ABSTRACT
Betaine is a bioactive peptide whose beneficial effects on diabetes complications have been considered, previously. The present study aimed to investigate the possible protective effects of betaine against hyperglycemia-induced steroidogenesis impairment and apoptosis in mice granulosa cells. Ovarian granulosa cells were isolated from C57/BL6 mice and cultured in steroidogenesis medium (SM) containing 30 ng/ml FSH and 0.5 µM testosterone. The cells were cultured in SM containing low (5 mM) or high (30 mM) glucose concentrations for 24 h in the presence or absence of betaine (5 mM). At the end of the experiment, estradiol and progesterone were measured by ELISA in the culture medium. Expression of apoptosis and steroidogenesis associated genes and caspase-3 activity were determined by qRT-PCR and colorimetric assays, respectively. Exposure of mice granulosa cells to high glucose concentration inhibited the steroidogenesis by decreasing estradiol and progesterone secretion and downregulation of steroidogenesis-related genes including 3βHSD, Cyp11a1, Cyp19a1, and StAR. Betaine treatment could ameliorate the steroidogenesis impairment at molecular and biochemical levels. High glucose concentration also enhanced apoptosis in mice granulosa cells that were characterized by elevation of caspase-3 activity, upregulation of bax gene and downregulation of bcl2 gene. Betaine treatment could attenuate the apoptotic-related changes induced by high glucose concentration in granulosa cells. According to the results of the present study, betaine could ameliorate the adverse effects of hyperglycemia on the physiological function of ovarian granulosa cells. The results highlight the potential role of betaine for the intervention of ovarian dysfunction in diabetic patients.
Abbreviations: AABA: Betaine-α-aminobutyric acid; AGEs: Advanced glycation end products; bax: bcl2 Associated X; bcl2: B-cell lymphoma 2; AMPK: AMP-activated protein kinase; BHMT: Betaine homocysteine methyltransferase; C/EBP: CCAAT-enhancer-binding proteins; Cyp11a1: Cholesterol side-chain cleavage cytochrome P450; Cyp19a1: Cytochrome P450 aromatase; DM: Diabetes mellitus; E2: Estradiol; ERS: Endoplasmic reticulum stress; GCs: Granulosa cells; GLUT: Glucose transporter; FSH: Follicle-stimulating hormone; 3βHSD: 3β-hydroxysteroid dehydrogenase; IL-1β: interleukin-1ß; LH: Luteinizing hormone; MDCK: Madin-Darby Canine Kidney cell; MT: Methionine synthase, MTT: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; NLRP3: NLR Family Pyrin Domain Containing 3; NF-κB: Nuclear factor κB; P4: Progesterone; ROS: Reactive oxygen species; SGLT: Sodium dependent glucose transporter; SLC7A6: Solute Carrier Family 7 Member 6; StAR: Steroidogenic acute regulatory protein; STZ: Streptozotocin; Tumor necrosis factor α: TNF-α; TXNIP: Thioredoxin interacting protein.
Introduction
Diabetes mellitus is a clinical metabolic disorder linked with reproductive complications of the female including impaired folliculogenesis, oligomenorrhea, and amenorrhea followed by ovulation failure and infertility (Tuncel et al. Citation2017; Wellons et al. Citation2017). During folliculogenesis, chronically high glucose levels have deleterious effects on the structure and function of the ovary, in particular the oocyte surrounded by granulosa cells. Tissue damage has been related to the increased formation of AGEs and the overproduction of ROS, which can cause oxidative stress, vascular endothelial cell damage, and inflammatory cytokine production (Fraunhoffer et al. Citation2018). During normal oocyte development, a mature antral follicle grows from a primordial follicle with a single oocyte surrounded by GCs. Normal proliferation and steroidogenesis of GCs are crucial for oocyte growth, maturation and fertilization, and subsequent embryonic development (Casarini et al. Citation2016).
GCs serve pivotal roles in the synthesis of ovarian steroids as well as the production of cytokines and growth factors required for oocyte development. FSH and LH along with intraovarian cytokines induce expression of steroidogenic enzymes in GCs, including the steroidogenic acute regulatory protein (StAR) that facilitates the translocation of cholesterol from the outer membrane to the inner membrane of mitochondria, the cholesterol side-chain cleavage cytochrome P450 (Cyp11a1), 3βHSD, and cytochrome P450 aromatase (Cyp19a1). FSH stimulates aromatization of androgens (derived from thecal cells) to estradiol (E2). After ovulation, the GCs transform into granulosa lutein cells that produce progesterone to help maintain a potential pregnancy and trigger production of thick cervical mucus that prevents sperm entry into the uterus (Casarini et al. Citation2016: Doblado et al. Citation2020).
Several reports provided evidence for an association of hyperglycemia and granulosa cell dysfunctions in animals or in vitro experimental models (Wu et al. Citation2015, Citation2017; Fraunhoffer et al. Citation2018; Kannan et al. Citation2019). Recent observation has shown that hyperglycemia decreases the expression of glucose transporters (GLUT 2–4, SGLT1-2) in mice, leading to disruption of GCs glucose uptake and energy deficiency (Xu et al. Citation2019). Moreover, elevated glucose levels impair production of progesterone and estradiol in both primary rat granulosa cells and rats treated with STZ (Chabrolle et al. Citation2008).
Apoptosis is a form of cell death, which takes part in the pathogenesis of many diabetes complications and is enhanced through various pathways such as the polyol pathway and endoplasmic reticulum stress pathway (Kato et al. Citation2019). Apoptosis of granulosa cells, a condition that is responsible for follicular atresia, controls cellular growth in the ovary. However, aberrant apoptosis of GCs has an adverse impact on ovarian follicular development. Emerging evidence has shown that oocytes from diabetic ovaries exhibit mitochondrial dysfunction, abnormal oocyte meiotic spindle formation, up-regulated cell cycle inhibitors and cell cycle arrest of GCs and increased granulosa cell apoptosis (Wu et al. Citation2015; Lee et al. Citation2019). Previous experiments have also shown that cleaved-caspase 8 and cleaved-caspase 3 in ovaries of diabetic mice are up-regulated, suggesting the incidence of apoptosis in GC under hyperglycemic stress (Wu et al. Citation2015; Fraunhoffer et al. Citation2018).
