ABSTRACT
As the final and critical step in in vitro fertilization (IVF), embryo transfer has always received much attention and deserves continuous optimization. In the present study, to explore the role of autocrine factors in embryo self-spent culture media, we prospectively compared embryo transfer with self-spent culture medium and fresh medium on clinical pregnancy outcomes. A total of 318 fresh IVF/intracytoplasmic sperm injection (ICSI) cycles were randomly allocated into two subgroups based on their transfer media (using a self-spent culture medium or new pre-equilibrated culture media), and the clinical outcomes were compared between groups. The implantation rates, clinical pregnancy rates and live birth rates for transfer using self-spent medium instead of new pre-equilibrated culture medium were slightly improved without statistical significance. Interestingly, however, biochemical pregnancy rate was found to be significantly decreased after transfer using self-spent medium for Day 3 embryos compared with new pre-equilibrated culture media. In short, embryo transfer with self-spent culture medium has shown some advantages, and large sample size studies are still needed to confirm these observations.
Abbreviations
ART: assisted reproductive technologies; ICSI: intracytoplasmic sperm injection; IVF: in vitro fertilization; ET: embryo transfer
Introduction
During the past four decades, assisted reproductive technology (ART) has undergone a series of tremendous developments and improvements from its inception to prosperity (Skakkebaek et al. Citation2019). Looking back, as the core system for embryo culture, the medium also undergoes a gradual optimization process from early simple balanced salt solutions to later complex culture system containing multiple components including phosphate, vitamins, amino acids, lipids, trace elements, and other biomolecules (Chronopoulou and Harper Citation2014; Sunde et al. Citation2016). Today, culture media for embryos has been completely commercialized, mainly divided into two categories, namely, single (single-step for the whole culture period) and sequential media systems (different media for days 0–3 and 3–6). However, subtle differences in culture effects caused by the application of different culture systems still exist (Morbeck et al. Citation2014). Therefore, until now, the influence of culture medium on embryo culture is still worth considering.
After obtaining high-quality embryos through embryo culture, the key to ensuring a successful pregnancy is to transfer the embryo into the uterine cavity in a suitable manner at the right time. Variables during embryo transfer (ET) have been confirmed to have an impact on subsequent pregnancy, such as expulsion of embryos, touching fundus, blood, or mucus on the catheter tip and bacterial contamination (Schoolcraft Citation2016). In addition, the choice of volume and type of transfer media during transplantation is also worthy of attention. Although commercialized transfer media supplemented with hyaluronan (HA) are now readily available, most reproductive medicine centers still use conventional fresh cleavage or blastocyst medium as a transfer media mainly due to the fact that controversy has always existed whether HA-enriched culture fluids can significantly improve clinical pregnancy (Urman et al. Citation2008; Nakagawa et al. Citation2012; Fancsovits et al. Citation2015). Therefore, research on transfer media still needs to be further performed, especially in terms of the effects of media components on embryo potential and endometrial receptivity.
In recent years, the development of diagnostics and metabolomics has prompted us to re-recognize the microenvironment of embryo culture. During 3–5 days of embryo culture, embryos release a variety of factors into the culture droplets, including different kinds of RNA and free DNA, proteins, small signal molecules, enzymes, and some metabolites, which can be used as targets for genetic diagnosis and embryo selection (Wallace et al. Citation2014; Capalbo et al. Citation2016; Belandres et al. Citation2019). In addition, some embryonic secretory factors also play biological roles in embryo implantation and maternal-fetal dialogue (Minas et al. Citation2005; Haouzi et al. Citation2011). Additionally, extracellular vesicles (EVs), nanosized, membrane-enclosed vesicles released by the cells that transport DNA, RNA, and proteins between cells, have been isolated from human embryo-conditioned media (Giacomini et al. Citation2017; Kurian and Modi Citation2019). A variety of miRNA species in embryo-derived EVs are predicted to mediate cellular activities such as adhesion and migration, suggesting that embryos could potentially modify the endometrial genome and mediate embryo-endometrial cross-talk (Kurian and Modi Citation2019). The number of EVs in embryo spent culture media has also been shown to be related to pregnancy outcomes (Abu-Halima et al. Citation2017). Therefore, we can conclude that self-spent culture medium droplets are valuable, and that transferring the droplets together with embryos into the uterus may be beneficial for embryo implantation and the continued pregnancy. However, due to the slight change in the pH and osmotic pressure of the culture droplets and the consumption of nutrient factors during the culture process, there may be potential adverse effects (Kleijkers et al. Citation2016). It remains unknown whether transferring embryos with droplets into the uterus can play a role in preimplantation embryos and endometrium, or whether it improves the pregnancy outcomes.
