ABSTRACT
Male diabetes mellitus (DM) can affect erectile function and sperm quality. In severe cases, DM can lead to retrograde or no ejaculation, so testicular sperm aspiration (TESA) is combined with intracytoplasmic sperm injection (ICSI) to treat subfertility and infertility for DM couples. However, the effect of TESA upon ICSI (TESA-ICSI) for DM patients remains unclear. This research investigated the effect of TESA-ICSI on first cycle ICSI-embryo transfer (ICSI-ET) for type 2 diabetic mellitus (T2DM) patients and the potential mechanisms. The subjects consisted of 1219 male patients with azoospermia or retrograde ejaculation who were treated with TESA-ICSI from 2015.01 to 2019.11. They were classified into two groups, the T2DM group (n = 54) and non-diabetic control group (n = 1165). Sperm selection for injection was performed using motile sperm organelle morphology examination criteria. The number of available embryos and the high-quality embryo rates following a single ET as well as cleavage, fertilization, implantation, clinical pregnancy and the abortion rates were noted. Compared with the non-diabetic group, the available embryo rate (75.20 ± 26.40% vs.78.36 ± 23.25%) and high-quality embryo rate (46.49 ± 30.37% vs. 47.55 ± 28.57%) in the T2DM group were lower and the abortion rate (20.83% vs. 8.88%) was higher, but these differences were not statistically significant. There were no significant differences in clinical pregnancy, implantation, normal fertilization, and cleavage rates between the two groups. The results show that TESA for male T2DM patients does not influence the effect of ICSI. For T2DM patients with severe oligozoospermia, asthenospermia, teratozoospermia, or retrograde ejaculation that do not meet ICSI criteria, TESA-ICSI may perhaps be considered for reproductive assistance.
Abbreviations
DM: diabetes mellitus; TESA: testicular sperm aspiration; ICSI: intracytoplasmic sperm injection; ICSI-ET; ICSI-embryo transfer; LH: luteinizing hormone; mL: milliliter; TES: testosterone; FSH: follicle-stimulating hormone; P: progesterone; HCG: human chorionic gonadotropin
Introduction
By the International Diabetes Federation, the number of people with diabetes in urban areas is expected to increase to 415.4 million in 2030, and to 538.8 million in 2045 (Cho et al. Citation2018). The incidence of T2DM is more rapid, and some scholars have recently estimated that the number of T2DM patients will increase to 700 million in the next 15 (Rocha et al. Citation2020) years. In the United States, more than one-third of the new cases of diabetes occur in young adolescents (Pinhas-Hamiel and Zeitler Citation2005; Xu et al. Citation2018). The phenomenon of the younger age of onset of diabetes is also worthy of attention, as it may affect the fertility of a growing number of diabetic males during their active reproductive period. Studies of the fertility rate in modern societies found that falling birth rates and fertility may be closely related to the increased incidence of DM (Hamilton and Ventura Citation2006; Lutz Citation2006).
Diabetes mellitus (DM) is a metabolic disorder that is characterized by chronic hyperglycemia and insufficient insulin secretion and/or dysfunction, leading to serious disorders of carbohydrate, fat and protein metabolism, as well as chronic injury, dysfunction and failure of various organs (Ramalho-Santos et al. Citation2014). Besides cardiovascular disease, nephropathy, neuropathy and retinopathy, the complications of DM can also include male infertility (Melendez-Ramirez et al. Citation2010; Niederberger Citation2015), which may be a sign of impaired male health status (Hamilton and Ventura Citation2006; Ventimiglia et al. Citation2015). Studies on male diabetic patients and animal models have found that diabetes can lead to changes in the pathophysiology of sexual and spermatogenic function, including low libido, altered spermatogenesis, apoptotic and degenerative changes in testes, reduced testosterone synthesis and secretion, and ejaculatory disorder (Sexton and Jarow Citation1997; Baccetti et al. Citation2002; Scarano et al. Citation2006; Agbaje et al. Citation2007; Ricci et al. Citation2009; Gazzaruso et al. Citation2011; Mallidis et al. Citation2011; La Vignera et al. Citation2012; Alves et al. Citation2013; Azar and Baba Citation2013).
Many adverse effects of DM upon male reproductive function are related to disorders of glucose metabolism, which is important for spermatogenesis for sperm motility and fertilization that require the participation of glucose (Ding et al. Citation2015). T2DM associated inflammation and increased oxidative stress, results in decreased sperm motility and increased sperm DNA fragmentation (Condorelli et al. Citation2018). It has also found that there exists an alteration in the gene expression of male diabetic patients which is involved in oxidative stress (Mallidis et al. 2009). The mechanism of male diabetes combined with azoospermia is related to hyperglycemia causing infection/inflammation of the male accessory gland (Patterson and Andriole Citation1997; Condorelli et al. Citation2013). It is well known that long-term chronic epididymitis can block epididymal duct and sperm release which will result in subfertility.