The application of natural bioactive compounds as an alternative treatment for diabetes-associated reproductive complications has been considered more due to their efficacy, fewer side effects, and easy availability (Gothai et al. Citation2016; Teodoro Citation2019). The previous finding has shown that the beneficial effects of bioactive compounds in treating diabetes complications may be due to various properties such as antioxidant, anti-inflammatory, and antiapoptotic of these compounds (Teodoro Citation2019). While many natural bioactive compounds may be potentially useful in diabetes, data related to molecular mechanisms involved in the beneficial effects of these compounds on ovarian health and function is limited.
Betaine (trimethylglycine) is produced in the inner mitochondrial membrane, primarily in the liver and kidneys and acts as an osmoprotectant and methyl group donor in various physiological processes in mammals (Zhao et al. Citation2018). Previous findings have also shown that betaine acts as an anti-inflammatory peptide in various diseases by reduction of endoplasmic reticulum stress and apoptosis and, also, inhibition of nuclear factor-κB signaling pathway and NLRP3 inflammasome activity (Zhao et al. Citation2018). Betaine can also improve insulin resistance and hyperglycemia in high-calorie diet-induced diabetes in experimental animals (Nazari et al. Citation2017). Betaine accumulates in granulosa cumulus cells by action of SLC7A6 transporter during the germinal vesicle stage oocyte development (Corbett et al. Citation2014). Several studies suggest that betaine may have a functional role in female reproductive health and fertility. It has been shown betaine elevates ovarian antioxidant enzyme activities and demonstrates methyl donor effect in non-pregnant rats (Alirezaei et al. Citation2012). Moreover, Salahi et al. (Citation2019) observed that betaine dietary supplementation could improve gestational diabetes mellitus in pregnant STZ-treated mice by increasing insulin levels, alleviating insulin resistance, and restoring normal serum concentration of homocysteine as well as lipid profile characteristics. Given the previous findings, it is therefore hypothesized that betaine may affect functions of granulosa cells in physiological and pathological conditions. Based on our knowledge, the impact of betaine on functions of ovarian granulosa cells undergoing hyperglycemia have not been determined yet. The present study aimed to investigate the effects of betaine on the expression of apoptosis and steroidogenesis related genes in mice granulosa cells exposed to a high concentration of glucose.
Results
Effect of betaine on the viability of granulosa cells cultured under high glucose concentration
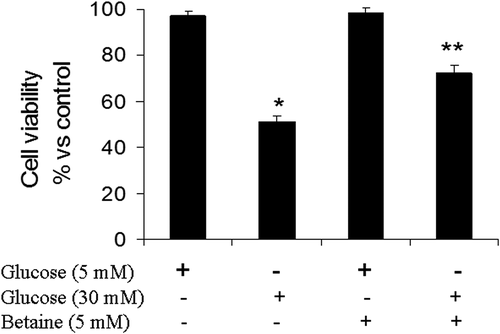
As presented in , exposure of granulosa cells to medium containing 30 mM glucose caused a reduction in cell viability in relation to cells that were cultured in basal medium containing 5 mM glucose (p < 0.05). MTT results showed that betaine (5 mM) increased the viability of granulosa cells exposed to 30 mM glucose compared to untreated cells in the same condition, significantly (p < 0.05) ().
Figure 1. Cell viability (MTT assay) of mice granulosa cells treated with betaine for 24 h. Granulosa cells from immature mice were cultured for 24 h in a medium containing 5 mM betaine in the presence of 5 mM or 30 mM glucose. The results are normalized with the untreated cells cultured in the presence of basal glucose concentration. Different number of asterisks demonstrate significant difference at p < 0.05
Effect of betaine on steroid hormone secretion under hyperglycemia condition
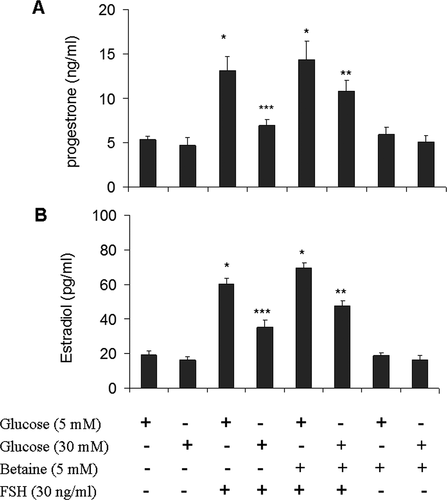
We first confirmed that FSH could induce steroid secretion in our cultured granulosa cells. We treated the cells with FSH (30 ng/mL) for 12 h and detected E2 and P4 production by ELISA assay. As expected, FSH induced E2 and P4 secretion in granulosa cells in different experimental protocols (). We then examined whether hyperglycemia was able to inhibit FSH stimulated steroid secretion in granulosa cells. As shown in , cultures supplemented with 30 mM glucose decreased E2 and P4 production in granulosa cells relative to cultures containing 5 mM glucose (P < 0.05) (). We then investigated whether the inhibitory effect of high glucose on the production of both E2 and P4 in granulosa cells could be improved by betaine. As shown in , simultaneous treatment of granulosa cells with glucose (30 mM) and betaine (5 mM) increased FSH stimulated E2 and P4 production. Betaine had no obvious effect on both E2 and P4 secretion in granulosa cells cultured in medium containing basal and high glucose levels without FSH stimulation.