In this study, we prospectively compared the efficacy and differences of the two embryo transfer medium (fresh or self-spent) on implantation and clinical pregnancy rates of cleavage stage embryos as well as blastocysts in fresh in vitro fertilization (IVF)/intracytoplasmic sperm injection (ICSI)–ET cycles.
Results
Patient characteristics
A total of 318 embryo transfer cycles [274 day 3 (D3) cycles and 44 day 5 (D5) cycles] met our inclusion criteria and were randomly allocated into the two groups. Mean female age, duration of infertility, indications for treatment, number of previous failed cycles, and the characteristics of ovarian stimulation were similar between the fresh and self-spent transfer medium groups as summarized in and shown in Figure S1.
Figure 1. Schematic diagrams of the different embryo loading protocol. At the right time, the selected embryos were pre-transferred into a 500 μl pre-equilibrated fresh culture medium (G1TM-PLUS or G2TM-PLUS) awaiting ET. (A) In the control group, the embryos were routinely loaded into a transfer catheter directly from the pre-equilibrated fresh culture medium for ET; (B) however, in the test group, until before ET, stretched thin Pasteur pipettes were used to absorb the self-spent droplets under the oil, and the droplets were transferred at least three times in a new dish to remove the oil floating on the surface. Then, the selected embryos were transferred to the combined droplet for catheter loading and ET.
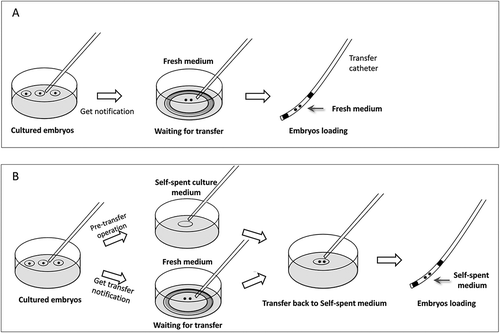
Table 1. Patient characteristics of embryo transfer with fresh or self-spent medium.
Embryo characteristics
The number and grade distribution of embryos selected for transfer were similar between the two groups (self-spent medium vs. fresh medium), including D3 cleavage stage embryos and D5 blastocyst stage embryos.
Specifically, in self-spent medium group, 256 D3 cleavage stage embryos (including 188 Grade 1 embryos and 68 Grade 2 embryos) and 22 blastocyst stage embryos (including 15 4/5AA embryos, 5 4/5AB/BA embryos, and 2 4/5BB embryos) were transferred; correspondingly, in the fresh medium group, 292 cleavage stage embryos (including 214 Grade 1 embryos and 78 Grade 2 embryos) and 22 blastocyst stage embryos (including 16 4/5AA embryos, 4 4/5AB/BA embryos, and 2 4/5BB embryos) were transferred.
Clinical outcomes
For D3 cleavage stage embryos transfer, the rates of positive human chorionic gonadotrophin (hCG) (60.15 vs. 57.53), implantation (35.54 vs. 30.48), clinical pregnancy (57.03 vs. 49.31), and live birth (53.13 vs. 44.52) were all slightly increased with the use of self-spent medium compared with fresh medium, but the differences did not reach statistical significance. The rate of biochemical pregnancy (5.19 vs. 14.18) was significantly decreased. The clinical abortion rate (8.21 vs. 9.72) was almost identical.