Thus, advanced progression of T2DM patients may lead to azoospermia or retrograde ejaculation. These patients have extremely low fertility, and assisted reproductive technology (ART) can be an option to improve the condition. Intracytoplasmic sperm injection (ICSI)-assisted reproductive technology can greatly reduce the impact of sperm factors of infertility (Palermo et al. Citation2017). In addition, ICSI technology does not require mature male gametes, and can directly use sperm taken from the epididymis or testis, which are mostly immature without complete flagella and developed cell membranes (Palermo et al. Citation1996). For male diabetic patients with azoospermia or severe retrograde ejaculation, the sperm required for ICSI to assist pregnancy must be surgically obtained from the testes. At present, there are three main types of surgical testicular sperm extraction in the clinic: TESA, testicular sperm extraction (TESE), microdissection of testicular sperm extraction (MD-TESE). MD-TESE is suitable for patients with non-obstructive azoospermia. Both TESA and TESE are appropriate for patients with a high probability of sperm extraction, such as obstructive azoospermia. TESE was markedly superior compared with TESA at obtaining sperm and in terms of the quantity and subsequent motility of the sperm found. However, TESE produces greater testicular damage. For patients with obstructive azoospermia and T2DM with azoospermia or retrograde ejaculation, only a very small amount of testicular tissue is needed to obtain the number of sperm that can meet the requirement for ICSI. Therefore, TESA-ICSI technology is more widely used in patients with obstructive azoospermia and T2DM with azoospermia or retrograde ejaculation, although the amount of testicular tissue obtained by TESA is less than that by TESE, the damage to testicular tissue is also smaller.
Some scholars have found that couples with diabetic male partners have accessed in vitro fertilization (IVF) (Maneesh et al. Citation2006; Agbaje et al. Citation2008; Costanzo et al. Citation2014), but no research till now has explored the influence of male T2DM on ICSI. It remains unclear if sperm from male T2DM patients will be effective for ICSI-embryo transfer (ICSI-ET). This study aims to explore whether TESA for male T2DM patients is effective with ICSI and this can provide fertility guidance for male diabetic patients as well as reduce the financial burden of patients and relieve psychological stress via better treatment outcomes.
Results
The research results show that there were no significant differences (p > 0.05) in follicle-stimulating hormone (FSH) and testosterone (TES) levels and testicular volumes between the two groups. For their partners, FSH, luteinizing hormone (LH) and progesterone (P) values on the day human chorionic gonadotropin (hCG) was measured were also not statistically different between the two groups.
shows the male age (32.42 ± 3.68vs.30.09 ± 3.71, p = 0.000) and the female age (29.35 ± 2.76vs.28.04 ± 3.20, p = 0.003) in T2DM group were significantly higher than those in the non-diabetic group. shows that the abortion rate of the T2DM group was significantly increased as compared to the non-diabetic group (p = 0.049). There were no significant differences (p > 0.05) in the available embryo rate, high-quality embryo rate, clinical pregnancy rate, implantation rate, fertilization rate, normal fertilization rate, cleavage rate between the two groups although the available embryo rate (75.20 ± 26.40%vs.78.36 ± 23.25%), high-quality embryo rate (46.49 ± 30.37% vs. 47.55 ± 28.57%), and clinical pregnancy rate (71.32%vs.75.00%) in the T2DM group was lower than those in the non-diabetic group.
Table 1. Demographics of study samples by T2DM status
Table 2. Comparison of clinical outcomes from TESA-ICSI treatment between T2DM and non-diabetic groups
In order to assess whether the age of the males and females affects the treating effect of TESA-ICSI, we adjusted the age of the males and females and established a model. In the adjusted model, we observed that there was no statistical difference in the available embryo rate (OR = −0.03, 95%CI: -0.09–0.04, p = 0.422), high-quality embryo rate (OR = −0.00, 95%CI: -0.08–0.08, p = 0.948), clinical pregnancy rate (OR = 1.23, 95%CI: 0.54–2.80, p = 0.613), fertilization rate (OR = 0.01, 95%CI: -0.05–0.06, p = 0.795), normal fertilization rate (OR = 0.01, 95%CI: -0.05–0.06, p = 0.853), cleavage rate (OR = 0.01, 95%CI: -0.04–0.06, p = 0.717) and the abortion rate (OR = 2.53,95%CI: 0.89–7.13, p = 0.080) between the two groups ().