Figure 2. Effect of betaine treatment on basal and FSH stimulated progesterone. (A) and estradiol (B) secretions by mice granulosa cells exposed to low (5 mM) or high (30 mM) glucose concentrations. Granulosa cells from immature mice were cultured for 12 h in a medium containing 5 mM betaine in the presence of 5 mM or 30 mM glucose and then again cultured in a medium with the same component along with FSH (30 ng/ml) for another 12 h. Hormonal analysis was performed by ELISA assay. Results are means ± SEM for three independent experiments with triplicated wells. Bars with different numbers of asterisks are significantly different at p < 0.05
Effect of betaine on the expression of key genes in steroidogenesis in granulosa cells under hyperglycemia condition
To determine whether the protective effect of betaine on restoration of steroid production in granulosa cells exposed to hyperglycemia resulted from the alteration of expression of the key genes in steroidogenesis, the mRNA levels of 3βHSD, Cyp11a1, and Cyp19a1enzymes and StAR, a major cholesterol carrier, were studied using qRT-PCR analysis.
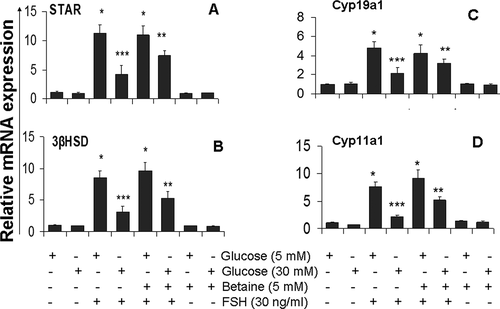
As shown in , glucose treatment (30 mM) in the presence of FSH resulted in a reduction of expression of 3βHSD (2.7 fold), Cyp19a1 (2.25 fold), Cyp11a1 (3.5 fold) and StAR (2.7 fold) relative to the values in the presence of FSH with 5 mM glucose. After treatment of FSH stimulated granulosa cells with 5 mM betaine in the presence of 30 mM glucose, the expression of 3βHSD, Cyp19a1, Cyp11a1, and StAR genes were increased compared to untreated cells (p < 0.05) (). Thus, the restoration of FSH induced E2 and P4 secretion in high glucose treated granulosa cells in response to betaine treatment appear to be caused by an elevation in the amounts of 3βHSD, Cyp11a1, StAR, and Cyp19a1 mRNA. In the basal state (no FSH or high glucose), betaine treatment did not affect the amount of mRNA levels of all steroidogenesis-related genes () (p > 0.05).
Figure 3. Effect of betaine treatment on mRNA levels of StAR (A), 3βHSD (B), Cyp11a1 (C) and Cyp11a1 (D) in mice granulosa cells exposed to low (5 mM) or high (30 mM) glucose concentrations. Granulosa cells from immature mice were cultured for 12 h in a medium containing 5 mM betaine in the presence of 5 mM or 30 mM glucose and then again cultured in a medium with the same component along with FSH (30 ng/ml) for another 12 h. qRT-PCR method was used to analysis of relative expression of studied gens. GAPDH was used as housekeeping gene. Results are means ± SEM for three independent experiments with triplicated wells. Bars with different numbers of asterisks are significantly different at p < 0.05
Effect of betaine treatment on apoptosis-related factors in granulosa cells cultured under hyperglycemic condition
To determine whether apoptosis has any role in the protective effects of betaine in granulosa cells exposed to high glucose medium, the expression of apoptosis-related genes and caspase-3 activity was measured in cells incubated with 30 mM glucose in the absence of FSH.
Significant downregulation of bcl2 antiapoptotic gene was observed after 24 h of 30 mM glucose treatment, whereas the bax apoptotic gene was upregulated at the same time point () (p < 0.05). The activity of caspase-3 was also increased in granulosa cells after exposure to 30 mM glucose compared to cells that were cultured in medium with basal glucose level () (p < 0.05). Altogether these results showed that high glucose concentration was able to induce apoptosis in granulosa cells.
Figure 4. Effect of betaine treatment on expression of apoptotic bax gene (A), antiapoptotic bcl2 gene (B), bax/bcl2 ratio (C) and caspase 3 activity (D) in mice granulosa cells exposed to low (5 mM) or high (30 mM) glucose concentrations. Granulosa cells from immature mice were cultured for 24 h in a medium containing 5 mM betaine in the presence of 5 mM or 30 mM glucose. Expression of apoptosis-related genes was analyzed using qRT-PCR method and GAPDH was used as housekeeping gene. The activity of caspase3 was determined in cell lysate of treated cells using a chromogenic substrate and expressed as IU/mg of cellular protein. Results are means ± SEM for three independent experiments with triplicated wells. Bars with different numbers of asterisks are significantly different at p < 0.05
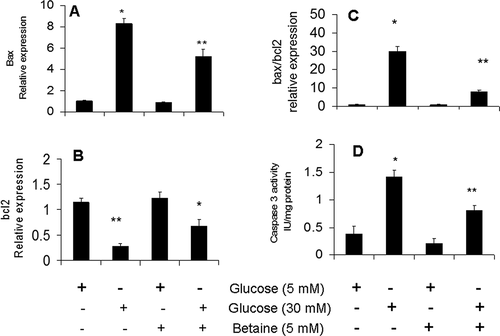
As shown in , apoptotic changes observed in granulosa cells exposed to 30 mM glucose were attenuated after their treatment with 5 mM betaine which were characterized by up-regulation of bcl2 and down-regulation of bax gene compared to untreated cells (p <.05) (). The increased activity of caspase-3 in granulosa cells cultured under hyperglycemic condition was attenuated after treatment with 5 mM betaine compared to untreated cells (p < 0.05) (). Betaine in culture medium containing basal glucose concentration had no significant effect on the expression of apoptosis-related genes (p > 0.05).
