ABSTRACT
Male germline-specific thioredoxin domain containing 8 (TXNDC8; alias SPTRX3) accumulates indefective human spermatozoa. We assessed the efficiency of two-step semen purification inremoving spermatozoa carrying TXNDC8, and examined the relationship of TXNDC8 with theoutcomes of assisted reproductive therapy (ART), conventional semen parameters, and sperm DNA integrity in sperm chromatin structure assay (SCSA). Semen samples (n = 255) from 91 ART couples were screened in two independent trials, both including a two-step, gradient-and-swim-up separation procedure yielding A-samples (raw semen), B-samples (gradient separated), and C-samples (gradient-and-swim-up). The C-samples were used for intracytoplasmic sperm injection (ICSI) with morphologically selected spermatozoa (IMSSI). Percentage of TXNDC8-positive spermatozoaincreased progressively from A to B/C-samples in both trials. In the first trial (35 couples), the TXNDC8 correlated positively with sperm DNA fragmentation index (%DFI; r = 0.66) measured before separation, and negatively with sperm concentration (r = −0.57) and motility (r = −0.67), also taken before separation. The high DNA stainability index (%HDS) correlated with the percentage of spermatozoa lacking TXNDC8 (r = 0.68). Both SCSA and TXNDC8 parameters showed moderate correlations (r = 0.33–0.66) with blood serum levels of hCG on day 11 (Beta 1) and day13 (Beta 2) after oocyte retrieval. In the second trial (56 couples), fathers of multiplets had a significantly lower percentage of TXNDC8-positive spermatozoa in B-sample (gradient separationonly) compared to men who conceived a singleton pregnancy (p = 0.01) and those who produced no pregnancy (p = 0.02). Those multiplets’ fathers also had a significantly higher sperm concentration while their SCSA parameters did not differ from others. It is concluded that theTXNDC8 levels correlate with SCSA and conventional raw semen parameters, and are predictive of pregnancy outcome and multiple births after ART. Two-step purification does not efficiently remove TXNDC8 carrying spermatozoa.
Abbreviations
ART– assisted reproductive therapy; DFI- DNA fragmentation index; FC- flow cytometry (FC); hCG: human chorionic gonadotropin; HDS: high DNA stainability index; HEPES- (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid); HTF- human tubal fluid; ICSI- intracytoplasmic sperm injection; IgG- immunoglobulin G; IMSSI- ICSI with morphologically selected spermatozoa; IVF- in vitro fertilization; IU-: intrauterine insemination; NGS- normal goat serum; PBS- phosphate buffered saline; PVP- polyvinylpyrrolidone; SAB- spontaneous abortion; SCSA- sperm chromatin structure assay; SPTRX3- spermatid specific thioredoxin 3; SSS- synthetic serum substitute; TRITC- tetramethyl rhodamine isothiocyanate; TX-100- Triton X-100; TXNDC- thioredoxin domain-containing proteins; TXNDC8- thioredoxin domain containing 8; TUNEL- Terminal deoxynucleotidyl transferase dUTP nick end labeling
Introduction
The thioredoxin domain-containing proteins (TXNDC/thioredoxins/) regulate the redox state of cells. During mammalian spermatogenesis, thioredoxins contribute to proper protein folding and disulfide bond (S-S) stabilization during the formation of sperm nucleus, acrosome and sperm accessory structures (Miranda-Vizuete et al. Citation2004). Furthermore, they participate in sperm cellular defense against oxidative stress, as indicated by increased sperm DNA defects in murine double knock-out of Txndc2 and Txndc3 genes (Smith et al. Citation2013). Human and other mammalian male germ cells carry three testis-specific thioredoxins, TXNDC2, TXNDC3, and TXNDC8, which may be carried over to fully differentiated spermatozoa as a result of normal or aberrant spermiogenesis (Jimenez et al. Citation2002, Citation2004; Sadek et al. Citation2003). In particular, TXNDC8 (originally designated SPTRX3) is not present in non-human mammalian spermatozoa after the completion of spermiogenesis. Similarly, TXNDC8 is not detectable by microscopy in most morphologically normal human spermatozoa, but accumulates in the nuclear vacuoles and superfluous cytoplasm in the tail midpiece of visibly defective ones (Jimenez et al. Citation2004; Buckman et al. Citation2009, Citation2013). A follow-up large TXNDC8 study (Buckman et al. Citation2013) validated this biomarker in 239 ART couples, including male, female, and idiopathic infertility, but did not examine its association with specific ART outcomes.
Previously, we used flow cytometry (FC) to investigate the relationship between TXNDC8 and treatment outcomes in 239 couples undergoing assisted reproductive therapy (ART) at an infertility clinic. We found elevated TXNDC8 levels (>15% TXNDC8 -positive spermatozoa) in 51% of male infertility patients, in 20% of men from couples with unexplained, idiopathic infertility and in 14% of men from couples previously diagnosed with female-only infertility. Couples with high semen content of TXNDC8 produced fewer two-pronuclear zygotes and had significantly reduced pregnancy and live birth rates. We concluded that the elevated semen content of TXNDC8 in men from ART couples coincided with reduced incidence of pregnancy by in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI), identifying TXNDC8 as a candidate biomarker reflective of ART outcome (Buckman et al. Citation2013).