Table 3. The association between T2DM and TESA-ICSI treatment outcomes
Discussion
Drugs are a common method of treating diabetes. Metformin is a biguanide compound isolated from Galega officinalis. It promotes the uptake of glucose by peripheral tissues and delays the absorption of glucose in the intestine, thereby reducing blood sugar. However, there is no consensus on whether metformin can improve or reduce male reproductive function. Some researchers found that sperm density and vitality could be improved after a 6-month study of metformin in the treatment of metabolic syndrome in patients with oligo-terato-male azoospermia (Morgante et al. Citation2011). In addition, it has also been reported that the normal sperm morphology of men with hyperinsulinemia can be improved after 3 months of metformin treatment (Bosman et al. Citation2014). However, other research provides an opposite conclusion that metformin not only does not improve sperm quality but also reduces sperm motility and even interferes with the normal testicular physiological process, resulting in spermatogenesis failure and obvious histological changes (Adaramoye et al. Citation2012; Tartarin et al. Citation2012; Calle-Guisado et al. Citation2018). Calle-Guisado et al. (Citation2018) consider that the reason for drawing the opposite conclusion is due to the differences in the research methods and testing instruments. Obviously, the safety of diabetic drugs on male reproductive capacity needs further research. Standardizing the research population, refining the dosage of drugs, unifying the medication methods and evaluation methods can help draw more reliable conclusions.
T2DM patients can develop azoospermia or retrograde ejaculation and further cause male infertility. The prevalence of infertility in men with diabetes is higher than that in the general population, and the use of ART in diabetic men has increased (Bener et al. Citation2009). At present, TESA-ICSI is a good choice for T2DM patients troubled with infertility due to ineffective drug treatment or unwillingness to continue drug treatment. Our study found that T2DM did not affect TESA-ICSI; and there are no significant differences in the embryo, high-quality embryo, and fertilization rates between the T2DM group and the non-diabetic groups. There are three possible explanations for these findings. First, there is no definitive evidence that T2DM affects testicular sperm. The studies of the effect of diabetes on sperm function have mostly involved mature sperm collected from an ejaculate obtained by masturbation, but there has been little research on testicular-extracted sperm. Second, diabetes may induce oxidative stress and nuclear DNA damage of mature sperm during transit through the male genital tract; whereas testicular sperm may not been exposed to this oxidative stress and DNA damage. Third, ICSI can reduce the dependence on sperm concentration, viability, and even DNA integrity because successful ICSI requires the selection of only a few motile sperms with normal morphology (Kidd et al. Citation2001). Recent studies have found that pregnancy from sperm with high levels of DNA damage is more likely with ICSI than IVF (Zhao et al. Citation2014; Zhang et al. Citation2015).
In our research, the abortion rate in T2DM group was higher than that in the non-diabetic group (p = 0.049). However, after adjusting for male and female age, the abortion rate between the two groups was not significant. The point estimate is still showing the direction of the negative effect of T2DM to abortion risk (OR = 2.53), but with a wide 95%CI, due to limited statistical power. However, diabetes is associated with an increased rate of sperm DNA fragmentation (Agbaje et al. Citation2007) possibly due to oxidative stress leading to sperm DNA damage (Agbaje et al. Citation2008; Aitken and Iuliis Citation2009). Past studies also have found that sperm DNA fragmentation has an impact on male fertility and the effectiveness of in vitro fertilization (Simon et al. Citation2017). As the rate of sperm DNA fragmentation increases, the rate of high-quality embryos significantly decreases. In particular, it was shown that a DNA fragmentation index >30% significantly reduced the clinical pregnancy rate, and significantly increased the early abortion rate (Robinson et al. Citation2012; Choi et al. Citation2017; Kim et al. Citation2019). These studies are in conflict. The small size and the limited varieties of sample might be a great factor influencing the result. To guarantee the accuracy of the research, we compared general clinical data between the two groups (male body mass index (BMI), smoking, testicular size, FSH, TES level, partner BMI, hCG day P, FSH, and LH values). To reduce the potential effects of the partner’s ovarian function, we excluded patients with <4 follicles retrieved. To reduce the impact of multiple transplantation cycles upon ICSI outcomes, we only analyzed data from the first fresh transplant cycle. However, our study still has limitations, such as the small sample size of the diabetes group, and the data limited to our center, with no data related to multiple centers in multiple countries. The small sample size also makes it impossible to classify embryos or blastocysts alone for the ETs of the two groups of patients. Therefore, it was also not possible to separately compare fresh ET and frozen ET (FET) as embryos are usually frozen at the 8-cell stage and FET embryos generally have better survival after freezing-thawing operations, suggesting these embryos may be stronger or of better quality.
In conclusion, we found that testicular sperm from male T2DM patients did not affect the effect of ICSI-assisted pregnancy. Considering the adverse effects of diabetes on male fertility and sexual function, it is desirable for male T2DM patients undergoing fertility treatment to carefully control blood sugar glucose levels. Couples with T2DM men should try for early pregnancy to avoid the longer-term effects of diabetes. Future research will require larger sample size and longer-term studies, as well as further examination of the regulation of sperm function in T2DM and non-diabetic men.