Changes in the expression ratio of bax/bcl2 at both basal and high concentrations of glucose in betaine-treated rat granulosa cells were shown in . Culture of granulosa cells under high glucose level significantly increased the expression ratio of bax/bcl2 (p < 0.05). Treatment of granulosa cells with betaine in the presence of 30 mM glucose reduced the bax/bcl2 ratio, significantly (p < 0.05). Betaine had no significant effect on the ratio of bax/bcl2 ratio in cells cultured in medium containing basal glucose concentration (p > 0.05).
Correlation analysis showed that there was a strong negative association between changes in bax/bcl2 ratio and cell viability, E2 and P4 levels in granulosa cells treated with betaine after exposure to hyperglycemic stress (p < 0.01). It was also found that cell viability had strong positive correlation with E2 and P4 concentrations in betaine treated cells under hyperglycemic condition (p < 0.01) ().
Table 1. Correlations between bax/bcl2 ratio, cell viability, and hormonal profiles in Betaine treated and untreated granulosa cells under hyperglycemic condition
Discussion
Hyperglycemia’s detrimental effects on the function of female reproductive tissue has been well documented (Wu et al. Citation2015; Wellons et al. Citation2017; Xu et al. Citation2019). Betaine is a widely distributed bioactive peptide in mammals, whose positive health effects have been shown in previous studies. Although it has been found that betaine accumulates in granulosa cumulus during germinal vesicle stage of oocyte development (Corbett et al. Citation2014), potential protective effects of betaine on functions of ovarian granulosa cells undergoing hyperglycemia are yet remained to be elucidated. In the present study, we studied the effect of betaine on gene expression linked to apoptosis and steroid hormone synthesis in mice granulosa cells exposed to high concentration of glucose.
Results of the current study indicated that a high concentration of glucose-induced granulosa cell apoptosis that was characterized by reduction of cell viability, decreased expression of the bcl2 gene and increased expression of both the bax gene and activity of caspase-3 in granulosa cells. Our observation confirmed the results of previous report that exposure of granulosa cells to a high concentration of glucose can lead to aberrant apoptosis of these cells (Wu et al. Citation2015). Moreover, in line with the results presented here, previous studies have shown that ovaries from diabetic mice had high bax gene expression; decreased expression of the bcl2 gene, and high activity of caspase-3 in granulosa cells (Wu et al. Citation2017; Fraunhoffer et al. Citation2018).
The findings of the current study revealed that culture of granulosa cells in the presence of high glucose for 24 h, reduced the expression of the key genes encoding steroidogenic enzymes including Cyp11a1, StAR, 3βHSD, and Cyp19a1 concurrent with reduced production of E2 and P4 into the culture medium. Chabrolle et al. (Citation2008) have previously reported a reduction in synthesis of both E2 and P4 as a result of exposure of rat primary granulosa cells to high glucose concentration and they also found that a decline in steroid hormone production is associated with downregulation of 3βHSD, Cyp11a1, StAR, and Cyp19a1 proteins. These results are consistent with a recent study that examined the effects of type 2 diabetes in mice in vivo and revealed a time-dependent decline in the levels of all these ovarian steroidogenic enzymes (Kannan et al. Citation2019). Based on the findings of current study and research performed in previous works, high concentrations of glucose have been shown to induce aberrant apoptosis of granulosa cells and minimize the expression of steroidogenic enzymes which can cause ovarian dysfunction leading to reproductive disorders such as oligomenorrhea, amenorrhea, polycystic ovary syndrome, and infertility.
The results of the current study, for the first time, indicated that when granulosa cells were treated with betaine under high glucose conditions, the harmful effects of high concentration of glucose on viability and steroidogenesis were attenuated. Cell viability of betaine-treated cells in the presence of 30 mM glucose was increased compared to untreated cells. Betaine treatment could also suppress the expression of bax apoptotic gene and the activity of caspase-3 gene and increased bcl2 gene expression and decreased bax/bcl2 ratios in granulosa cells exposed to high glucose concentration. These findings confirmed the protective effect of betaine against hyperglycemia-induced cytotoxicity in granulosa cells.
Several mechanisms may be suggested for protective effects of betaine in granulosa cells under high glucose conditions. In hyperglycemic conditions, glucose acts as an efficient serum osmolyte due to reduced absorption and entrapping in the extracellular compartment (Norris and Schermerhorn Citation2019). Cells are prone to hypertonic stress and shrinkage during hyperglycemia and diabetes (Burgos et al. Citation2019; Norris and Schermerhorn Citation2019). Cell shrinkage, by mechanical stress can induce endoplasmic reticulum stress (ERS), cytochromes c release and subsequent caspase-3 or caspase-12 activation and cellular apoptosis (Pihán et al. Citation2017; Burgos et al. Citation2019) Betaine is an important osmoprotectant which can be stored in large quantities in cells, increases the free water content of cells and the cytoplasmic cell volume to avoid shrinking under hyperosmotic conditions and inhibits different proteins related to hyperosmotic apoptosis by stabilizing proteins’ basic structures (Kempson et al. Citation2014; Zhao et al. Citation2018; Zhang et al. Citation2018). Taken together, it is possible that betaine may act as an osmoprotectant and prevent the activation of apoptotic factors and cell cytotoxicity in GCs under hyperosmotic conditions caused by high concentration of glucose.
Previous work of others has shown that hyperhomocysteinemia in diabetic patients can inhibit insulin signals and raise insulin resistance through activation of inflammatory cytokines, and also can induce cellular apoptosis by induction of ERS and elevation of oxidative damage (Tripathi et al. Citation2016; Feng and Xu Citation2017; Qi et al. Citation2017; Ansari et al. Citation2020). BHMT catalyzes conversion of homocysteine to methionine by addition of a methyl group from betaine (Zhao et al. Citation2018). This reaction along with methionine synthase (MS) is critical in animals for detoxification of homocysteine. It is therefore suggested that betaine may attenuate cellular apoptosis associated with hyperhomocysteinemia in granulosa cells under hyperglycemic condition. This hypothesis should be considered in future investigations.