Notably, TXNDC8 accumulates in the sperm head nuclear vacuoles, thus marking spermatozoa with abnormal chromatin structure. These and previous studies of others found a relationship between sperm parameters, measured by the flow cytometric sperm chromatin structure assay (SCSA), and couples’ ART outcomes (Sutovsky et al. Citation2015; Simon et al. Citation2016). Our own meta-analysis of infertility treatment outcomes including the incidence of spontaneous abortion (SAB) and multiple births in 233 couples that underwent treatment by ICSI or intrauterine insemination (IUI) revealed a statistically significant correlation between SCSA parameters and SAB (Kennedy et al. Citation2011). Thus, the relationship of novel sperm quality biomarkers such as TXNDC8 with sperm chromatin structure and ART outcomes is clinically relevant.
Beyond diagnosing male infertility in ART couples, progress has been made in the purification of spermatozoa for ART, which is increasingly important since the vast majority of ART cycles are now realized by ICSI, and require subjective selection of single spermatozoa to be injected in oocytes (Said and Land Citation2011; Sutovsky et al. Citation2015). Consequently, removal of TXNDC8-carrying spermatozoa with abnormal, vacuolated chromatin may be desirable during sperm preparation for ICSI. Need for the improvement of semen processing and sperm pre-selection is amplified by the drive toward fewer embryos transferred per cycle, aimed at reducing the incidence of multiple births (Ahlering and Sutovsky Citation2015). With regard to multiple pregnancies, significantly lower levels of SCSA measured chromatin damage were observed in men from couples having triplet pregnancies versus SAB in the aforementioned study (Kennedy et al. Citation2011). While the link between sperm TXNDC8 and couples’ ability to conceive by ART has been established, no such relationship was examined for potential impact on multiple births after multi-embryo transfer. Consequently, the present study provides first evidence of such relationship in raw and gradient/swim-up prepared spermatozoa, while also examining the correlation of semen TXNDC8 content with sperm chromatin structure and conventional semen parameters in two separate cohorts of ART couples.
Results
Effect of two-step purification on semen TXNDC8 levels
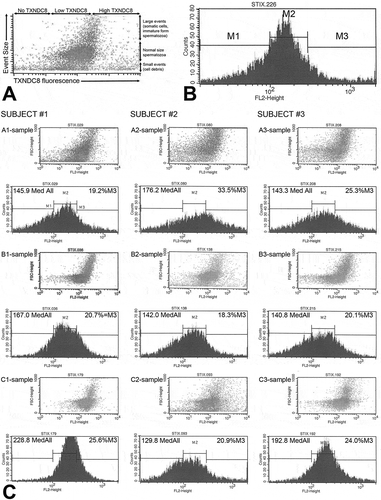
The A- (raw semen), B- (gradient purified), and C (swim up)-samples from 35 subjects () were analyzed by TXNDC8 FC in the first trial. Three types of purification outcomes were observed by analyzing FC histograms and TXNDC8 fluorescence intensities. Prevalently, the median fluorescence intensity of TXNDC8 and percentage of positive spermatozoa increased in B- and C-samples, but in six subjects, TXNDC8 fluorescence decreased or remained constant in B- and C-samples (). Based on visible light scatter (), both purification steps tended to enrich for FC events consistent with the size and fluorescence intensity of fully differentiated spermatozoa, while eliminating very large and very small FC events, attributable to abnormally large, immature forms of spermatozoa, somatic cell contaminants and cellular debris. Moderate-to-high level correlation of TXNDC8-induced fluorescence was observed between B- and C-samples, and to a lesser extent between A and B, and, A- and C-samples (Supplemental Data (SD) Table SD1). The highest correlation was observed between the percentages of TXNDC8-positive spermatozoa (within marker area (M) containing positive spermatozoa %M3 TXNDC8) of B- and C-samples (r = 0.84; Table SD1). As the cellular debris and contaminating somatic cells were removed by gradient separation and swim-up, the median fluorescence of TXNDC8-positive spermatozoa increased, in some subjects as much as five times between A-samples compared to B-/C-samples ( and Table SD2). Accordingly, the average median relative TXNDC8-induced fluorescence (TXNDC8 Median ALL; no units) rose from 63.3 ± 2.5 in A-samples to 92.4 ± 4.6 in B-samples and 90.9 ± 5.8 in C-samples. We concluded that two-step purification efficiently removed cellular debris and large spermatozoa/somatic cell contaminants, but the efficiency of the removal of TXNDC8 spermatozoa varied among subjects. In most subjects, the enrichment of purified semen fractions with spermatozoa coincided with increased percentage of spermatozoa bearing high content of TXNDC8.
Table 1. Sperm, SCSA, TXNDC8 and Beta 1&2 parameters in B-samples of 35 couples from first trial.
Figure 1. Two-step purification procedure altered the composition and TXNDC8 content of subjects’ semen, as documented by flow cytometry. (A) Scatter diagram combining the TXNDC8-induced fluorescence (x-axis) with forward scatter of visible light (y-axis) reflective of the size of cells passing through cytometer. Flow cytometric events in a semen sample are visualized as individual dots, collectively representing a mixture of morphologically normal and defective spermatozoa, cellular debris and contaminating somatic cells aggregating in distinct foci based on their size. (B). Histogram of TXNDC8-induced fluorescence is arbitrarily divided into marker areas M1-M3, reflecting, from left to right, very low background fluorescence/autofluorescence (M1), low level of TXNDC8 (M2), and elevated/high TXNDC8 fluorescence (M3). Cellular content of TXNDC8 in a sample is visualized in form of peaks reflecting the TXNDC8-induced fluorescence levels of individual flow cytometric events. (C) Scatter diagrams and histograms of raw semen (sample A1-3, top row), gradient purified semen (samples B1-3, middle row) and gradient + swim-up purified semen (samples C1-3, bottom row) in three subjects with varied semen purification outcomes, arranged in columns. In subject #1, purification removed most of the large cells and small debris but enriched the population of spermatozoa positive for TXNDC8, as the median relative fluorescence attributed to TXNDC8 (Med All; no units) increased from 145.9 to 228.8 and the percentage of cells with high TXNDC8 fluorescence (%M3) rose from 19.2% to 25.6%. In subject #2, purification was inefficient at removing TXNDC8-positive spermatozoa, but also failed to remove large cells and debris with low/no TXNDC8, thus reducing both median fluorescence and % TXNDC8-positive spermatozoa in proportion to debris and cells. In subject #3, the percentage of high TXNDC8 spermatozoa remained relatively constant but the median fluorescence increased dramatically as the sample was enriched in normal size spermatozoa and cleared of most debris and large cells that would lower the TXNDC8 fluorescence. Typical examples are from the second trial.
