and external spermatic veins and spermatic arteries did not differ between MHIV and MSIV (Tuccar et al. Citation2009).
Noteworthy, both the anatomic relationship between the vas deferens and the internal spermatic fascia, and the choice of surgical approach in varicocelectomy are important for the success of the operation. Yet, only few studies have been undertaken to improve microsurgical varicocelectomy techniques. Conventional methods, such as cadaveric and surgical observations cannot reveal the complexity of the micro-structures of the spermatic cord. Previously, a combination of continuous biopsy and computer-assisted anatomic dissection (CAAD) was used in micro-anatomic studies because they provide high resolution and magnification of microstructures which allows a more detailed view of many micro-anatomic entities in a three-dimensional manner (Kay et al. Citation1998; Fujimura and Nozaka Citation2002).
Based on this background, the present study attempted to explore the microanatomy of the spermatic cord by histological staining and three-dimensional reconstruction using a combination of continuous biopsy and CAAD. The aim of our study was to provide a better understanding of the sophisticated micro-structures of the spermatic cord to provide the basis to improve the surgical treatment for varicocele.
Results
Fascia and vessels
After staining, the histological transverse sections clearly displayed the microstructures of the spermatic cord (). The fascia was clearly observed in the polarimicroscope images of the sections after sirius red staining which showed typical characteristics of type II collagen (). Under stereo microscopy, we observed that the outermost layer of the irregular cylindrical spermatic cord was the external spermatic fascia and the cremaster muscle inside which were two thin and translucent sheaths (). The large sheath which wrapped the internal spermatic vessels was identified as the well-known internal spermatic fascia while the smaller sheath which wrapped the vas deferens and its associated vessels was termed as the vas deferens fascia. Most of the two delicate circular sheaths with different contours and sizes were stuck laterally to the inner contour of the cremaster or the external spermatic fascia. They connected closely at the middle, with each other leaving little space between them. The two sheaths and their contents ran in parallel inside the external spermatic fascia and the cremaster muscle. The existence of two separate sheaths was also confirmed by the 3D reconstruction images ().
Figure 1. Histologic transverse sections showing micro-structures of the sperm cord under a low-magnification microscope. VD: vas deferens, ESF: external spermatic fascia, ISF: internal spermatic fascia, ISV: internal spermatic vessels, DF:deferens fascia, DA: deferens artery, DV: deferens vein. (A & B) The sperm cord under a light microscope (HE staining, ×4). (C) The sperm cord under a stereo microscope (sirius red staining, ×2). (D) The morphology of fascia under a polarimicroscope (sirius red staining, ×4). The light red color shows the dense type II collagen of the fascia which is characterized by strong refractivity.
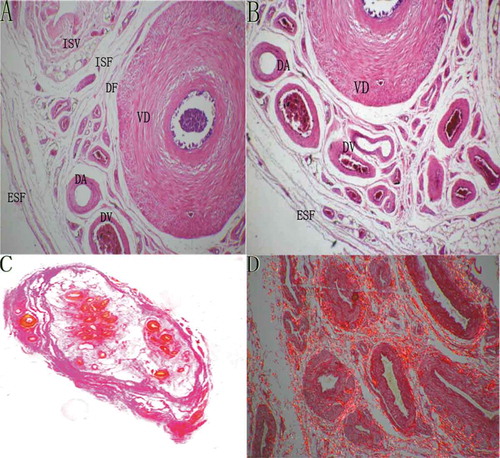
Figure 2. The microanatomy of the vas deferens and the internal spermatic fascia. (A) A histologic transverse section of the whole sperm cord under stereo microscopy (sirius red staining, ×2). The black arrows point to the internal spermatic fascia which wraps around the internal spermatic vessels; the green arrows point to the vas deferens fascia which wraps around the vas deferens plus its accompanying vessels. CM: cremaster, ISV: internal spermatic vessels, VD: vas deferens, ESF: external spermatic fascia, DV: deferens vessels. (B) A histologic transverse section of the whole sperm cord from another sperm cord specimen under stereo microscopy (decolored after sirius red staining, ×2). The green arrows point to the internal spermatic fascia which wraps around the internal spermatic vessels; the red arrows point to the vas deferens fascia which wraps around the vas deferens plus its accompanying vessels.