Growing evidence (Boots and Jungheim Citation2015) has shown that aberrant inflammation and cytokine release in diabetes leads to impaired oocyte competence, anovulation, and associated infertility. Based on earlier studies it has been suggested that betaine attenuates the inflammation through suppression of NLRP3 inflammasome activation and inhibition of NF-κB signaling pathway; pathway that governs transcription of several pro-inflammatory genes and triggers apoptosis by activation of caspase-3 in hyperglycemic condition in ovary (Nayki et al. Citation2016; Zhao et al. Citation2018). The protective effect of betaine against apoptosis in granulosa cells under hyperglycemic conditions may be attributed to inhibition of such inflammatory pathways. The precise identification of these mechanisms in granulosa cells needs further investigation.
The results of this study, for the first time, demonstrated that betaine could improve steroidogenesis in granulosa cells under a hyperglycemic condition. Betaine increased the expression of Cyp11a1, StAR, 3βHSD, and Cyp19a1 genes, as well as the synthesis and secretion of E2 and P4 by granulosa cells. Our results showed that GCs cultured in basal medium (5 mM glucose) supplemented with FSH and betaine could secrete nonsignificant higher levels of E2 and P4 compared to GCs that cultured in FSH-free basal medium with betaine. A similar pattern was observed for 3β-HSD and Cyp19a1 gene expression. These findings suggest that betaine can not affect steroidogenesis pathway of granulosa cells at normal glucose condition but it can potentiate FSH induced steroid synthesis in GCs under physiological concentrations of glucose.
Data related to the impact of betaine on steroidogenesis are very limited. Recently, Abobakr et al. (Citation2017) found that StAR mRNA expression, a limiting step in the biosynthesis of steroid hormones that facilitates the translocation of cholesterol into the mitochondria, was significantly increased in adrenal tissue of laying hens after application of supplementary betaine.
Several mechanisms may be suggested for restoration of steroidogenesis by betaine in granulosa cells under high glucose condition. The previous study showed that hyperglycemia induces homocysteinylation of proteins and induces their accumulation or malfolding in the ER leading to a set of responses referred to as ERS (Tripathi et al. Citation2016). Hyperhomocysteinemia can suppress serum estrogen levels in homocysteine-treated animals (Qi et al. Citation2017). It has also been reported that promoting inflammatory factors such as IL-6, IL-8 and TNF-α suppress estradiol secretion by GCs and this effect is reversed by 4-PBA an ERS inhibitor or TAK242, a TLR4/NFκB pathway inhibitor (Lei et al. Citation2019). These data make it clear that hyperglycemia can interfere with steroidogenesis by inducing ERS and inflammation. Considering betaine’s function in reducing inflammation, hyperhomocysteinemia, and oxidative stress (Zhao et al. Citation2018); it is possible that improvement of adverse effects of hyperglycemia explained here, may have a mechanistic role in restoring steroid synthesis in granulosa cells under high glucose condition. Additional studies are needed to clarify the underlying cellular and molecular mechanisms related to the improvement of steroid synthesis by betaine in granulosa cells under hyperglycemic condition.
Materials and methods
Experimental animals and ethics
Immature female mice (25 days old) were obtained from the laboratory animal house of faculty of veterinary medicine of Shahid Chamran University of Ahvaz and housed under a 12 h light/dark schedule with food and water ad libitum. All protocols of the present study were approved by the research ethics committee of Shahid Chamran University of Ahvaz (EE/98.24.3.2.26576/scu.ac.ir). Working with them was carried out on the basis of the guideline for the care and use of laboratory animals (NIH publication no. 86–23).
Granulosa cell isolation and culture
The female mice were injected subcutaneously with 20 IU pregnant mare serum gonadotropin; PMSG (Sigma, USA) to induce follicular growth. Forty-eight hours later, the mice were euthanized and the ovaries were collected, separated from the associated fat, oviduct, and bursa ovary, and then placed in ice-cold Dulbecco’s modified Eagle medium: nutrient mixture F-12 (DMEM/F-12) (Gibco® Life Technologies, USA) containing 10 mM HEPES, 1% penicillin/streptomycin, and 10% fetal bovine serum, pH 7.4. Granulosa cells were liberated from large follicles to the medium by puncturing the antral follicle with a 26-gauge needle; cell dissociation was facilitated by efflux and influx of the cell suspension through a glass pipet. The cells were washed twice with culture medium and centrifuged at 1500 × RPM for 10 min, after which the supernatant was discarded. The collected cells were counted in the presence of Trypan blue staining. The purity of isolated GCs was determined using immunostaining for FSH receptor (FSHR) as a specific marker of granulosa cells (Supplementary material; S1).
Treatment protocol
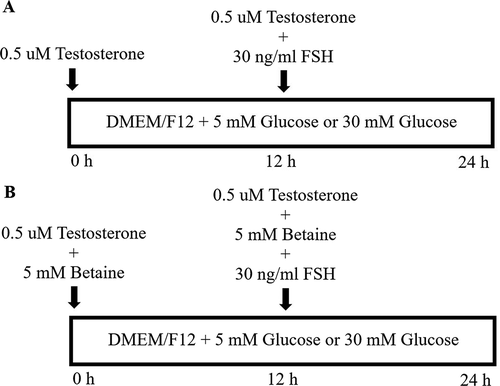
GCs were seeded onto a six well plate at a density of 106 cells/ml. The cells were pre-incubated for 24 h before the experiment. The medium was then replaced with fresh growth medium containing the appropriate reagents according to each experimental design. The basal medium used for various assays was DMEM/F-12-LG (low glucose; 5 mM) supplemented with 2% FBS, 1% antibiotics and 2 mM glutamine. To examine the effects of betaine on apoptosis and cell viability of GCs under high glucose condition, the cells were treated with betaine (5 mM) (Sigma, USA) for 24 h in the presence of low (basal medium; 5 mM) and high (30 mM) glucose concentrations. To determine the effects of betaine on steroidogenesis of GCs under high glucose condition, the cells were treated with betaine (5 mM) (Sigma, USA) for 12 h in the presence of basal medium (5 mM glucose) and high glucose condition (30 mM glucose) supplemented with 0.5 µM testosterone as substrate for estradiol synthesis. Subsequently, the steroidogenesis was induced by treatment of cells for 12 h with similar concentrations of betaine, glucose and testosterone, and FSH (30 ng/ml) (Ortega et al. Citation2012). Schematic diagram of steroidogenesis protocol is shown in . The dosage for betaine treatment was taken from previous cell culture studies in mice (Villa et al. Citation2017; Xiao et al. Citation2017).