For D5 blastocyst stage embryos transfer, due to the small number of patients undergoing blastocyst transfer in fresh cycles, only 44 blastocyst transfer cycles (22 cycles with self-spent medium and 22 cycles with fresh medium) were included. Compared with the use of fresh medium, the rates of positive hCG (59.09 vs. 54.55), implantation (54.55 vs. 45.45), clinical pregnancy (54.55 vs. 45.45), and live birth (50.00 vs. 40.90) were slightly increased and the rate of biochemical pregnancy (7.69 vs. 16.67) was slightly decreased with the use of self-spent medium, where the clinical abortion rate (8.33 vs. 10.00) was almost identical. No significant differences for blastocyst transfer were found between the two groups ().
Table 2. Clinical outcomes of embryo transfers with fresh or self-spent medium.
Discussion
As a key factor in ART technology, culture medium has always been a research hotspot in the field. Although the culture medium has undergone considerable development and the outcome of embryo culture is good, it still cannot fully simulate in vivo environment. What effect this difference in culture conditions will have on future generations is still unclear, which is worthy of attention (Chronopoulou and Harper Citation2014). In this study, we focused on autocrine factors of the embryo, comparing the differences of clinical pregnancy outcomes after embryo transfer using fresh medium and self-spent medium. Interestingly, the biochemical pregnancy rate was found to be significantly decreased after transfer using self-spent medium for D3 embryos. Although no other significant difference between the two was revealed, some potential benefits were brought about by the change of embryo transfer using spent medium, in terms of embryo implantation rate, clinical pregnancy rate, and other aspects, which we will continue to investigate.
Implantation is a limiting step in ART procedures, but innovations in embryo transfer are lagging behind other aspects (Schoolcraft Citation2016). At present, most studies on transfer medium focus on the addition of HA and its concentration as well as the addition of protein and its concentration; however, research on the application of factors secreted by embryos in the transfer process of ART and their effects on clinical pregnancy are relatively rare (Urman et al. Citation2008; Nakagawa et al. Citation2012; Fancsovits et al. Citation2015). In fact, the factors secreted by the embryo during development and the small molecules secreted by endometrium play an important role in the early maternal-embryo dialogue and subsequent implantation, which has been confirmed in both human and animal models (Genbacev et al. Citation2003; Herrler et al. Citation2003). However, embryo of transfer using fresh media has virtually eliminated these factors, especially the cytokines secreted by early embryos.
Factors secreted by the embryo in spent medium have been repeatedly reported. Sher and Yie reported that soluble human leukocyte antigen (HLA-G) in embryo culture medium was associated with higher IVF implantation and pregnancy rate (Sher et al. Citation2005; Yie et al. Citation2005); Sheth et al. (Citation1991) reported that interleukin-1 (IL-1) levels in spent medium could be a useful parameter for predicting implantation. Moreover, microRNAs secreted from human blastocysts in culture media could be profiled with high reproducibility and some of them could be used for embryo reproductive competence assessment (Rosenbluth et al. Citation2014; Capalbo et al. Citation2016). Mitochondrial DNA in medium on D3 of culture was considered a noninvasive biomarker of blastocyst potential, and a high mtDNA/gDNA ratio in spent medium was associated with successful implantation outcome (Stigliani et al. Citation2014). Therefore, the usefulness of the spent medium has been confirmed.
In our study, no statistical differences were found in pregnancy outcomes between the two protocols of embryo transfer except biochemical pregnancy rate which was significantly decreased after transfer using self-spent medium for D3 embryos. Although the exact etiology of biochemical pregnancy remains unknown; the failure of maternal-embryo crosstalk should be critical (Annan et al. Citation2013; Kolte et al. Citation2014). Numerous studies have shown that cytokine and growth factors act in an autocrine/paracrine manner to regulate embryonic development as well as adhesion to maternal endometrial epithelium (Minas et al. Citation2005; Haouzi et al. Citation2011). The maternal endometrial response to implantation may be that more factors secreted by the preimplantation embryo partly explaining the decreased biochemical pregnancy upon transfer with self-spent medium. Therefore, whether embryo transfer with spent medium is beneficial for implantation cannot be simply concluded, and large sample studies will still be necessary.