Correlation between sperm levels of TXNDC8, conventional semen parameters, establishment of pregnancy and sperm chromatin structure measured by SCSA
In addition to patients’ age and conventional semen parameters of sperm concentration and motility, the SCSA parameters were made available for all 35 couples in the first trial ( and Table SD2). The SCSA is a commercially outsourced clinical flow cytometric test of denatured, acridine orange stained spermatozoa, reflective of sperm quality, DNA damage, and fertility in ART settings (Evenson et al. Citation1999). Pregnancy outcomes were not made available for this cohort, but blood serum levels of human chorionic gonadotropin (hCG) on day 11 (Beta 1/B1) and day 13 (Beta 2/B2) after oocyte retrieval were provided for 19/28 couples, reflecting the establishment of chemical pregnancy. The B1 and B2 values were closely correlated with each other (r = 0.91). Comparing men from couples with B1 values above zero with those having B1 = 0 showed that the former had significantly younger female partners, but not significantly different SCSA, TXNDC8, or conventional semen parameters. Against expectation, both SCSA parameters trended higher in men from B1 > 0 couples () and positively correlated with B1- and B2-values (). On the contrary, most FC parameters reflecting high TXNDC8 fluorescence (TXNDC8 Median All, Median M3 and %M3) trended higher in men from couples with B1 = 0 (Table SD2).
Table 2. Correlation matrix (Pearson’s r-coefficients) of conventional semen parameters, flow cytometric TXNDC8 and SCSA values, and Beta values in a cohort of 35 ART couples in first trial.
Correlation analysis (, Table SD3, Table SD4) revealed a positive correlation (r = 0.66) between median fluorescence of TXNDC8 in the sperm fraction with high TXNDC8 content (TXNDC8 Median M3) and sperm DNA fragmentation index (%DFI; % spermatozoa with fragmented DNA) measured by SCSA. Furthermore, negative correlations were observed between median fluorescence of TXNDC8 positive spermatozoa, and sperm concentration (r = −0.57) and motility (r = −0.67; ). Median fluorescence of TXNDC8-positive spermatozoa correlated weakly with the second SCSA parameter, the high DNA stainability index or %HDS (r = 0.20). However, there was a robust correlation between %HDS and the percentage of spermatozoa lacking TXNDC8 (TXNDC8%M1; r = 0.68) in C-samples. Highest recorded correlations with %HDS were r = −0.60 for sperm concentration and r = −0.75 for sperm motility. The DFI also correlated negatively with sperm concentration (r = −0.27) and motility (r = −0.51).
The B1 and B2 values did not show a strong correlation with conventional semen parameters but correlated with median fluorescence of presumably normal spermatozoa that lacked TXNDC8 labeling (TXNDC8 Median M1; r = −0.44 for B1 and r = 0.47 for B2). In agreement with elevated SCSA %HDS values in B1 = 0 couples, positive correlations were observed between B2 and %HDS (r = 0.57), and B1 and both %HDS (r = 0.42) and %DFI (r = 0.46). Beta values showed negative albeit low-level correlations with female and male age (), as would be expected because the couples with Beta values above 0 were on average 3–4 years younger on both the male and female side (). Male and female age were closely correlated with each other (r = 0.80; Table SD3). Against expectation, both male and female age were negatively correlated with SCSA %DFI and %HDS (Table SD3). As expected, older couples and couples with B1 = 0 had fewer spermatozoa with low TXNDC8 levels (TXNDC8 %M2) and more spermatozoa with high TXNDC8 (%M3; ). Consequently, TXNDC8%M2 correlated negatively and TXNDC8%M3 positively with age, and reverse correlations were seen with Beta values (Table SD3).