At the end of the experiment, the growth media were collected and stored at −30°C until the subsequent hormone assay. Granulosa cells were frozen in 0.5 ml RNXTM Reagent (SinaClon Inc, Iran) and stored at −80°C until qRT-PCR analysis and caspase3 assay. All experiments were carried out in triplicate.
Cell viability assay
The viability of granulosa cells at the end of different experimental protocols was determined by the MTT assay. Briefly, cells were plated in 96 well culture plates (8 × 103 cells/well) and were grown in medium containing the appropriate reagents according to each experimental design for 24 h, then 10 µl of 5 mg/ml MTT (Sigma-Aldrich, MO, USA) stock solution was added to each well and the plate was incubated at 37°C for 3 h. The medium was replaced with 100 µl DMSO, and the absorbance was read at 570 nm using a microplate reader (BioTek, USA). Data are displayed as the percentage of viable cells in treated groups compared with control cells. The analysis was performed in three independent experiments with triplicated wells.
RNA isolation and cDNA synthesis
Total RNA was isolated using RNX TM reagent according to the manufacturer’s procedure (SinaClon, Iran). The purity of RNA at 260/280 OD ratio and the RNA integrity was evaluated using an Eppendorf µCuvette G1.0 microvolume measuring cell (Eppendorf, Germany). Reverse transcription was performed with the YTA cDNA synthesis kit (Yekta Tajhiz, Iran) using 1 µg of RNA and random hexamer primer as recommended by the manufacturer.
Primer design
The sequences of bax, Bcl2, Cyp11a1, StAR, 3βHSD, Cyp19a1and GAPDH genes were obtained from NCBI database and primer sets were designed via GeneRunner and Primer Express software v.3.0 (Applied Biosystems, Foster City, USA) and analyzed in Basic Local Alignment Search Tool (https://blast.ncbi.nlm.nih.gov/Blast.cgi) to avoid homology with other genome regions. Specific sets of primers (Bioneer, South Korea) designed for this study are shown in .
Table 2. Oligonucleotide sequences of primers used in the current study
Real-time quantitative RT-PCR
To evaluate the expression levels of apoptosis-related genes including bax and bcl2 and steroidogenesis-related genes, including Cyp11a1, StAR, 3βHSD, and Cyp19a1 in granulosa cells, real-time PCR analysis was performed using Roche Light-Cycler detection system (Basel, Switzerland) by the qPCRTM Green Master Kit for SYBR Green I® (Yekta Tajhiz, Iran). The relative expression levels of target transcripts were compared to mice GAPDH as a housekeeping gene. Reactions were performed in a 12.5 μL mixture containing 6.25 μL qPCRTM Green Master Kit for SYBR Green I® (YektaTajhiz, Iran), 0.25 μL of each primer (200 nM), 3 μL cDNA (100 ng) and 2.25 μL nuclease-free water. The PCR protocol used consisted of a 5 min denaturation at 94°C followed by 45 cycles of 94°C for 10 sec, 60°C for 15 sec and 72°C for 25 sec. Reactions were performed in triplicate. Two separate reactions without cDNA or with RNA were performed in parallel as controls. Relative quantification was performed according to the comparative 2−ΔΔCt method and using Lightcycler 96® software. Amplification efficiency for the target and reference genes was validated using fivefold dilution series of control cDNA template as 1:16, 1:8, 1:4, 1:2, and 1. Then, standard curve was drawn by plotting the logarithmic input cDNA dilution factor versus mean CT and the slope was determined. PCR reaction efficiency was calculated via the following formula: E = (10–1/slope)-1.
Estradiol and progesterone assay
The concentration of progesterone (P4) and estradiol (E2) in the culture medium of granulosa cells was measured at the end of the experiment by commercial ELISA kit according to the manufacturer’s instructions (DiaMetra, Italy). All samples were run in one assay to avoid inter-assay variation. The limits of detection of P4 and E2 were 0.05 ng/ml and 5 pg/ml, respectively. The intra-assay coefficients of variation of both hormones were less than 5%. All assays were carried out in duplicate.
Assay of caspase-3 activity
The caspase-3 activity was measured using a caspase-3 colorimetric assay kit according to the manufacturer’s instructions (Biovision, Mountain View, USA) by a spectrophotometer. The caspase-3 colorimetric assay was based on the hydrolysis of the peptide substrate acetyl-Asp-Glu-Val-Asp p-nitroanilide (Ac-DEVD-pNA) by caspase-3, resulting in the release of the p-nitroaniline (pNA) moiety. p-Nitroaniline has a high absorbance at 405 nm (εmM = 10.5). The concentration of the pNA released from the substrate is calculated from the absorbance values at 405 nm. Enzyme activity was defined as produced micromole pNA/min/mg tissue protein and expressed as IU/mg protein. Protein concentration was measured using Bradford method and bovine serum albumin as standard.
Statistical analysis
Statistical analysis was conducted using SPSS 22 software (SPSS Inc., Chicago, IL, USA). All experiments were done in triplicate and data are presented as the mean ± SEM. Statistical differences were determined by one-way analysis of variance (ANOVA). Tukey test was used to estimate the significance of the results. The relationship between bax/bcl2 ratio and cell viability and E2 and P4 levels was measured by Pearson correlation test. The level of significance for all tests was set at P < 0.05.