There are still some issues to be addressed. Firstly, in order to reduce the impact of embryos quality on implantation and data bias, we only included high-quality embryos in this study. However, for some patients without high-quality embryos, inferior embryo transfer is acceptable. Whether transfer with spent medium has an effect on the implantation of poor-quality embryos remains unclear. Additionally, self-spent of one-step media for blastocyst transfer requires further study. Moreover, in the process of ART, the external environment that embryos are exposed to may have an epigenetic effect on the offspring, which has been growing much attention (de Ziegler et al. Citation2018). The impacts of the two different transfer medium on epigenetics could also worth considering.
In conclusion, this study focused on the role of autocrine factors in embryo self-spent culture medium and proposed a new transfer strategy. For the selected samples in the study, although biochemical pregnancy was reduced for D3 embryo transfer, we did not find obvious significant differences in embryo implantation and clinical pregnancy between the two transfer strategies. Further studies involving larger sample sizes and some other variables noted above require more investigation in the future.
Materials and methods
Patient selection
A total of 335 couples participated in this study, which was performed between January 2018 and December 2019 at the Reproductive Medicine Department, Jinan Central Hospital Affiliated to Shandong University, and Reproductive Medicine Center, Gansu Provincial Maternity and Child-Care Hospital. The trial (NCT03728140) was registered at ClinicalTrials.gov. Among them, 12 participants were bleeding during transfer and 5 patients undergone secondary transplantation due to embryonic residues. The remaining 318 cases were included in the study, 150 patients completed embryo transfer with self-spent medium, while the other 168 patients experienced embryo transfer with fresh medium (see the flow diagram in Supp. Figure S1), and the clinical pregnancy outcomes between the two groups were subsequently analyzed and compared.
Patients met the following inclusion criteria: (i) ≤ 35 years old; (ii) regular menstrual cycles; (iii) baseline follicle stimulating hormone (FSH) <12 IU/L; (iv)infertility with simple tubal or/and male factors; (v) did not experience more than two prior failed ET cycles; (vi) at least two good-quality D3 embryo or one good-quality D5 blastocyst for ET. Exclusion criteria were as follows: (i) uterine abnormalities, such as adenomyosis, endometriosis and endometrial polyps; (ii) ovarian abnormalities, such as low ovarian response and polycystic ovary syndrome (PCOS); (iii) endometrial thickness <7 mm on transfer day.
Ovarian stimulation and IVF/ICSI procedure
Controlled ovarian hyperstimulation was carried out with uninterrupted injection of recombinant FSH (Gonal-F; Merck Serono; Switzerland) following pituitary down-regulation by GnRHa as described previously (Huang et al. Citation2016). When the dominant follicles measured ≥ 18 mm in diameter, hCG (, Lizhu Inc, China) or recombinant hCG (Ovidrel, Merck Sereno, Switzerland) was administrated, and oocyte retrieval was performed approximately 36.5 h later. Sperms were prepared using density gradient centrifugation and swim-up techniques before fertilization, and IVF/ICSI procedures were applied 4–6 h after oocyte retrieval, which was typical at our institution. Fertilization was assessed by checking the appearance of two pronuclei 16–18 h later.
Embryo culture
After fertilization, embryos were cultured individually in G1TM-PLUS medium drops (20 μL, Vitrolife, V.Frölunda, Sweden) containing human serum albumin and gentamicin covered with OVOILTM (Vitrolife, V.Frölunda, Sweden) in the culture dish until D3, G2TM-PLUS medium was sequential used from D3 to D5 if the embryos need to blastocyst culture. All media for embryo culture were pre-equilibrated at 37°C and 6% CO2 before use.