Methods and materials
Population
A total of 1219 couples were recruited into this longitudinal research (). They were divided into two groups: the T2DM group (54 couples) and the non-diabetic group (1165 couples). All the subjects were treated via TESA-ICSI at the Reproductive Center of Northwest Women’s and Children’s Hospital (2015.01.01–2019.11.30). Each patient signed a written informed consent form before TESA surgery. This research was approved by the Research Ethics Committee of the Northwest Women’s and Children’s Hospital.
Figure 1. Flow-chart of the study cohort characteristics. CBAVD, congenital bilateral absence of the vas deferens; TESA-ICSI, testicular sperm aspiration-intracytoplasmic sperm injection; T2DM: type 2 diabetic mellitus
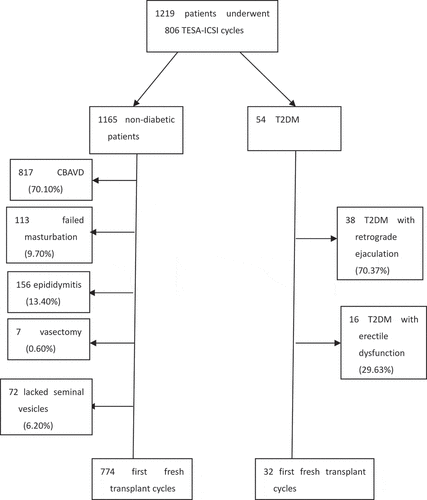
For the T2DM group, the duration of the disease was about 3.5–8 years. Seventeen cases were treated with regular insulin therapy and 24 cases with regular oral medication (acarbose and metformin). Among the T2DM group, the sperm of 38 cases with retrograde ejaculation was obtained by centrifugation of urine and were evaluated by the IVF laboratory staff in the Reproductive Center of Northwest Women’s and Children’s Hospital, but the results did not meet the ICSI criteria. Meanwhile, 16 cases failed to ejaculate due to erectile dysfunction. Finally, in the T2DM group, first fresh transplant cycles were performed in 32 cases because of azoospermia or retrograde ejaculation.
For the non-diabetic group, due to large sample, the situation presented differently: 817 cases exhibited congenital bilateral absence of the vas deferens (70.10%); 113 cases failed masturbation during partner egg collection (9.70%); 156 cases displayed epididymitis (13.40%); 7 cases (0.60%) had previous vasectomy (6 cases remarried and had a history of childbearing; 1 case was the first marriage and the couple wanted another child); and 72 cases lacked seminal vesicles (6.18%). One hundred and fifty-two cases in the non-diabetic group (excluding 113 patients that failed masturbation) and 16 T2DM men with non-ejaculation underwent TESA operation before ART to assess whether the testicular sperm met ICSI criteria (). The TESA sperm was evaluated for ICSI criteria by the laboratory staff in the Reproductive Center of Northwest Women’s and Children’s Hospital. Finally, in the non-diabetic group, 774 cases were performed with first fresh transplant cycles.
Figure 2. Flowchart for cohort selection.T1DM: type 1 diabetic mellitus; T2DM: type 2 diabetic mellitus
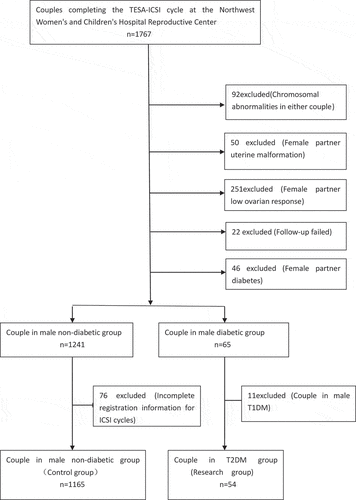
The inclusion criteria were as follows: (1) All the cases succeeded in sperm collection by masturbation for at least three times except that three cases of T2DM failed due to non-ejaculation; (2) For the T2DM group, only the non-ejaculation and retrograde ejaculation cases were included but the sperm collected from the retrograde ejaculation cases by urine centrifugation did not meet ICSI criteria. Other diabetic patients with oligospermia were not included. (3) No reproductive-infected diseases, such as mycoplasma, chlamydia and gonococcal bacteria, occurred in the past 3 months; (4) The chromosomes of the couples appeared normal, and the male patient’s Y chromosome microdeletion region was normal; (5) The female partner had no uterine malformation or low ovarian response (<4 follicles obtained on the day of retrieval), was ≤36 years of age, and had no polycystic ovary syndrome and diabetes; (6) There were no diseases of hereditary and familial history. (7) 54 men were diagnosed as T2DM by a professional doctor and all the cases of 1165 non-diabetic patients had no abnormalities in fasting blood glucose tests; (8) The researchers followed up for 12 months after embryo/blastocyst transfer and 22 cases were excluded because of fail to follow-up.