Ethics approval
All protocols of the present study were approved by the research ethics committee of Shahid Chamran University of Ahvaz (EE/98.24.3.2.26576/scu.ac.ir).
Author contributions
All authors contributed to the study conception and design. MRT and MA designed the study. KAS performed the study and collected the data. MRT and MA analyzed the results. MRT and KAS drafted the article. MRT and MA revised the article critically for important intellectual content. All authors gave final approval of the version to be published. MRT is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Supplemental Material
Download MS Word (1.2 MB)Acknowledgments
This work was funded by a grant from Shahid Chamran University of Ahvaz Research Council (Grant No: SCU.VB98.231).
Disclosure statement
Authors declare that there is no conflict of interest.
Supplementary material
Supplemental data for this article can be accessed here.
References
- Abobakr H, Hu Y, Hou Z, Sun Q, Idriss AA, Omer NA, Zong Y, Zhao R. 2017. Dietary betaine supplementation increases adrenal expression of steroidogenic acute regulatory protein and yolk deposition of corticosterone in laying hens. Poult Sci. 96(12):4389–4398. doi:https://doi.org/10.3382/ps/pex241.
- Alirezaei M, Niknam P, Jelodar G. 2012. Betaine elevates ovarian antioxidant enzyme activities and demonstrates methyl donor effect in non-pregnant rats. Int J Pep Res Ther. 18(3):281–290. doi:https://doi.org/10.1007/s10989-012-9300-5.
- Ansari T, Jami A, Rabbani B, Khalil M, Jamal Q. 2020. Frequency of elevated plasma homocysteine (Hcy) levels among type 2 Diabetes mellitus (T2DM) patients. Professional Med. 27(3):635–640. doi:https://doi.org/10.29309/TPMJ/2020.27.03.4169.
- Boots CE, Jungheim ES. 2015. Inflammation and human ovarian follicular dynamics. Sem Reprod Med. 33(4):270–275. doi:https://doi.org/10.1055/s-0035-1554928.
- Burgos JI, Morell M, Mariángelo JI, Petroff MV. 2019. Hyperosmotic stress promotes endoplasmic reticulum stress-dependent apoptosis in adult rat cardiac myocytes. Apoptosis. 24(9–10):785–797. doi:https://doi.org/10.1007/s10495-019-01558-4.
- Casarini L, Riccetti L, De Pascali F, Nicoli A, Tagliavini S, Trenti T, La Sala GB, Simoni M. 2016. Follicle-stimulating hormone potentiates the steroidogenic activity of chorionic gonadotropin and the anti-apoptotic activity of luteinizing hormone in human granulosa-lutein cells in vitro. Mol Cel Endocrin. 422:103–114. doi:https://doi.org/10.1016/j.mce.2015.12.008.
- Chabrolle C, JeanPierre E, Tosca L, Ramé C, Dupont J. 2008. Effects of high levels of glucose on the steroidogenesis and the expression of adiponectin receptors in rat ovarian cells. Reprod Biol Endocrinol. 6(1):11. doi:https://doi.org/10.1186/1477-7827-6-11.
- Corbett HE, Dubé CD, Slow S, Lever M, Trasler JM, Baltz JM. 2014. Uptake of betaine into mouse cumulus-oocyte complexes via the SLC7A6 isoform of y+ L transporter. Biol Reprod. 90(4):811–819. doi:https://doi.org/10.1095/biolreprod.113.116939.
- Doblado M, Zhang L, Toloubeydokhti T, Garzo GT, Chang RJ, Duleba AJ. 2020. Androgens modulate rat granulosa cell steroidogenesis. Reprod Sci. 27(4):1002–1007.
- Feng X, Xu Y. 2017. Hyperhomocysteinemia as a metabolic risk factor for glucose intolerance among high-risk groups of chinese adults. Med Sci Monit. 23:2775–2781. doi:https://doi.org/10.12659/MSM.905002.
- Fraunhoffer NA, Abuelafia AM, Barrientos MA, Cimerman KV, Olmos MF, Chuluyan E, Barrios M. 2018. Long-term apoptosis-related protein expression in the diabetic mouse ovary. PLoS One. 13(9):e0203268. doi:https://doi.org/10.1371/journal.pone.0203268.
- Gothai S, Ganesan P, Park SY, Fakurazi S, Choi DK, Arulselvan P. 2016. Natural phyto-bioactive compounds for the treatment of type 2 diabetes: inflammation as a target. Nutrients. 8(8):461. doi:https://doi.org/10.3390/nu8080461.
- Kannan S, Srinivasan D, Raghupathy PB, Bhaskaran RS. 2019. Association between duration of obesity and severity of ovarian dysfunction in rat-cafeteria diet approach. J Nutr Biochem. 71:132–143. doi:https://doi.org/10.1016/j.jnutbio.2019.05.012.
- Kato A, Tatsumi Y, Yako H, Sango K, Himeno T, Kondo M, Kato Y, Kamiya H, Nakamura J, Kato K. 2019. Recurrent short-term hypoglycemia and hyperglycemia induce apoptosis and oxidative stress via the ER stress response in immortalized adult mouse Schwann (IMS32) cells. Neurosci Res. 147:26–32. doi:https://doi.org/10.1016/j.neures.2018.11.004.
- Kempson SA, Zhou Y, Danbolt NC. 2014. The betaine/GABA transporter and betaine: roles in brain, kidney, and liver. Front Physiol. 5:159. doi:https://doi.org/10.3389/fphys.2014.00159.
- Lee J, Lee HC, Kim SY, Cho GJ, Woodruff TK. 2019. Poorly-controlled type 1 diabetes mellitus impairs LH-LHCGR signaling in the ovaries and decreases female fertility in mice. Yonsei Med J. 60(7):667–678. doi:https://doi.org/10.3349/ymj.2019.60.7.667.