Cleavage stage embryos were morphologically classified according to the number and size of blastomeres, the percentage of fragmentation as well as multinucleation on D3, as following: Grade 1, no or <5% fragmentation with at least six equal‑sized blastomeres and no multinucleation; Grade 2, <20% fragmentation with slightly unequal-sized blastomeres and no evidence of multinucleation; Grade 3, 20%–50% fragmentation with obviously unequal-sized cells; and Grade 4, >50% damage or severely unequal-sized cells or visible multinucleation. Blastocysts were classified according to development stage (staging 1–5) and morphology of inner cell mass (Grading A-C) and trophectoderm (Grading A-C), as described elsewhere (Hill et al. Citation2013).
During embryo culture, individual embryos were cultured in individual droplets covered with paraffin oil. Such consumed embryo culture droplet was defined as the self-spent medium of the particular embryo.
Embryo transfer
Two D3 fresh cleavage stage embryos scored better than grade 2 or single D5 fresh blastocyst stage embryo scored better than 4BB was selected for intrauterine transfer. When the patient was ready for embryo transfer, after receiving the transplant notice from the clinical staff, the selected embryos were pretransferred into a 500 μl pre-equilibrated fresh culture medium (G1TM-PLUS or G2TM-PLUS) awaiting ET (less than 5 min, time for clinical placement of graft tube to uterine). In the test group, the stretched thin Pasteur pipettes were used to absorb the self-spent droplets under the oil, and the droplets were transferred at least three times in a new dish to remove the oil floating on the surface. Then, the selected embryos were immediately transferred to the combined droplet for catheter loading. Correspondingly, the embryos of the control group were loaded directly in the pre-equilibrated fresh culture medium (operation schematic diagram see ). Luteal support by progesterone administration was provided uniformly to all patients after transfer.
Outcome measures
Chemical pregnancy was confirmed by detecting serum β-hCG level 14 days after embryo transfer, and clinical pregnancy was defined as presence of at least one gestational sac identified by ultrasound 5 weeks after transfer. Biochemical pregnancy rate was defined as the number of early pregnancy losses divided by the total number of positive serum β-hCG. Clinical pregnancy rate was defined as the number of clinical pregnancies divided by the number of transfer cycles. Implantation rate was calculated as the number of geastational sacs divided by the number of embryos transferred. Clinical abortion rate was defined as the number of clinical pregnancy losses before 28 weeks of gestation divided by the total number of clinical pregnancies. Live birth rate was defined as a ratio between healthy living newborns and embryo transfer cycles.
Statistical analysis
Data were analyzed using student’s t-test and chi-square test. P <.05 was considered statistically significant.
Ethics approval
The couples underwent ICSI/IVF treatment and their high-quality embryos were randomized to embryo transfer using either fresh or self-spent medium with informed consents and ethics approvals from Central Hospital Affiliated to Shandong University or Gansu Provincial Maternity and Child-care Hospital.
Author contributions
Study design: YZ, YW, BW, BY, ST, RH. Techniques performing in IVF labs: BW, BY, ST, RH. Clinical intervention and tracking: YZ, ST, YN. Data collection: BW, BY, YN, YZ. Statistical analysis: BW, YZ. Manuscript writing: BW, YZ. Manuscript revising: BW, YZ, YL. All authors approved revisions and the final manuscript.
Supplemental Material
Download MS Word (36.5 KB)Acknowledgments
This work was financially supported in part by the National Key Basic Research Program of China (2018YFC1003600), Gansu Provincial Science and Technology Department Grant (18YF1WA045), and Jinan Science and Technology Development Plan (201907014). We thank all the staff at the participating IVF centers.
Disclosure statement
The authors declare no conflicts of interest.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Abu-Halima M, Häusler S, Backes C, Fehlmann T, Staib C, Nestel S, Nazarenko I, Meese E, Keller A. 2017. Micro-ribonucleic acids and extracellular vesicles repertoire in the spent culture media is altered in women undergoing in vitro fertilization. Sci Rep. 7:13525. doi:10.1038/s41598-017-13683-8.
- Annan JJ, Gudi A, Bhide P, Shah A, Homburg R. 2013. Biochemical pregnancy during assisted conception: a little bit pregnant. J Clin Med Res. 5:269–274. doi:10.4021/jocmr1008w.