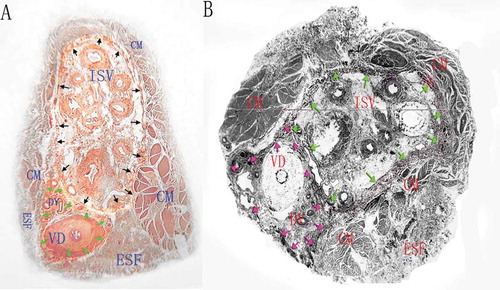
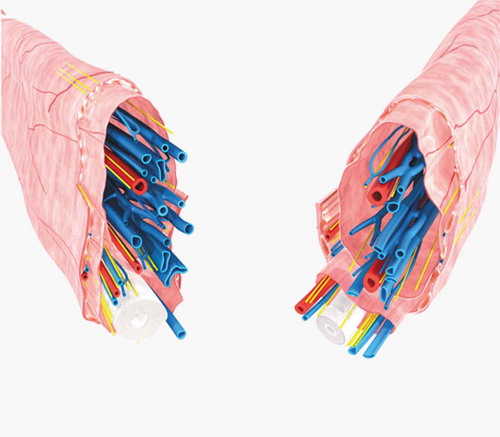
Figure 3. The 3D reconstruction images of the vessels, cord, fascias and muscle of the sperm cord based on the histological transverse sections from two different specimens. The external spermatic fascia and the cremaster constitute the outmost layer which wraps around two separate sheaths and their contents. The white tubular structure indicates the vas deferens, the blue tubular ones indicate the veins and the red ones the arteries.
The number of veins, arteries and lymphatic vessels did not differ between the subinguinal and inguinal levels or between left and right sides (all P > 0.05) ().
Table 1. Mean number of the spermatic vein, arteries and lymphatics at subinguinal and inguinal levels in 13 cadaveric specimens (t-test).
Nerves
At the vas deferens fascia, the majority of nerve fiber bundles were distributed posteriorly or laterally to the vas deferens while few were distributed around the vas deferens artery (). At the external compartment of the spermatic cord, including the external spermatic fascias and the cremaster, nerve fiber bundles mainly traveled among the muscle bundles (). In the internal spermatic fascia, most nerves were located around the internal spermatic artery (). Only a few nerve fiber bundles were found in the adipose tissue (). In general, the nerve fiber bundles in the spermatic cord were mainly distributed around the vas deferens, arteries and muscle bundles, attached to the fascia or the connective tissue (). The number of nerve fibers was not markedly different among the external compartment, the vas deferens fascia and the internal spermatic fascia (P > 0.05) ().
Table 2. Mean number of nerve fibers at left and right side in 13 cadaveric specimens (t-test).
Figure 4. Histological sections showing the neural morphology and distribution in the sperm cord. VD: vas deferens, N: nerve fibers, CM: cremaster, ISV: internal spermatic vessels, ESV: external spermatic vessels, FC: fat cells. (A) A histologic transverse section of vas deferens under light microscopy (HE staining, ×4) showing nerve fibers located around the vas deferens. (B) A histologic transverse section of external spermatic compartment under light microscopy (HE staining, ×4) showing nerve fibers located between muscles. (C) Nerve fibers around the internal spermatic vessels under light microscopy (HE staining, ×4). (D) Nerve fibers in adipose tissue under light microscopy (HE staining, ×4). (E & F) Nerve fibers under light microscopy stained by silver staining and immunohistochemical staining (Mouse anti-human S100 monoclonal antibody, ×4).