Relationship between multiple births, sperm chromatin structure and TXNDC8 in ART couples
In the second trial, the A-, B- and C-samples from 56 couples (total of 168 samples) were processed for FC with the anti-TXNDC8 antibody. Clinical data available in this cohort included sperm concentration, motility, % normal WHO morphology and SCSA data for A-samples, and ART pregnancy outcomes. The most informative TXNDC8 data with regard to pregnancy outcome were derived from the analysis performed on gradient-purified spermatozoa (B-samples), as opposed to raw or gradient and swim-up isolated spermatozoa (see breakdown of sperm parameters between A-, B-, and C-samples in Table SD5). Fathers of multiples had significantly fewer TXNDC8-positive spermatozoa (%M3) compared to fathers of singletons (p = 0.01) and men from couples that failed to conceive (p = 0.02; ). Inversely, fathers of multiplets had a significantly higher proportion of spermatozoa with low, background levels of TXNDC8 (TXNDC8 %M1; p = 0.03 multiples vs. no pregnancy; p = 0.01 multiples vs. singleton); they also had a significantly lower overall intensity of median TXNDC8-induced fluorescence (TXNDC8 Median ALL; p = 0.004 singleton vs. multliplets; p = 0.03 multiples vs. no pregnancy). Among conventional semen parameters, fathers of multliplets had a significantly higher sperm concentration compared with those who did not conceive (p = 0.02), but not with fathers of singletons (p > 0.05). Sperm motility and percentage of normal morphology did not differ significantly between pregnancy-sorted groups. Both of the SCSA indexes (SCSI), %DFI/SCSI-1 and %HDS/SCSI-2, were numerically higher in the multiplets group (), although this increase was not statistically significant (p > 0.05), likely due to the combination of a small subject number and large standard deviation within the multiplets group. Also, it should be noted that the SCSA parameters were not available for B-samples in which most significant differences in TXNDC8 content were recorded. Neither male nor female age was significantly different between pregnancy groups.
Table 3. Relationship between multiple births, sperm chromatin structure and relative TNXDC8-induced sperm fluorescence levels (no units) in 56 ART couples in second trial.
For the purpose of correlation analysis (, Table SD6 and Table SD7), the C-samples were most informative, followed by A and B samples. The TXNDC8 parameters were correlated most closely with sperm concentration, motility, and percentage normal morphology. The TXNDC8 parameters reflective of a high percentage of cells with low, background levels of TXNDC8 fluorescence (TXNDC8 %M2 and, to a lesser extent, TXNDC8 Median M2) showed a positive correlation with sperm concentration (r = 0.57), motility (r = 0.54), and morphology (r = 0.48; ). Male and female partners’ age correlated with each other (r = 0.71), but not with conventional or flow cytometric sperm parameters. The number of children per couple showed only low-level correlations (highest r = −0.30 for TXNDC8 %M3, r = 0.28 for sperm concentration and r = −0.27 for TXNDC8 Median ALL). Such trends support the thesis that high sperm content of TXNDC8 had negative impact on ART outcomes. Among SCSA parameters, which did not correlate with each other, % DFI showed a moderate negative correlation with sperm motility (r = −0.42), and with TXNDC8 %M3 (r = −0.35) and TXNDC8 Median M2 (r = −0.37).
Table 4. Correlation matrix (Pearson’s r-coefficients) of conventional semen parameters, flow cytometric TXNDC8 and SCSA values and pregnancy values in a cohort of 56 ART couples in second trial.
Discussion
This study examined the relationship between semen levels of TXNDC8 and the incidence of successful conception and pregnancies of multiplets in couples from a general infertility clinic population treated by ICSI with morphologically selected spermatozoa (IMSSI); (Bartoov et al. Citation2003). The hypothesis tested proposed that the female partners of men with low levels of TXNDC8 are more likely to conceive by ART but also more prone to multiple births when 2–3 embryos are transferred. Study design included a two-step sperm purification procedure. Two significant observations were made: First, the percentage of TXNDC8-positive spermatozoa in most subjects' raw semen (A-samples), consistent with previously established 15% cutoff (Buckman et al. Citation2013) increased dramatically after gradient and swim-up purification that removed cellular debris (see Table SD2). This increase in the proportion of TXNDC8-positive spermatozoa was likely due to the removal of cellular debris and somatic cells from semen that read as negative flow cytometric events in TXNDC8 analysis. Second, men who conceived multiplets(i.e., those who presumably produced most viable embryos in vitro) had a significantly lower percentage of TXNDC8-positive spermatozoa, compared to men from couples who conceived a singleton pregnancy and those who failed to conceive. No more than three embryos were transferred in any of the treated couples. Besides sperm concentration, none of the available conventional and SCSA semen parameters showed statistically significant differences between parents of multiplets versus singletons or couples that failed to conceive.
Pregnancy data were only made available for the second of two patient cohorts examined, with the first cohort restricted to having beta-values (B1 and B2) as the sole indicators of ART outcome. Although this cohort was relatively small, the comparison between couples with B1 = 0 vs. B1 > 1 are meaningful. As expected, women from couples who established pregnancy (B1 > 1) were significantly younger than their counterparts with B1 = 0 (31.0 vs. 35.7 years; p = 0.03). Such an observation agrees with the established trend of female fertility declining at the threshold age of 35 years. Though not statistically different between B1 = 0 versus B1 > 0 couples, semen content of TXNDC8 and chromatin defect-bearing spermatozoa identified by SCSA trended higher in the B1 = 0 group. Perhaps, lower female age compensated for reduced sperm quality, which was further mitigated by the use of two-step sperm purification and ICSI/IMSSI.
The most significant finding from the second trial, this time with available pregnancy data, was that low TXNDC8 levels in men from treated couples were predictive of an increased chance of multiple pregnancies after the transfer of up to three embryos. These findings are of significance for clinical decision-making regarding the number of embryos to be transferred. As illustrated by the highly publicized case of ‘octomom,’ there are major ethical and medical concerns regarding multiple embryo transfer after assisted fertilization (Rosenthal Citation2010). We show that the TXNDC8 test can identify a presumably superior sperm sample most likely to produce multiple implantations and pregnancies, providing infertility clinicians with a useful tool for deciding on single versus multiple embryo transfer. The second trial reaffirmed that the two-step purification method eliminated cellular debris but was not efficient in removing defective spermatozoa that carry over TXNDC8. Various methods of sperm purification are increasingly used for sperm preparation prior to ICSI procedure (Ahlering and Sutovsky Citation2015). Our finding of high TXNDC8 levels in purified sperm fractions implies that even the two-step sperm purification alone may not be an efficient procedure to avoid the use of defective spermatozoa for ICSI treatment. With further improvements including routine IMSSI application and, perhaps, the modified (for clinical use) semen nanopurification techniques originally developed in livestock species (Odhiambo et al. Citation2014; Feugang et al. Citation2015), may permit the efficient removal of the TXNDC8-positive spermatozoa and spermatozoa with fragmented DNA from the ICSI sperm pool that could increase the confidence in transferring fewer embryos and ultimately reduce the incidence of multiple births while maintaining high pregnancy rates.
