ABSTRACT
Prenatal tobacco-smoke exposure negatively affects the reproductive functions of female offspring and oxidative stress plays a major role at this point. Alpha-lipoic acid (ALA), well known as a biological antioxidant, has been used as a nutritional supplement and as a therapeutic agent in the treatment of certain complications during pregnancy. We aimed to investigate the effects of maternal tobacco-smoke exposure and/or ALA administration on puberty onset, sexual behavior, gonadotrophin levels, apoptosis-related genes, apoptotic cell numbers and oxidative stress markers in the adult female rat offspring. Sprague-Dawley rats were divided into four groups; control, tobacco smoke (TS), TS+ALA and ALA groups. Animals were exposed to TS and/or ALA for 8 weeks before pregnancy and throughout pregnancy. All treatments ended with birth and later newborn female rats were selected for each experimental group. The experiment ended at postnatal day 74–77. Maternal tobacco smoke advanced the onset of puberty in the female offspring of the TS group (p < 0.05). In all treatment groups; the mean number of anogenital investigations and lordosis quality scores showed a decline, serum luteinizing hormone levels significantly increased (p < 0.05) and several histopathological changes in ovaries were observed compared to the control group. In addition, an increase in apoptotic marker levels and apoptotic cell numbers was detected in the ovaries of all treatment groups. Decreased TAS and increased TOS levels were detected in all treatment groups compared to control. These findings suggested that maternal tobacco smoke and/or ALA administration may be leading to the impaired reproductive health of female offspring.
Abbreviations: ALA: alpha-lipoic acid; LH: luteinizing hormone; FSH: follicle-stimulating hormone; TAS: total antioxidant status; TOS: total oxidant status; Apaf1: apoptotic protease-activating factor 1; Casp3: caspase 3; Casp9: caspase 9; CF: cyst follicles; 4-HNE: 4-Hidroxynonenal; 8-OHdG: 8-hydroxydeoxyguanosine; TUNEL: terminal deoxynucleotidyl transferase-mediated deoxyuridine-biotin nick end labeling; ROS: reactive oxygen species; GnRHR: gonadotropin-releasing hormone receptor; HPG: hypothalamic–pituitary–gonadal; AMPK: AMP-activated protein kinase; ELISA: enzyme-linked immunosorbent assay; cDNA: complementary DNA; qPCR: quantitative real-time PCR; FC: follicular cysts; PF: primary follicle; SF: secondary follicle; GF: graafian follicle; CL: corpus luteum; DF: degenerated follicle; AF: atretic follicle
Introduction
Exposure to maternal cigarette smoke during pregnancy causes harmful consequences such as preterm birth, fetal growth retardation and increased mortality-morbidity. In addition, it has been documented that one of its long-term effects on children is reduced fertility (Habek et al. Citation2002; Rogers Citation2008). Studies showed that prenatal exposure to cigarette smoke adversely affects fertility by inducing early onset menarche, decreasing conception rates, changing the ovarian size and the number of follicles, disrupting endocrine hormone levels and early menopause of female offspring (Ernst et al. Citation2012; Camlin et al. Citation2016). Reproductive health and sexuality issues have a role in our day to day living and not much has been documented about the sexual behavior of adolescents exposed to prenatal tobacco smoke. Pathophysiological mechanisms related to sexual dysfunction caused by prenatal cigarette smoke exposure in children are still not clear. Cigarette smoking may affect sexual behaviors by negatively affecting sex hormone levels which are necessary for sexual desire and sexual differentiation (Park et al. Citation2011). Many experimental and clinical observations show that free radicals play a potential role in the occurrence of this event (Park et al. Citation2011; Yalın and Mete Citation2011). Alpha-lipoic acid (ALA) is an important coenzyme of mitochondrial multienzyme complexes and is known as an antioxidant involved in various biological processes (Packer et al. Citation1995). The administration of ALA in pregnancy is based on the following scientific evidence. ALA is a natural molecule that is very useful in preventing miscarriages and premature birth thanks to its antioxidant, anti-inflammatory and immunomodulatory properties. However, information on the safety of ALA use during pregnancy is still unclear. Until now, a few studies have been conducted with a significant number of pregnant women to confirm the safety of its continuous administration. One study reported that 600 mg of ALA administered to pregnant mothers for at least 7 weeks of pregnancy did not show any adverse effects on the mother and newborns. (Parente et al. Citation2017). In animal studies, it has been demonstrated that the use of ALA has a protective effect on the fetus in mothers exposed to toxic pollutants (Al Ghafli et al. Citation2004; Koga et al. Citation2012). Besides, studies on the use of ALA in pregnant women are mostly related to congenital malformations caused by diabetes (Sugimura et al. Citation2009; Tomo et al. Citation2015). There are limited studies suggesting that ALA supports ovarian follicle development (Talebi et al. Citation2012). There has been no study on how maternal ALA administration affects reproductive health and sexual behavior in female offspring. However, reports indicated above suggest that ALA may have a protective effect on maternal tobacco exposure.