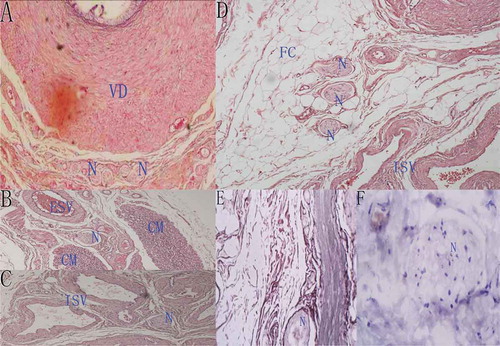
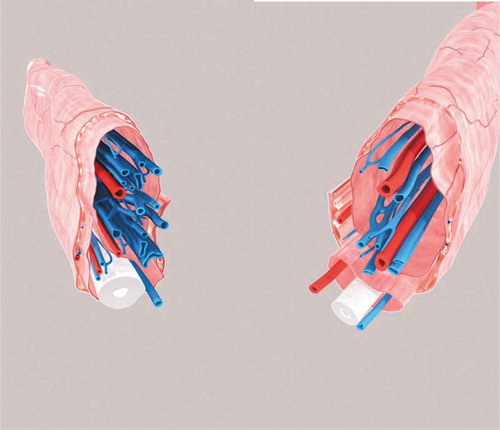
Figure 5. The 3D reconstruction images of the vessels, cord, fascias, muscle and nerves of the sperm cord based on the histological transverse sections from two different specimens. The external spermatic fascia and the cremaster constitute the outmost layer which wraps around two separate sheaths and their contents. The white tubular structure indicates the vas deferens, the blue tubular ones indicate the veins, the red ones the arteries, and the yellow lines the nerves.
Discussion
Different from previous studies in which anatomic examination of the spermatic cord was performed during surgery, the present study used histological sections and 3D reconstruction of serial sections to explore the microanatomy of the spermatic cord. By examining all the fascias in the spermatic cord through histological staining and CAAD, we observed that the internal spermatic vessels and the vas deferens together with its neurovascular plexus were surrounded by two separate sheaths. We hold the view that the larger sheath is the internal spermatic fascia as found by previous research. For the first time, we name the smaller sheath as the vas deferens fascia. These two fascias, which are wrapped by the external compartment, are connected to each other and ran in parallel in the spermatic cord. We did not observe any pedicles linking the fascias to the external compartment.
Gaudin et al. described, for the first time, a ‘delicate translucent vascular sheath’ surrounding the internal spermatic vessels and the vas deferens together with its neurovascular plexus located externally to the sheath (Gaudin et al. Citation1988). Beck et al. interpreted the sheath as the internal spermatic fascia and regarded the vas deferens as the external compartment, a view that is held in many studies (Baazeem and Zini Citation2009; Beck et al. Citation1992; Zini et al. Citation2006; Zini Citation2007). Current anatomic observations have shown that internal spermatic vessels are surrounded by the internal spermatic fascia, and that the vas deferens is located at the posterior of the fascia. This is consistent with our finding although the observation that the vas deferens and its neurovascular plexus are also wrapped around by a sheath was not observed in their study. In addition, our view is that the vas deferens is a part of the internal compartment and not the external compartment.
Mirilas et al. provided a different point of view regarding the internal spermatic fascia (Mirilas et al. Citation2008; Mirilas Citation2011). They suggested that the sheath around the internal spermatic vessels is a continuation of the membranous layer of the extraperitoneal fascia from above the internal inguinal ring. Similarly, the vas deferens and its vessels are surrounded by a membranous layer. These findings are in agreement with ours, except that, in their study, the two membranous layers are located inside the internal spermatic fascia. They concluded that the two membranous layers attach posteriorly to the internal spermatic fascia by two short pedicles. Our findings are dissimilar to their interpretation of the internal spermatic fascia and also with their finding on the pedicles. In our study, the internal spermatic fascia refers to the sheath wrapping around the internal spermatic vessels, an observation that is held by many studies. However, in their study, the internal spermatic fascia refers to the sheath that surrounds the two membranous layers and their contents. Here, we found that the two membranous layers and their contents are surrounded by the external spermatic fascia and the cremaster rather than by the internal spermatic fascia as interpreted by Mirilas et al.