TESA operation process
Before the TESA operation, the reasons for using testicular sperm, as well as the possible benefits and complications (such as hematoma and infection), were explained to all patients and partners. A written informed consent was obtained from the patient/couple. After routine disinfection of the perineum using iodophor, the area was washed with physiological saline. Under local anesthesia with 2% lidocaine, a 21-gauge needle attached to a 10-mL syringe was inserted through the scrotal skin and into the testicle to perforate the tunica albuginea. A small amount of seminiferous tissue was collected and placed in culture medium, and some tissue was placed in an embryo culture chamber to examine for the presence of motile mature sperm using an inverted microscope. The puncture wound was compressed to stop bleeding, and the patients were informed of matters that needed attention after the operation. There were three cases of testicular hematoma and one case of epididymitis after the operation. After conservative treatment, all the patients recovered without sequelae.
Testicular sperm collection and selection process
The extracted testicular tissue was placed in a glass-bottomed dish containing 10% human albumin serum (HSA; Vitrolife, Sweden) and minced into testicular cell suspensions. Morphological selection of spermatozoa was performed under a Nikon Eclipse Ti-U inverted microscope (Nikon, Florence, Italy). Selection targeted relatively normal sperm, with the sperm head exhibiting a regular oval shape and a single vacuole no more than 4% of the total area of the sperm head, as well as sperm that moved forward. If there were many immobile sperms, selection targeted sperm that were moving slightly or exhibited a curved tail.
Oocyte retrieval
When the follicle met the maturity criteria: diameter>15 mm, serum E2 level>100 pg/mL, subcutaneous injection of 6000–10000IU hCG (Livzon Pharmaceutical, Zhuhai, China) or 250 μgrhCG (Aize, Merck Serono, Germany) intradermal injection were carried out to trigger ovulation. Thirty-six hours after the administration of hCG, an ultrasound-guided vaginal probe was used for follicular aspiration with or without sedation or anesthesia.
Fertilization, embryo cleavage and embryo transfer
For ICSI, oocyte maturity was assessed after denudation. After TESA, morphologically normal and motile sperm was injected into a mature oocyte containing visible polar bodies. Fertilization of oocytes was assessed 18–20 h after ICSI, as determined by the presence of two pronuclei. Embryo quality was evaluated by the root Peter cleavage scoring system (Brinsden Citation1999). The embryo rating was divided into three grades: Grade I, II and III. All of the grades were available embryos and grades I and II were high-quality embryos. If there were more than four high-quality embryos, the embryos were subsequently cultured to blastocysts (culture day 5–6). Blastocysts were graded according to Gardner and Schoolcraft (Citation1999) using the developmental stages (compaction stage, early stage, complete blastocyst and hatching blastocyst) and morphology of the trophoblast and inner cell mass. After a comprehensive assessment of the partner’s physical condition, 1–2 embryos/blastocysts were transferred to the uterine cavity on day 3/5–6 day after oocyte retrieval. If women exhibited ovarian hyperstimulation syndrome, endometrial abnormalities or hydrosalpinx, the freshly transplanted embryos/blastocysts were removed. Embryos/blastocysts were cryopreserved and then thawed when women were ready for transplantation. All the cases included in this study are the first fresh transplant cycle.
Serum human chorionic gonadotropin (hCG) concentrations were measured 14 days after ET. Clinical pregnancy was defined as the presence of a gestational sac confirmed by ultrasound examination in the fourth week after ET. All the transplants in this study were the first fresh transplant cycle.
Laboratory analysis
All the serum were separated by 3500 rpm centrifuge 5 min and were kept at room temperature for 1 h. The levels of FSH, LH, P and TES were measured with a Chemiluminescence Microparticle Immuno Assay (Beckman DXI 800, American). The ranges of sex hormone assays in male patients were as follows: FSH, 1.27–19.26 mIU/ml; LH3, 1.24–8.62 mIU/ml; TES, 1.75–7.81 ng/ml.
Statistical analysis
Descriptive statistics are presented as mean ± standard deviation (SD). Continuous and categorical variables were compared between the diabetes and non-diabetic groups using student‘s T-test and Chi-square/Fisher’s exact tests, respectively. In order to control the influences of unbalanced age differences for the results, we conducted additional analysis by stratifying male and female ages. Linear regression model was employed to assess the associations between diabetes and individual available embryo rate, high-quality embryo rate, fertilization rate and cleavage rate by calculating β-coefficient and 95% confidence interval (CI). Logistic regression model was used to calculate odds ratio (OR) and 95%CI. P values less than 0.05 were considered significant. All the analyses were conducted using Stata/SE 16.1.