- Belandres D, Shamonki M, Arrach N. 2019. Current status of spent embryo media research for preimplantation genetic testing. J Assist Reprod Genet. 36:819–826. doi:10.1007/s10815-019-01437-6.
- Capalbo A, Ubaldi FM, Cimadomo D, Noli L, Khalaf Y, Farcomeni A, Ilic D, Rienzi L. 2016. MicroRNAs in spent blastocyst culture medium are derived from trophectoderm cells and can be explored for human embryo reproductive competence assessment. Fertil Steril. 105:225–235. e3. doi:10.1016/j.fertnstert.2015.09.014.
- Chronopoulou E, Harper JC. 2014. IVF culture media: past, present and future. Hum Reprod Update. 21:39–55. doi:10.1093/humupd/dmu040.
- de Ziegler D, Pirtea P, Poulain MP, Vanlieferinghen S, MarcAyoubi J. 2018. Time to think about neonatal outcome in assisted reproductive technology. Fertil Steril. 109:789–790. doi:10.1016/j.fertnstert.2018.03.018.
- Fancsovits P, Lehner A, Murber A, Kaszas Z, Rigo J, Urbancsek J. 2015. Effect of hyaluronan-enriched embryo transfer medium on IVF outcome: a prospective randomized clinical trial. Arch Gynecol Obstet. 291:1173–1179. doi:10.1007/s00404-014-3541-9.
- Genbacev OD, Prakobphol A, Foulk RA, Krtolica AR, Ilic D, Singer MS, Yang ZQ, Kiessling LL, Rosen SD, Fisher SJ. 2003. Trophoblast L-selectin-mediated adhesion at the maternal-fetal interface. Science. 299:405–408. doi:10.1126/science.1079546.
- Giacomini E, Vago R, Sanchez AM, Podini P, Zarovni N, Murdica V, Rizzo R, Bortolotti D, Candiani M, Viganò P. 2017. Secretome of in vitro cultured human embryos contains extracellular vesicles that are uptaken by the maternal side. Sci Rep. 7:5210. doi:10.1038/s41598-017-05549-w.
- Haouzi D, Dechaud H, Assou S, Monzo C, de Vos J, Hamamah S. 2011. Transcriptome analysis reveals dialogues between human trophectoderm and endometrial cells during the implantation period. Hum Reprod. 26:1440–1449. doi:10.1093/humrep/der075.
- Herrler A, von Rango U, Beier HM. 2003. Embryo-maternal signaling: how the embryo starts talking to its mother to accomplish implantation. Reprod Biomed Online. 6:244–256. doi:10.1016/S1472-6483(10)61717-8.
- Hill MJ, Richter KS, Heitmann RJ, Graham JR, Tucker MJ, DeCherney AH, Browne PE, Levens ED. 2013. Trophectoderm grade predicts outcomes of single-blastocyst transfers. Fertil Steril. 99:1283–1289. e1. doi:10.1016/j.fertnstert.2012.12.003.
- Huang J, Chen H, Lu X, Wang X, Xi H, Zhu C, Zhang F, Lv J, Ge H. 2016. The effect of protein supplement concentration in embryo transfer medium on clinical outcome of IVF/ICSI cycles: a prospective, randomized clinical trial. Reprod Biomed Online. 32:79–84. doi:10.1016/j.rbmo.2015.10.004.
- Kleijkers SH, van Montfoort AP, Bekers O, Coonen E, Derhaag JG, Evers JL, Dumoulin JC. 2016. Ammonium accumulation in commercially available embryo culture media and protein supplements during storage at 2–8° C and during incubation at 37° C. Hum Reprod. 31:1192–1199. doi:10.1093/humrep/dew059.
- Kolte AM, van Oppenraaij RH, Quenby S, Farquharson RG, Stephenson M, Goddijn M, Christiansen OB, ESHRE Special Interest Group Early Pregnancy. 2014. Non-visualized pregnancy losses are prognostically important for unexplained recurrent miscarriage. Hum Reprod. 29:931–937. doi:10.1093/humrep/deu042.