These discrepancies may have resulted from the different investigation methods. Nonetheless, we believe that our histological examination of sections and 3D reconstruction of the serial sections provides a more precise characterization of the anatomy of the spermatic cord compared to gross observations during surgery. 3D reconstruction also could provide a more intuitive picture than the information from cord specimens that helped to further understand the external spermatic fascia, vas deferens fascia and internal spermatic fascia. From the 3D models, the external spermatic fascia and the cremaster constitute the outmost layer were showed clearly, which wraps around two separate sheaths and their contents. The vessels were also wrapped by the separate sheath. Different understanding of the microstructures of the spermatic cord may result in different procedures in microsurgical varicocelectomy which was introduced by Marmar in 1985 and then modified by Goldstein in 1992 (Marmar et al. Citation1985; Goldstein et al. Citation1992). This microsurgery was further modified by Zini on the basis that internal spermatic vessels are surrounded by the internal spermatic fascia and the vas deferens is located at the posterior of the fascia (Zini et al. Citation2006).
In this micro-anatomic study on cadaveric specimens, we also compared the number of arteries, veins and lymphatic vessels between the inguinal and subinguinal levels because these parameters influence the choice of surgical approaches. At present, MHIV and MSIV are widely used in clinical andrology. Some surgeons prefer MHIV over MSIV because the latter deals with more internal and external spermatic veins (diameter ≥ 2 mm) and has to encounter the internal spermatic artery which is firmly wrapped by complicated reticular veins in many cases compared to MHIV (95% for MSIV versus 30% for MHIV). Consequently, MSIV is thought to be more complicated and it may increase the risk of injury to the arteries (Hopps et al. Citation2003). However, considering that the number of internal and external spermatic veins and arteries is not notably different between the inguinal and subinguinal levels as observed by Tuccar et al., others believe that MSIV will not increase operational injuries. In fact, they prefer MSIV due to its advantage of reducing pain and postoperative recovery time (Tuccar et al. Citation2009). Although the use of 13 cadavers was a small size sample and we did not observe differences in the number of veins, arteries, and lymphatic vessels between the subinguinal and inguinal levels in Chinese male cadaveric specimens. This finding is also consistent with our previous clinical trial in which we found no differences in the number of veins, arteries, and lymphatic vessels between the two regions. Therefore, we agree with Tuccar et al. that MSIV may not increase the difficulty of operation. However, additional studies are required to explore whether ethnicity may cause micro-anatomical differences in the distribution of vessels.
Understanding the nerve distribution patterns in the spermatic cord is important for the microsurgery involving nerves. This study was based on cross sections rather than spermatic cord biopsies, which was likely to be more accurate. Following its first use by Choa to treat chronic orchalgia in 1992, microsurgical varicocelectomy has been proved to be effective by several subsequent studies (Choa and Swami Citation1992; Levine et al. Citation1996). This operation aims to ligate all the nerves in the spermatic cord. Yet, current anatomical studies on the location of nerves in the spermatic cord have not revealed their precise distribution to facilitate the microsurgical varicocelectomy. Therefore, one of our goals was to examine the microanatomy of the spermatic cord nerves. We found that many nerves were distributed in all tissues except tabular structures and their distribution or location in the spermatic cord was not constant. This microanatomic finding is of interest to microsurgical varicocelectomy. Studies on the use of varicocelectomy to treat chronic orchalgia reported that 9.2% of the patients had only partial pain relief and 8% still felt pain after surgery (Biggers and Soderdahl Citation1981; Yeniyol et al. Citation2003). We postulate that the unsuccessful pain relief might have been caused by incomplete ligation of nerve fiber bundles. Consequently, we suggest that skeletonized ligation should be performed during varicocelectomy to relieve refractory chronic testicular pain. A study on clinical use in microsurgical spermatic cord denervation procedure will be performed in future.