Ethics approval
Each patient signed a written informed consent form before TESA surgery. This study was approved by the Research Ethics Committee of the Northwest Women’s and Children’s Hospital.
Authors’ contributions
Each author has contributed to the research process. Conceived and designed the experiments: XJp, SJz, LX; Performed the experiments: GM, SZ, SJ, SJh; Analyzed the data: XX; Wrote the manuscript: LX; Critically revised, and completed/corrected the manuscript, and approved the final version: XJp, SJz, ZZ.
Acknowledgments
The authors acknowledge the Reproductive Center of the Northwest Women and Children’s Hospital. We received strong support from the entire team during the writing process. We want to send our special thanks to Li Xiaomian (School of International Studies of Xi’an Jiaotong University) who has contributed a lot in revising and polishing the paper. We also thank Charles Allan, PhD, from Liwen Bianji, Edanz Editing China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Adaramoye O, Akanni O, Adesanoye O, Labo-Popoola O, Olaremi O. 2012. Evaluation of toxic effects of metformin hydrochloride and glibenclamide on some organs of male rats. Niger J Physiol Sci. 27(2):137–144.
- Agbaje IM, McVicar CM, Schock BC, McClure N, Atkinson AB, Rogers D, Lewis SE. 2008. Increased concentrations of the oxidative DNA adduct 7,8-dihydro-8-oxo-2-deoxyguanosine in the germ-line of men with type 1 diabetes. Reprod Biomed Online. 16(3):401–409. eng. doi:https://doi.org/10.1016/S1472-6483(10)60602-5.
- Agbaje IM, Rogers DA, McVicar CM, McClure N, Atkinson AB, Mallidis C, Lewis SE. 2007. Insulin dependant diabetes mellitus: implications for male reproductive function. Hum Reprod. 22(7):1871–1877. doi:https://doi.org/10.1093/humrep/dem077.
- Aitken RJ, Iuliis GND. 2009. On the possible origins of DNA damage in human spermatozoa. Mol Hum Reprod. 16(1):3–13. doi:https://doi.org/10.1093/molehr/gap059.
- Alves MG, Martins AD, Cavaco JE, Socorro S, Oliveira PF. 2013. Diabetes, insulin-mediated glucose metabolism and Sertoli/blood-testis barrier function. Tissue Barriers. 1(2):e23992. doi:https://doi.org/10.4161/tisb.23992.
- Azar ST, Baba KE. 2013. Low testosterone and diabetes. Curr Diabetes Rev. 9(5).
- Baccetti B, La Marca A, Piomboni P, Capitani S, Bruni E, Petraglia F, De Leo V. 2002. Insulin-dependent diabetes in men is associated with hypothalamo-pituitary derangement and with impairment in semen quality. Hum Reprod. 17(10):2673–2677. eng. doi:https://doi.org/10.1093/humrep/17.10.2673.
- Bener A, Al-Ansari AA, Zirie M, Al-Hamaq AO. 2009. Is male fertility associated with type 2 diabetes mellitus? Int Urol Nephrol. 41(4):777–784. eng. doi:https://doi.org/10.1007/s11255-009-9565-6.
- Bosman E, Esterhuizen AD, Rodrigues FA, Becker PJ, Hoffmann WA. 2014. Effect of metformin therapy and dietary supplements on semen parameters in hyperinsulinaemic males. Andrologia. n/a-n/a.
- Brinsden. 1999. Textbook of in vitro fertilization. 2(2807):667–668.
- Calle-Guisado V, Gonzalez-Fernandez L, Martin-Hidalgo D, Garcia-Marin LJ, Bragado MJ. 2018. Metformin inhibits human spermatozoa motility and signalling pathways mediated by protein kinase A and tyrosine phosphorylation without affecting mitochondrial function. Reprod Fertil Dev.
- Cho NH, Shaw JE, Karuranga S, Huang Y, JDD RF, Ohlrogge AW, Malanda B. 2018. IDF diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. S0168822718302031.
- Choi HY, Kim SK, Kim SH, Choi YM, Jee BC. 2017. Impact of sperm DNA fragmentation on clinical in vitro fertilization outcomes. Clin Exp Reprod Med. 44(4):224–231.
- Condorelli R,A, Calogero AE, Vicari E, Duca Y. 2013. Prevalence of male accessory gland inflammations/infections in patients with Type 2 diabetes mellitus. J Endocrinol Invest.
- Condorelli RA, La Vignera S, Mongioì LM, Alamo A, Calogero AE. 2018. Diabetes mellitus and infertility: different pathophysiological effects in type 1 and type 2 on sperm function. Front Endocrinol (Lausanne). 9:268. eng. doi:https://doi.org/10.3389/fendo.2018.00268.