In general, high TXNDC8 values correlated both with SCSA and conventional semen parameters. Considering that relationship between abnormal chromatin structure/DNA fragmentation and low ART success is well documented (Simon et al. Citation2016), the positive correlations between SCSA parameters and high sperm TXNDC8 content are as anticipated. Similarly, negative correlations of sperm count, motility, and morphology with both SCSA and TXNDC8 values are not surprising. More puzzling is the observation that fathers of multiplets in the second trial had the numerically highest SCSA parameters of A-samples, while their conventional sperm parameters were similar to fathers of singletons and men from couples that failed to conceive. Also surprising is the negative correlation between SCSA DFI and male age, though this has been reported by others as well (Yang et al. Citation2019). On the contrary, the percentage of TXNDC8-positive spermatozoa in fathers of multiplets trended lower in A-samples and were significantly lower in B-samples. Similarly, men from couples with Beta 1 > 0 in first trial had elevated SCSA parameters but lower %TXNDC8-positive spermatozoa in A-samples. To fully understand this phenomenon, future studies should assess side-by-side SCSA and TXNDC8 parameters in both raw and purified semen samples and replicate the above. At present, these observations suggest the existence of partially overlapping but distinctly divergent sperm subpopulations when sperm chromatin structure and TXNDC8 carryover are considered.
Illustrating the consistency and repeatability of TXNDC8 assay, the general trend toward higher TXNDC8 content associated with lesser sperm quality and ART success agreed between two described trials. Also, the highest correlation coefficients in both trials were recorded in C-samples (Tables SD3 and SD7). To maximize repeatability, ART treatments in both trails were administered by the same physician and embryologist, and flow cytometric analyses were done at the same facilities with the same equipment. Nevertheless, differences between trials may have been due to the different sizes of cohorts (35 vs. 56 couples) and the fact that only 24/35 first trial subjects generated a C-sample. Also considered should be possible modifications of ART protocols and operating procedures during the time period between first and second trial, and the average male age being higher in the first trial (43.4 ± 0.9 yr) compared to second trial (36.3 ± 0.7 yr). Female age was slightly lower in first trial 1 (33.2 ± 0.8 yr) than in the second (35.1 ± 0.7 yr).
Contrary to C-samples appearing most informative in correlation analysis, most significantly different live birth outcomes in the second trial were derived from the analysis performed on gradient-only separated spermatozoa (B-samples). This disparity could be due to varied content of sperm-derived TXNDC8-containing cellular debris in A-samples and to the overall enrichment of C-samples with motile spermatozoa, with or without carryover of TXNDC8. In other words, gradient purification procedure removed a substantial portion of flow cytometric events other than spermatozoa (debris, somatic cells, residual bodies), while retaining most of the TXNDC8 containing spermatozoa in the post-separation pellet. Next, swim-up removed dead spermatozoa and any remaining debris, but retained and apparently even increased the proportion of TXNDC8 positive to TXNDC8-free spermatozoa. Such findings indicate that TXNDC8 is present in motile spermatozoa, some of which may appear morphologically normal in conventional semen analysis. Thus, it may be prudent in a clinical setting to apply the biomarker-based sperm tests to samples obtained after initial gradient purification, perhaps in combination with IMSSI to maximize benefit.
As exemplified by , gradient and swim up purification does remove a portion of presumably defective spermatozoa with carryover of TXNDC8 in some subjects. Along these lines, gradient purification procedure also removed spermatozoa that lacked sperm head proteins PAWP/WBP2NL and PLCZ, implicated in the process of oocyte activation at fertilization (Tavalaee et al. Citation2017b). Furthermore, the aforementioned oocyte activating factors were diminished in semen of ART patients with high DNA fragmentation (by TUNEL assay) and reduced ICSI fertilization rates (Tavalaee et al. Citation2017a). It is thus possible that a negative relationship exists between sperm content of such proteins and TXNDC8. Such hypotheses could be tested by multiplex image-based FC, as was recently done in bulls used for artificial insemination wherein sperm content of PAWP correlated with several biomarkers related to sperm quality (Kennedy et al. Citation2014).
Altogether, the findings presented here may have value for clinical decision-making regarding the preparation of spermatozoa for ART and the number of embryos to be transferred. A TXNDC8-based test could potentially identify a superior sperm sample most likely to produce multiple implantations and pregnancies, providing infertility clinicians with a useful tool for deciding on single versus multiple embryo transfer. Removing the TXNDC8-positive spermatozoa from the ICSI pool could lead to more confidence in transferring fewer embryos, ultimately lowering the multiple birth rates while maintaining high pregnancy rates.