One of the limitations of this study is that 13 cadavers was a small size sample. Moreover, we failed to measure the diameters of veins in the spermatic cord in the histological sections. In addition, the use of cadaver material may be different from fresh sample because the vessels can dilate and contract in fresh sample. In addition, we did not characterize the nature of nerve fibers in the cadaveric specimens. Further studies should be performed on the two sheaths wrapping the internal spermatic vessels and the vas deferens together with its neurovascular perplex to determine their origin by tracing them to the tissues above the inguinal ring. A well designed large-size prospective randomized control clinical trial is required.
Materials and methods
Specimens
We examined specimens of the spermatic cord (including the left and right sides) obtained from 13 healthy male cadavers at the Department of Anatomy, Southern Medical University. The exact causes of death were unknown, but macroscopic examination showed that the spermatic cords, testis, kidneys and their vessels were free from obvious pathological changes and surgical intervention.
Histologic observation
The sections were stained for histological examination by two methods: hematoxylin-eosin (HE) staining and sirius red staining for vessels, fascias and nerves. All the stained sections were investigated in sequence under an optical microscope to identify the morphology of each structural entity and to explore the relationships among fascias, blood vessels, and vas deferens. Vessels and nerves were counted at the inguinal and subinguinal levels, and in the right and left spermatic cords. All fascias, arteries, veins, and nerves were verified by histologic examination by an experienced pathologist. Statistical analysis was performed using SPSS 16.0 (IBM, USA) to compare the number of vessels and nerves between the two levels and two side spermatic cords.
Three-dimensional reconstruction
All the reconstruction steps were manually performed. Histological sections were digitized by stereo microscopy (OLYMPUS SZ61-SET, Germany). Histological images were reassembled with Photoshop software 7.0 and realigned for sequential representation. Vessels, fascias, and nerve fibers were outlined manually and reconstructed three-dimensionally using the Minics software10.0 (Materialise, Belgium). A complete 3D reconstruction was performed only for the two left and two right spermatic cords.
Ethical approval
All study protocols were approved by the Medical Ethics Committee of Zhujiang Hospital and Southern Medical University, Guangzhou.
Authors’ Contributions
Participated in the design of the study, carried out the study: XMM. Carried out the study: performed statistical analysis, prepared figures and data tables: BKL. Carried out the study, participated in statistical analysis and wrote the manuscript: YY, XQW and QL. Helped to carry out the study. All authors read and approved the final manuscript: WS, SW, RWX, XMZ, DJL.
Disclosure statement
All authors declare no competing interests.
Additional information
Funding
References
- Avila-Vergara MA, Balderas-Ariza JA, Cordova-Gonzalez K, Hernandez-Guerrero C. 2001. Effect of palomo procedure on the quality of the semen in infertile patients with varicocele and oligoastenospermia. Ginecol Obstet Mex. 69:262–267.
- Baazeem A, Zini A. 2009. Surgery illustrated – surgical atlas microsurgical varicocelectomy. BJU Int. 104(3):420–427. doi:10.1111/j.1464-410X.2009.008768.x.
- Beck EM, Schlegel PN, Goldstein M. 1992. Intraoperative varicocele anatomy: a macroscopic and microscopic study. J Urol. 148(4):1190–1194. doi:10.1016/S0022-5347(17)36857-X.
- Biggers RD, Soderdahl DW. 1981. The painful varicocele. Mil Med. 146(6):440–441. doi:10.1093/milmed/146.6.440.
- Bryniarski P, Taborowski P, Rajwa P, Kaletka Z, Życzkowski M, Paradysz A. 2017. The comparison of laparoscopic and microsurgical varicocoelectomy in infertile men with varicocoele on paternity rate 12 months after surgery: a prospective randomized controlled trial. Andrology. 5(3):445–450. doi:10.1111/andr.12343.