- Costanzo PR, Suárez SM, Scaglia HE, Zylbersztein C, Litwak LE, Knoblovits P. 2014. Evaluation of the hypothalamic-pituitary-gonadal axis in eugonadal men with type 2 diabetes mellitus. Andrology. 2(1):117–124. doi:https://doi.org/10.1111/j.2047-2927.2013.00163.x.
- Ding GL, Liu Y, Liu ME, Pan JX, Guo MX, Sheng JZ, Huang HF. 2015. The effects of diabetes on male fertility and epigenetic regulation during spermatogenesis. Asian J Androl. 17(6):948–953. eng. doi:https://doi.org/10.4103/1008-682X.150844.
- Gardner DK, Schoolcraft WB. 1999. Culture and transfer of human blastocysts. Curr Opin Obstet Gynecol. 11(3):307–311. doi:https://doi.org/10.1097/00001703-199906000-00013.
- Gazzaruso C, Coppola A, Giustina A. 2011. Erectile dysfunction and coronary artery disease in patients with diabetes. Curr Diabetes Rev. 7(2):143–147. doi:https://doi.org/10.2174/157339911794940693.
- Hamilton BE, Ventura SJ. 2006. Fertility and abortion rates in the United States, 1960-2002. Int J Androl. 29(1):34–45. eng. doi:https://doi.org/10.1111/j.1365-2605.2005.00638.x.
- Kidd SA, Eskenazi B, Wyrobek AJ. 2001. Effects of male age on semen quality and fertility: A review of the literature. Fertil Steril. 75(2):237–248. doi:https://doi.org/10.1016/S0015-0282(00)01679-4.
- Kim SM, Kim SK, Jee BC, Kim SH. 2019. Effect of sperm DNA fragmentation on embryo quality in normal responder women in in vitro fertilization and intracytoplasmic sperm injection. Yonsei Med J. 60(5):461–466. eng. doi:https://doi.org/10.3349/ymj.2019.60.5.461.
- Kriegel TM, Heidenreich F, Kettner K, Pursche T, Hoflack B, Grunewald S, Poenicke K, Glander HJ, Paasch U. 2009. Identification of diabetes- and obesity-associated proteomic changes in human spermatozoa by difference gel electrophoresis. Reprod Biomed Online. 19(5):660–670. eng. doi:https://doi.org/10.1016/j.rbmo.2009.07.001.
- La Vignera S, Condorelli R, Vicari E, D’Agata R, Calogero AE. 2012. Diabetes mellitus and sperm parameters. J Androl. 33(2):145–153. doi:https://doi.org/10.2164/jandrol.111.013193.
- Lutz W. 2006. Fertility rates and future population trends: will Europe\”s birth rate recover or continue to decline?
- Mallidis C, Agbaje I, Mcclure N, Kliesch S. 2011. The influence of diabetes mellitus on male reproductive function. A poorly investigated aspect of male infertility. Der Urologeausga. 50(1):33–37. doi:https://doi.org/10.1007/s00120-010-2440-3.
- Mallidis C, Agbaje I, O’Neill J, McClure N. 2009a. The influence of type 1 diabetes mellitus on spermatogenic gene expression. Fertil Steril. 92(6):2085–2087. eng. doi:https://doi.org/10.1016/j.fertnstert.2009.06.006.
- Mallidis C, Green BD, Rogers D, Agbaje IM, Hollis J, Migaud M, Amigues E, McClure N, Browne RA. 2009b. Metabolic profile changes in the testes of mice with streptozotocin-induced type 1 diabetes mellitus. Int J Androl. 32(2):156–165. eng. doi:https://doi.org/10.1111/j.1365-2605.2007.00829.x.
- Maneesh M, Jayalakshmi H, Singh TA, Chakrabarti A 2006. Impaired hypothalamic-pituitary-gonadal axis function in men with diabetes mellitus.
- Melendez-Ramirez LY, Richards RJ, Cefalu WT. 2010. Complications of type 1 diabetes. Endocrinol Metab Clin. 39(3):625–640.
- Morgante G, Tosti C, Orvieto R, Musacchio MC, Piomboni P, De Leo V. 2011. Metformin improves semen characteristics of oligo-terato-asthenozoospermic men with metabolic syndrome. Fertil Steril. 6:95.
- Mulholland J, Mallidis C, Agbaje I, McClure N. 2011. Male diabetes mellitus and assisted reproduction treatment outcome. Reprod Biomed Online. 22(2):215–219. eng. doi:https://doi.org/10.1016/j.rbmo.2010.10.005.
- Niederberger C. 2015. Re: relationship between physical occupational exposures and health on semen quality: data from the longitudinal investigation of fertility and the environment (LIFE) study. J Urol. 194(6):1711. eng. doi:https://doi.org/10.1016/j.juro.2015.07.035.