Materials and methods
Patient cohort
Sperm samples were collected separately from two cohorts of men from couples attending infertility clinic during time periods ending in May (n = 35 couples) and November (n = 56 couples) of the same year. There were no specific couple inclusion criteria set based on diagnosis, etiology, or andrological parameters. Only subjects with sufficient sperm numbers after SCSA, two-step purification and treatment were included. All couples were treated by ICSI with single spermatozoa selected at a high magnification (intracytoplasmic morphologically selected sperm injection/IMSSI); (Bartoov et al. Citation2003). No more than three embryos were transferred in any of the treated couples.
Sperm samples and pregnancy data
Available conventional WHO sperm parameters in the first trial included sperm concentration and motility. Such parameters were also made available for second trial subjects, with addition of sperm morphology (WHO % normal morphology). In both trials, data from SCSA (Evenson and Jost Citation2000), performed by a commercial provider (SCSA Diagnostics. Inc., Brookings, SD), were correlated with TXNDC8 parameters. Two different parameters representing only partially overlapping sperm cohorts within a semen sample (Evenson Citation2016) were measured: the DNA fragmentation index (%DFI or SCSI-1) was the proportion of cells containing denatured DNA with strand breaks and protamination defects, and was calculated from the DFI frequency histogram obtained from the ratio between the red and total (red + green) fluorescence intensity. High DNA stainability (% HDS or SCSI-2) was calculated based on the percentage of spermatozoa with high levels of green fluorescence, which are thought to represent immature spermatozoa with DNA damage and incomplete chromatin condensation (Giwercman et al. Citation2003, Citation2010). Both DFI and HDS were informative for the purpose of the present study as both DNA-damaged spermatozoa and immature sperm forms have the potential to contain TXNDC8. Available treatment outcomes in the first trial were limited to blood serum levels of human chorionic gonadotropin (hCG) on day 11 and day 13 after oocyte retrieval, further defined as Beta 1 (B1) and Beta 2 (B2) values in international units (I.U.). Values above 0 can indicate either normal or ectopic pregnancy. In second trial, actual pregnancy data established by ultrasound were provided instead of hCG values and live births had been recorded in couples that achieved pregnancy. There was no information provided on initial clinical diagnosis of male, female, combined, or idiopathic infertility, although it is possible to infer from sperm parameters that male infertility was represented in both trials.
Semen gradient purification and swim-up
As a part of sperm preparation for ART treatment cycle, each raw semen sample, designated A-sample, was subjected to a two-step purification procedure. First, the A-sample was centrifuged through a discontinuous gradient (45/90 AllGrad, Life Global), generating a B-sample, which was then subjected to sperm swim-up procedure. Raw semen, A-samples as well as purified B- and C-samples were subjected to flow cytometric analysis of TXNDC8. No conventional sperm parameters or SCSA values were available for B- and C-samples. In the first trial, a total of 35 couples provided an A-sample, of which 28 were able to yield a B-sample, of which 24 were also able to yield a C-sample, 19 of which had available Beta values. All 56 couples in second trial provided A-, B- and C-samples, and all had available pregnancy/live birth records.
Intracytoplasmic sperm injection
Intracytoplasmic morphologically selected sperm injection (IMSSI) procedure utilized differential interference (DIC) at 600x or Hoffman contrast at 400x to visualize imperfections in spermatozoa selected for ICSI not normally seen at 200x. Narishige hydraulic micromanipulators utilized for procedures were mounted on an Olympus IX-71 microscope. Research Instruments (RI) air injectors were used for all manipulations. No camera was used for this modified IMSSI procedure. Manipulation was performed in a 10 µl drop of modified human tubal fluid (HTF) with HEPES buffer (InVitroCare, Inc.) supplemented with 10% synthetic serum substitute (SSS; Irvine Scientific) and oil overlay. Spermatozoa were immobilized and evaluated in Sage (Cooper Surgical) medium with 7% polyvinylpyrrolidone (PVP). Injected oocytes were placed in 20 µl drops of embryo continuous culture media (Irvine Scientific) and incubated for 5–6 days at 37°C in a humidified atmosphere of medical mix gas, 6% CO2, 5% O2, balanced N2.
Flow cytometric sample preparation and microscopy
Samples were centrifuged to remove seminal plasma and fixed in 2% electron microscopy grade formaldehyde in phosphate-buffered saline (PBS), then washed by centrifugation in 8 ml of PBS and resuspended in 0.3 ml of PBS for storage at 4°C until processing for FC. All centrifugations during fixation and processing were done for 5 min at 350 x g in a hinged rotor centrifuge using 15 ml polypropylene tubes (Falcon), to minimize sample loss by clumping and sperm adhesion to tube wall. To detect TXNDC8, samples were permeabilized on day 1 for 40 min by the addition of 2% Triton X-100 (TX) in PBS to resuspended pellets to reach final TX-concentration of 0.1% (v/v). Next, normal goat serum (NGS, Sigma) was added at 2% v/v and 25 min later, the rabbit anti-TXNDC8 antibody was added to achieve 1/200 dilution (v/v) for overnight incubation at 4°C. This antibody, developed in coauthor Miranda Vizuete’s, laboratory, was characterized in detail and shown to be specific by Western blotting, FC, immunohistochemistry, and immunofluorescence in our previous studies (Jimenez et al. Citation2004; Buckman et al. Citation2009, Citation2013). On day 2, concentrated goat anti-rabbit IgG-TRITC (Zymed/Thermo Fisher Scientific) was added to each pellet at 1/400 final dilution (v/v), which was tested by us to produce minimal background autofluorescence even in the absence of washing between the first and second antibodies.. Following incubation for 40 min at room temperature, 8 ml of PBS with 0.1% TX was added per tube and removed by centrifugation. Final pellets were resuspended in 0.3 ml pure PBS (no TX) and transferred into cell strainer-capped (to remove cytometer clogging-causing sperm clumps) polystyrene FC tubes (BD Falcon).