- Lei L, Ge J, Zhao H, Wang X, Yang L. 2019. Role of endoplasmic reticulum stress in lipopolysaccharide-inhibited mouse granulosa cell estradiol production. J Rep Dev. 65:459–465. doi:https://doi.org/10.1262/jrd.2019-052.
- Nayki U, Onk D, Balci G, Nayki C, Onk A, Gunay M. 2016. The effects of diabetes mellitus on ovarian injury and reserve: an experimental study. Gynecol Obstet Invest. 81(5):424–429. doi:https://doi.org/10.1159/000442287.
- Nazari M, Moghimipour E, Tabandeh MR. 2017. Betaine down regulates apelin gene expression in cardiac and adipose tissues of insulin resistant diabetic rats fed by high-calorie diet. Int J Pept Res Ther. 23(2):181–190. doi:https://doi.org/10.1007/s10989-016-9551-7.
- Norris OC, Schermerhorn T. 2019. The mean cell volume difference (dMCV) reflects serum hypertonicity in diabetic dogs. PLoS One. 14(7):e0219864. doi:https://doi.org/10.1371/journal.pone.0219864.
- Ortega I, Wong DH, Villanueva JA, Cress AB, Sokalska A, Stanley SD, Duleba AJ. 2012. Effects of resveratrol on growth and function of rat ovarian granulosa cells. Fertil Steril. 98(6):1563–1573. doi:https://doi.org/10.1016/j.fertnstert.2012.08.004.
- Pihán P, Carreras-Sureda A, Hetz C. 2017. BCL-2 family: integrating stress responses at the ER to control cell demise. Cell Death Differ. 24(9):1478–1487. doi:https://doi.org/10.1038/cdd.2017.82.
- Qi X, Zhang B, Zhao Y, Li R, Chang HM, Pang Y, Qiao J. 2017. Hyperhomocysteinemia promotes insulin resistance and adipose tissue inflammation in PCOS mice through modulating M2 macrophage polarization via estrogen suppression. Endocrinology. 158(5):1181–1193. doi:https://doi.org/10.1210/en.2017-00039.
- Salahi P, Alirezaei M, Kheradmand A, Rocky A. 2019. Betaine: a promising micronutrient in diet intervention for ameliorating maternal blood biochemical alterations in gestational diabetes mellitus. Int J Pept Res Ther. 26:1177–1184.
- Teodoro AJ. 2019. Bioactive compounds of food: their role in the prevention and treatment of diseases. Oxid Med Cell Longev. 2019:1–4. doi:https://doi.org/10.1155/2019/3765986.
- Tripathi M, Zhang CW, Singh BK, Sinha RA, Moe KT, DeSilva DA, Yen PM. 2016. Hyperhomocysteinemia causes ER stress and impaired autophagy that is reversed by Vitamin B supplementation. Cell Death Dis. 7(12):e2513. doi:https://doi.org/10.1038/cddis.2016.374.
- Tuncel E, Durgun O, Peynirci H, Ersoy C. 2017. Sexual dysfunction in female patients with type 2 diabetes mellitus: a cross-sectional single-centre study among Turkish patients. Hum Fertil. 20(3):192–199. doi:https://doi.org/10.1080/14647273.2016.1266039.
- Villa I, Senesi P, Montesano A, Ferraretto A, Vacante F, Spinello A, Bottani M, Bolamperti S, Rubinacci A, Luzi L, et al. 2017. Betaine promotes cell differentiation of human osteoblasts in primary culture. J Transl Med. 15(1):132. doi:https://doi.org/10.1186/s12967-017-1233-5.
- Wellons MF, Matthews JJ, Kim C. 2017. Ovarian aging in women with diabetes: an overview. Maturitas. 96:109–113. doi:https://doi.org/10.1016/j.maturitas.2016.11.019.
- Wu Y, Li Y, Liao X, Wang Z, Li R, Zou S, Jiang T, Zheng B, Duan P, Xiao J. 2017. Diabetes induces abnormal ovarian function via triggering apoptosis of granulosa cells and suppressing ovarian angiogenesis. Int J Biol Sci. 13(10):1297–1308. doi:https://doi.org/10.7150/ijbs.21172.
- Wu Y, Zhang Z, Liao X, Wang Z. 2015. High fat diet triggers cell cycle arrest and excessive apoptosis of granulosa cells during the follicular development. Biochem Biophys Res Commun. 466(3):599–605. doi:https://doi.org/10.1016/j.bbrc.2015.09.096.
- Xiao Y, Rungruang S, Hall LW, Collier JL, Dunshea FR, Collier RJ. 2017. Effects of niacin and betaine on bovine mammary and uterine cells exposed to thermal shock in vitro. Int J Dairy Sci. 100(5):4025–4037. doi:https://doi.org/10.3168/jds.2016-11876.
- Xu B, Hu Y, Luo YT, Xu H, Qi SH, Zhang YY, Zhao D, Yuan DZ. 2019. Changes in expression of follicular glucose transporters may be involved in ovarian function impairment during diabetic hyperglycemia. Ann Clin Lab Sci. 49(6):785–793.
- Zhang D, Jing H, Dou C, Zhang L, Wu X, Wu Q, Song H, Li D, Wu F, Liu Y, et al. 2018. Supplement of betaine into embryo culture medium can rescue injury effect of ethanol on mouse embryo development. Sci Rep. 8(1):1–13. doi:https://doi.org/10.1038/s41598-017-17765-5.
- Zhao G, He F, Wu C, Li P, Li N, Deng J, Zhu G, Ren W, Peng Y. 2018. Betaine in inflammation: mechanistic aspects and applications. Front Immunol. 9:1070. doi:https://doi.org/10.3389/fimmu.2018.01070.