- Palermo GD, Colombero LT, Schattman GL, Davis OK, Rosenwaks Z. 1996. Evolution of pregnancies and initial follow-up of newborns delivered after intracytoplasmic sperm injection. Jama. 276(23):1893–1897. eng. doi:https://doi.org/10.1001/jama.1996.03540230043033.
- Palermo GD, O’Neill CL, Chow S, Cheung S, Parrella A, Pereira N, Rosenwaks Z. 2017. Intracytoplasmic sperm injection: state of the art in humans. Reproduction. 154(6):F93–f110. eng. doi:https://doi.org/10.1530/REP-17-0374.
- Patterson JE, Andriole VT. 1997. Bacterial urinary tract infections in diabetes. Infect Dis Clin North Am. 11(3):735–750. doi:https://doi.org/10.1016/S0891-5520(05)70383-4.
- Pinhas-Hamiel O, Zeitler P. 2005. The global spread of type 2 diabetes mellitus in children and adolescents. J Pediatr. 146(5):693–700. eng. doi:https://doi.org/10.1016/j.jpeds.2004.12.042.
- Ramalho-Santos J, Amaral S, Oliveira P. 2014. Diabetes and the impairment of reproductive function: possible role of mitochondria and reactive oxygen species. Curr Diabetes Rev. 4(1):46–54. doi:https://doi.org/10.2174/157339908783502398.
- Ricci G, Catizone A, Esposito R, Pisanti FA, Vietri MT, Galdieri M. 2009. Diabetic rat testes: morphological and functional alterations. Andrologia. 41(6):361–368. eng. doi:https://doi.org/10.1111/j.1439-0272.2009.00937.x.
- Robinson L, Gallos ID, Conner SJ, Rajkhowa M, Miller D, Lewis S, Kirkman-Brown J, Coomarasamy A. 2012. The effect of sperm DNA fragmentation on miscarriage rates: a systematic review and meta-analysis. Hum Reprod. 27(10):2908–2917. eng. doi:https://doi.org/10.1093/humrep/des261.
- Rocha M, Apostolova N, Diaz-Rua R, Muntane J, Victor VM. 2020. Mitochondria and T2D: role of Autophagy, ER Stress, and Inflammasome. Trends Endocrinol Metab. doi:https://doi.org/10.1016/j.tem.2020.03.004
- Scarano WR, Messias AG, Oliva SU, Klinefelter GR, Kempinas WG. 2006. Sexual behaviour, sperm quantity and quality after short-term streptozotocin-induced hyperglycaemia in rats. Int J Androl. 29(4):482–488. eng. doi:https://doi.org/10.1111/j.1365-2605.2006.00682.x.
- Sexton WJ, Jarow JP. 1997. Effect of diabetes mellitus upon male reproductive function. Urology. 49(4):508–513. doi:https://doi.org/10.1016/S0090-4295(96)00573-0.
- Simon L, Emery BR, Carrell DT. 2017. Review: diagnosis and impact of sperm DNA alterations in assisted reproduction. Best Pract Res Clin Obstet Gynaecol. 44:38–56. eng. doi:https://doi.org/10.1016/j.bpobgyn.2017.07.003.
- Tartarin P, Moison D, Guibert E, Dupont J, Habert R, R-f V, Frydman N, Pozzi S, Frydman R, Lecureuil C. 2012. Metformin exposure affects human and mouse fetal testicular cells. Hum Reprod. (11):11.
- Ventimiglia E, Capogrosso P, Boeri L, Serino A, Colicchia M, Ippolito S, Scano R, Papaleo E, Damiano R, Montorsi F. et al. 2015. Infertility as a proxy of general male health: results of a cross-sectional survey. Fertil Steril. 1041:48–55. eng. doi:https://doi.org/10.1016/j.fertnstert.2015.04.020.
- Whiting DR, Guariguata L, Weil C, Shaw J. 2011. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 94(3):0–321.
- Xu G, Liu B, Sun Y, Du Y, Snetselaar LG, Hu FB, Bao W. 2018. Prevalence of diagnosed type 1 and type 2 diabetes among US adults in 2016 and 2017: population based study. BMJ. 362:k1497. eng. doi:https://doi.org/10.1136/bmj.k1497.
- Zhang Z, Zhu L, Jiang H, Chen H, Chen Y, Dai Y. 2015. Sperm DNA fragmentation index and pregnancy outcome after IVF or ICSI: a meta-analysis. J Assist Reprod Genet. 32(1):17–26. eng. doi:https://doi.org/10.1007/s10815-014-0374-1.
- Zhao J, Zhang Q, Wang Y, Li Y. 2014. Whether sperm deoxyribonucleic acid fragmentation has an effect on pregnancy and miscarriage after in vitro fertilization/intracytoplasmic sperm injection: a systematic review and meta-analysis. Fertil Steril. 102(4):998–1005.e1008. eng. doi:https://doi.org/10.1016/j.fertnstert.2014.06.033.