Negative control sample was prepared by the replacement of anti-TXNDC8 with a nonimmune rabbit serum (Sigma) at an equivalent immunoglobulin concentration, which was performed on a pooled sample from multiple subjects and screened both by FC and epifluorescence microscopy. Screening of both the positive (to confirm signal) and the negative (to confirm specificity) samples was done under a Nikon Eclipse 800 microscope with epifluorescence and differential interference contrast (DIC) optics. Representative images were recorded using MetaMorph software, as described (Buckman et al. Citation2013).
Flow cytometry and statistical analysis
Flow cytometric analysis of TXNDC8 was conducted as described (Buckman et al. Citation2013) using FACScan cytometer (BD Biosciences). Ten thousand FC events, which besides spermatozoa, may include cellular debris of spermatogenic and somatic origin, immature sperm forms (spermatids and spermatid-derived residual bodies) and somatic cells (leukocytes, epithelial cells), were recorded per sample. Each sample was rendered as a scatter diagram of visible light and a histogram of relative fluoresce (no units) induced by TXNDC8 content of the sample. The histograms were divided arbitrarily by marker areas M1, M2 and M3, and the percentages of FC events (TXNDC8 %M1/M2/M3) and their median relative fluorescence intensities were recorded within each marker in addition to overall median fluorescence value of the entire sample (TXNDC8 Med ALL). All values were correlated with conventional and SCSA sperm parameters, and compared between couples that failed to conceive versus singleton parents versus multiplets' parents. Statistical analysis, supervised by a professional statistician, was performed using Microsoft Excel and SAS tools to test for statistical significance (p < 0.05), including ANOVA and Pearson’s correlation.
Authors’ contributions
Conceptualized and supervised the study, and co-wrote the article: PS, PA; clinician working with patients: PA; embryologist performing sperm preparation and assessment, and ICSI/IMSSI: DG; processed the sperm samples after purification and prepared them for shipping and flow cytometric analysis: KB; labeled and analyzed samples for flow cytometry and light microscopy: MS; developed and validated the TXNDC8 antibody, and contributed to study design: AMV.
Ethical approval
Both male and female partners signed informed consent and the samples were coded as to make the study subjects unidentifiable to research team at the University of Missouri, wherein all samples were handled and processed strictly as stipulated by an approved Internal Review Board (MU IRB) protocol.
Supplemental Material
Download PDF (411.3 KB)Acknowledgments
We appreciate the generosity of anonymous ART patients whom consented to donate the sperm samples for this research. We thank the staff of Cell & Immunology Core, School of Medicine, University of Missouri, for assistance with FC.
Disclosure statement
PA is the founder and owner of MCRM, Chesterfield, MO. PS is the co-founder and chief scientific officer of AndroLabb LLC, Columbia MO, and chief scientific officer of International Boar Semen Inc., Eldora, IA.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Ahlering P, Sutovsky P. 2015. Biomarker-based flow-cytometric semen analysis for male infertility diagnostics and clinical decision making in ART.. In: Sills ES, editor. Screening for the single euploid embryo - molecular genetics in reproductive medicine. New York, NY: Springer Science+Media LLC; p. 33–51.
- Bartoov B, Berkovitz A, Eltes F, Kogosovsky A, Yagoda A, Lederman H, Artzi S, Gross M, Barak Y. 2003. Pregnancy rates are higher with intracytoplasmic morphologically selected sperm injection than with conventional intracytoplasmic injection. Fertil Steril. 80(6):1413–1419. doi:10.1016/j.fertnstert.2003.05.016.
- Buckman C, George TC, Friend S, Sutovsky M, Miranda-Vizuete A, Ozanon C, Morrissey P, Sutovsky P. 2009. High throughput, parallel imaging and biomarker quantification of human spermatozoa by ImageStream flow cytometry. Syst Biol Reprod Med. 55(5–6):244–251. doi:10.3109/19396360903056224.
- Buckman C, Ozanon C, Qiu J, Sutovsky M, Carafa JA, Rawe VY, Manandhar G, Miranda-Vizuete A, Sutovsky P. 2013. Semen levels of spermatid-specific thioredoxin-3 correlate with pregnancy rates in ART couples. PLoS One. 8(5):e61000. doi:10.1371/journal.pone.0061000.
- Evenson D, Jost L. 2000. Sperm chromatin structure assay is useful for fertility assessment. Methods Cell Sci. 22(2–3):169–189. doi:10.1023/A:1009844109023.
- Evenson DP. 2016. The Sperm Chromatin Structure Assay (SCSA((R))) and other sperm DNA fragmentation tests for evaluation of sperm nuclear DNA integrity as related to fertility. Anim Reprod Sci. 169:56–75. doi:10.1016/j.anireprosci.2016.01.017.
- Evenson DP, Jost LK, Marshall D, Zinaman MJ, Clegg E, Purvis K, de Angelis P, Claussen OP. 1999. Utility of the sperm chromatin structure assay as a diagnostic and prognostic tool in the human fertility clinic. Hum Reprod. 14(4):1039–1049. doi:10.1093/humrep/14.4.1039.
- Feugang JM, Liao SF, Crenshaw MA, Clemente H, Willard ST, Ryan PL. 2015. Lectin-functionalized magnetic iron oxide nanoparticles for reproductive improvement. JFIV Reprod Med Genet. 3(2):145.