- Kurian NK, Modi D. 2019. Extracellular vesicle mediated embryo-endometrial cross talk during implantation and in pregnancy. J Assist Reprod Genet. 36:189–198. doi:10.1007/s10815-018-1343-x.
- Minas V, Loutradis D, Makrigiannakis A. 2005. Factors controlling blastocyst implantation. Reprod Biomed Online. 10:205–216. doi:10.1016/S1472-6483(10)60942-X.
- Morbeck DE, Krisher RL, Herrick JR, Baumann NA, Matern D, Moyer T. 2014. Composition of commercial media used for human embryo culture. Fertil Steril. 102:759–766. e9. doi:10.1016/j.fertnstert.2014.05.043.
- Nakagawa K, Takahashi C, Nishi Y, Jyuen H, Sugiyama R, Kuribayashi Y, Sugiyama R. 2012. Hyaluronan-enriched transfer medium improves outcome in patients with multiple embryo transfer failures. J Assist Reprod Genet. 29:679–685. doi:10.1007/s10815-012-9758-2.
- Rosenbluth EM, Shelton DN, Wells LM, Sparks AE, Van Voorhis BJ. 2014. Human embryos secrete microRNAs into culture media—a potential biomarker for implantation. Fertil Steril. 101:1493–1500. doi:10.1016/j.fertnstert.2014.01.058.
- Schoolcraft WB. 2016. Importance of embryo transfer technique in maximizing assisted reproductive outcomes. Fertil Steril. 105:855–860. doi:10.1016/j.fertnstert.2016.02.022.
- Sher G, Keskintepe L, Batzofin J, Fisch J, Acacio B, Ahlering P, Ginsburg M. 2005. Influence of early ICSI-derived embryo sHLA-G expression on pregnancy and implantation rates: a prospective study. Hum Reprod. 20:1359–1363. doi:10.1093/humrep/deh758.
- Sheth KV, Roca GL, Al-Sedairy ST, Parhar RS, Hamilton CJ, al-Abdul Jabbar F. 1991. Prediction of successful embryo implantation by measuring interleukin-1-alpha and immunosuppressive factor(s) in preimplantation embryo culture fluid. Fertil Steril. 55:952–957. doi:10.1016/S0015-0282(16)54305-2.
- Skakkebaek NE, Jørgensen N, Andersson AM, Juul A, Main KM, Jensen TK, Toppari J. 2019. Populations, decreasing fertility, and reproductive health. Lancet. 393:1500–1501. doi:10.1016/S0140-6736(19)30690-7.
- Stigliani S, Persico L, Lagazio C, Anserini P, Venturini PL, Scaruffi P. 2014. Mitochondrial DNA in Day 3 embryo culture medium is a novel, non-invasive biomarker of blastocyst potential and implantation outcome. Mol Hum Reprod. 20:1238–1246. doi:10.1093/molehr/gau086.
- Sunde A, Brison D, Dumoulin J, Harper J, Lundin K, Magli MC, Van den Abbeel E, Veiga A. 2016. Time to take human embryo culture seriously. Hum Reprod. 31:2174–2182. doi:10.1093/humrep/dew157.
- Urman B, Yakin K, Ata B, Isiklar A, Balaban B. 2008. Effect of hyaluronan-enriched transfer medium on implantation and pregnancy rates after day 3 and day 5 embryo transfers: a prospective randomized study. Fertil Steril. 90:604–612. doi:10.1016/j.fertnstert.2007.07.1294.
- Wallace M, Cottell E, Cullinane J, McAuliffe FM, Wingfield M, Brennan L. 2014. 1H NMR based metabolic profiling of day 2 spent embryo media correlates with implantation potential. Syst Biol Reprod Med. 60:58–63. doi:10.3109/19396368.2013.854426.
- Yie S, Balakier H, Motamedi G, Librach CL. 2005. Secretion of human leukocyte antigen-G by human embryos is associated with a higher in vitro fertilization pregnancy rate. Fertil Steril. 83:30–36. doi:10.1016/j.fertnstert.2004.06.059.