- Choa RG, Swami KS. 1992. Testicular denervation. a new surgical procedure for intractable testicular pain. Br J Urol. 70(4):417–419. doi:10.1111/j.1464-410X.1992.tb15800.x.
- Fujimura A, Nozaka Y. 2002. Analysis of the three-dimensional lymphatic architecture of the periodontal tissue using a new 3D reconstruction method. Microsc Res Tech. 56(1):60–65. doi:10.1002/()1097-0029.
- Gaudin J, Lefevre C, Person H, N’Guyen H, Senecail B. 1988. The venous hilum of the testis and epididymis: anatomic aspect. Surg Radiol Anat. 10(3):233–242. doi:10.1007/BF02115243.
- Goldstein M, Gilbert BR, Dicker AP, Dwosh J, Gnecco C. 1992. Microsurgical inguinal varicocelectomy with delivery of the testis: an artery and lymphatic sparing technique. J Urol. 148(6):1808–1811. doi:10.1016/S0022-5347(17)37035-0.
- Hopps CV, Lemer ML, Schlegel PN, Goldstein M. 2003. Intraoperative varicocele anatomy: a microscopic study of the inguinal versus subinguinal approach. J Urol. 170(6):2366–2370. doi:10.1097/01.ju.0000097400.67715.f8.
- Kay PA, Robb RA, Bostwick DG. 1998. Prostate cancer microvessels: a novel method for three-dimensional reconstruction and analysis. Prostate. 37(4):270–277. doi:10.1002/()1097-0045.
- Levine LA, Matkov TG, Lubenow TR. 1996. Microsurgical denervation of the spermatic cord: a surgical alternative in the treatment of chronic orchialgia. J Urol. 155(3):1005–1007. doi:10.1016/S0022-5347(01)66369-9.
- Marmar JL, DeBenedictis TJ, Praiss D. 1985. The management of varicoceles by microdissection of the spermatic cord at the external inguinal ring. Fertil Steril. 43(4):583–588. doi:10.1016/S0015-0282(16)48501-8.
- Masarani M, Cox R. 2003. The aetiology, pathophysiology and management of chronic orchialgia. BJU Int. 91(5):435–437. doi:10.1046/j.1464-410X.2003.04094.x.
- Mirilas P. 2011. Do not get lost in the spaces of the spermatic cord during subinguinal microsurgical varicocelectomy. BJU Int. 107(3):496–497. author reply 497. doi:10.1111/j.1464-410X.2011.10093_1.x.
- Mirilas P, Mentessidou A. 2012. Microsurgical subinguinal varicocelectomy in children, adolescents, and adults: surgical anatomy and anatomically justified technique. J Androl. 33(3):338–349. doi:10.2164/jandrol.111.013052.
- Mirilas P, Mentessidou A, Skandalakis JE. 2008. Secondary internal inguinal ring and associated surgical planes: surgical anatomy, embryology, applications. J Am Coll Surg. 206(3):561–570. doi:10.1016/j.jamcollsurg.2007.09.022.
- Tuccar E, Yaman O, Erdemli E, Zeyrek T, Esmer AF, Kilic O, Avunduk MC. 2009. Histomorphological differences of spermatic cords regarding subinguinal versus inguinal levels: a cadaveric study. Urol Int. 82(4):444–447. doi:10.1159/000218535.
- Yeniyol CO, Tuna A, Yener H, Zeyrek N, Tilki A. 2003. High ligation to treat pain in varicocele. Int Urol Nephrol. 35(1):65–68. doi:10.1023/A:1025972601213.
- Zini A. 2007. Varicocelectomy: microsurgical subinguinal technique is the treatment of choice. Can Urol Assoc J. 1(3):273–276.
- Zini A, Fischer A, Bellack D, Noss M, Kamal K, Chow V, Mak V. 2006. Technical modification of microsurgical varicocelectomy can reduce operating time. Urology. 67(4):803–806. doi:10.1016/j.urology.2005.10.044.