- Giwercman A, Lindstedt L, Larsson M, Bungum M, Spano M, Levine RJ, Rylander L. 2010. Sperm chromatin structure assay as an independent predictor of fertility in vivo: a case-control study. Int J Androl. 33(1):e221–e227. doi:10.1111/j.1365-2605.2009.00995.x.
- Giwercman A, Richthoff J, Hjollund H, Bonde JP, Jepson K, Frohm B, Spano M. 2003. Correlation between sperm motility and sperm chromatin structure assay parameters. Fertil Steril. 80(6):1404–1412. doi:10.1016/S0015-0282(03)02212-X.
- Jimenez A, Johansson C, Ljung J, Sagemark J, Berndt KD, Ren B, Tibbelin G, Ladenstein R, Kieselbach T, Holmgren A, et al. 2002. Human spermatid-specific thioredoxin-1 (Sptrx-1) is a two-domain protein with oxidizing activity. FEBS Lett. 530(1–3):79–84. doi:10.1016/S0014-5793(02)03417-8.
- Jimenez A, Zu W, Rawe VY, Pelto-Huikko M, Flickinger CJ, Sutovsky P, Gustafsson JA, Oko R, Miranda-Vizuete A. 2004. Spermatocyte/spermatid-specific thioredoxin-3, a novel Golgi apparatus-associated thioredoxin, is a specific marker of aberrant spermatogenesis. J Biol Chem. 279(33):34971–34982. doi:10.1074/jbc.M404192200.
- Kennedy C, Ahlering P, Rodriguez H, Levy S, Sutovsky P. 2011. Sperm chromatin structure correlates with spontaneous abortion and multiple pregnancy rates in assisted reproduction. Reprod Biomed Online. 22(3):272–276. doi:10.1016/j.rbmo.2010.11.020.
- Kennedy CE, Krieger KB, Sutovsky M, Xu W, Vargovic P, Didion BA, Ellersieck MR, Hennessy ME, Verstegen J, Oko R, et al. 2014. Protein expression pattern of PAWP in bull spermatozoa is associated with sperm quality and fertility following artificial insemination. Mol Reprod Dev. 81(5):436–449. doi:10.1002/mrd.22309.
- Miranda-Vizuete A, Sadek CM, Jimenez A, Krause WJ, Sutovsky P, Oko R. 2004. The mammalian testis-specific thioredoxin system. Antioxid Redox Signal. 6(1):25–40. doi:10.1089/152308604771978327.
- Odhiambo JF, DeJarnette JM, Geary TW, Kennedy CE, Suarez SS, Sutovsky M, Sutovsky P. 2014. Increased conception rates in beef cattle inseminated with nanopurified bull semen. Biol Reprod. 91(4):97. doi:10.1095/biolreprod.114.121897.
- Rosenthal MS. 2010. The Suleman octuplet case: an analysis of multiple ethical issues. Womens Health Issues. 20(4):260–265. doi:10.1016/j.whi.2010.04.001.
- Sadek CM, Jimenez A, Damdimopoulos AE, Kieselbach T, Nord M, Gustafsson JA, Spyrou G, Davis EC, Oko R, van der Hoorn FA, et al. 2003. Characterization of human thioredoxin-like 2. A novel microtubule-binding thioredoxin expressed predominantly in the cilia of lung airway epithelium and spermatid manchette and axoneme. J Biol Chem. 278(15):13133–13142. doi:10.1074/jbc.M300369200.
- Said TM, Land JA. 2011. Effects of advanced selection methods on sperm quality and ART outcome: a systematic review. Hum Reprod Update. 17(6):719–733. doi:10.1093/humupd/dmr032.
- Simon L, Zini A, Dyachenko A, Ciampi A, Carrell DT. 2016. A systematic review and meta-analysis to determine the effect of sperm DNA damage on in vitro fertilization and intracytoplasmic sperm injection outcome. Asian J Androl. 19(1):80–90.
- Smith TB, Baker MA, Connaughton HS, Habenicht U, Aitken RJ. 2013. Functional deletion of Txndc2 and Txndc3 increases the susceptibility of spermatozoa to age-related oxidative stress. Free Radic Biol Med. 65:872–881. eng. doi:10.1016/j.freeradbiomed.2013.05.021.
- Sutovsky P, Aarabi M, Miranda-Vizuete A, Oko R. 2015. Negative biomarker based male fertility evaluation: sperm phenotypes associated with molecular-level anomalies. Asian J Androl. 17(4):554–560. doi:10.4103/1008-682X.153847.
- Tavalaee M, Kiani-Esfahani A, Nasr-Esfahani MH. 2017a. Relationship between potential sperm factors involved in oocyte activation and sperm DNA fragmentation with intra-cytoplasmic sperm injection clinical outcomes. Cell J. 18(4):588–596. doi:10.22074/cellj.2016.4725.
- Tavalaee M, Parivar K, Shahverdi AH, Ghaedi K, Nasr-Esfahani MH. 2017b. Status of sperm-born oocyte activating factors (PAWP, PLCzeta) and sperm chromatin in uncapacitated, capacitated and acrosome-reacted conditions. Hum Fertil (Camb). 20(2):96–103.
- Yang H, Li G, Jin H, Guo Y, Sun Y. 2019. The effect of sperm DNA fragmentation index on assisted reproductive technology outcomes and its relationship with semen parameters and lifestyle. Transl Androl Urol. 8(4):356–365. doi:10.21037/tau.2019.06.22.