Therefore, we purposed to investigate the effects of maternal tobacco exposure and ALA administration on pubertal maturation, sexual behaviors, gonadotropin levels (FSH and LH), ovarian tissue, oxidative stress markers (TAS/TOS), apoptotic cell numbers and apoptosis-related genes in the adult female rat offspring. In particular, we aimed to investigate the long-term reproductive health outcomes of female offspring exposed to maternal tobacco smoke.
Results
Effects of TS and/or ALA on puberty parameters
The mean control age at vaginal opening was 52.3 ± 2.8 days (range: 50–57 days, ). Maternal tobacco smoke significantly advanced vaginal opening compared to control female rats (46.6 ± 2.9 days for the TS group, p < 0.05). All of the female rats in the TS group completed vaginal opening on PND 50, while about 40% of the control female rats showed complete canalization of the vagina at the same age (). However, the day of vaginal opening of female rats was unaffected by ALA or TS+ALA exposure compared to the control female rats (49.6 ± 3.3 days, 52.7 ± 3.7 days and 52.3 ± 2.8 days, respectively, ).
Figure 1. Effects of maternal tobacco smoke and/or ALA treatment on pubertal maturation in female offspring. (A) Cumulative percentage of female rats showing vaginal opening according to age. In the tobacco-smoke group, significantly advanced vaginal opening compared to control female rats (p < 0.05). However, there was no significant difference in ALA or TS + ALA groups compared to the control group. (B) Pubertal weights of female offspring. There is a significant increase in pubertal weight belonging to the ALA group compared to control, TS and TS+ALA groups. There is no significant difference between TS, TS + ALA and control groups. Each experimental group consisted of 7 rats. Data are given as mean ± SD. * p < 0.001 (one-way ANOVA and post hoc Tukey test). VO: Vaginal opening, ALA: Alfa lipoic acid, TS: Tobacco smoke.
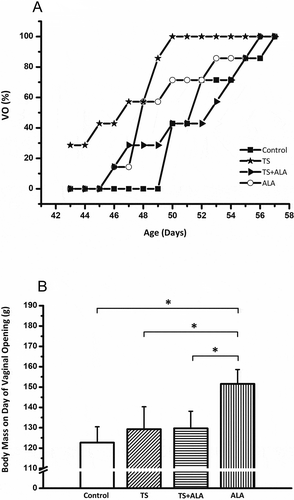
There was a significant increase in pubertal weight in the ALA group compared to the control group (151.6 ± 7.1 g and 122.6 ± 7.8 g, respectively, p < 0.001, ). However, when compared to the control group, it was shown that pubertal weights did not change in the TS or TS+ALA groups (122.6 ± 7.8 g, 129.3 ± 11 g and 129.7 ± 8.4 g, respectively, ). In addition, pubertal weight was significantly higher in the ALA group when compared with the TS and TS+ALA groups (p < 0.001, ).
Effects of TS and/or ALA on estrous cyclicity
The estrous cycle of female rats treated with TS and/or ALA was not significantly affected. Therefore, the mean total number of estrous cycles (2.4 ± 0.5, 2 ± 1, 2 ± 0.6 and 2.2 ± 0.8, respectively, p > 0.05 ) and average duration of proestrus, estrus, metestrus and diestrus phases were not significantly different in all treatment groups compared to the control group (proestrus: 5 ± 0.8, 4.3 ± 1.2, 4.2 ± 0.6 and 4.4 ± 1.1; estrus: 2.4 ± 0.5, 2.6 ± 1, 3.3 ± 1 and 2.8 ± 0.8; metestrus: 2.8 ± 0.4, 3.7 ± 1.6, 2 ± 1.1 and 2.8 ± 2.5; diestrus: 4.2 ± 1.3, 4 ± 1.5, 5.6 ± 1 and 4.4 ± 2.4, respectively, p > 0.05 ).
Table 1. Effects of TS and/or ALA on estrous cyclicity in female rats
Effects of TS and/or ALA on sexual behavior parameters
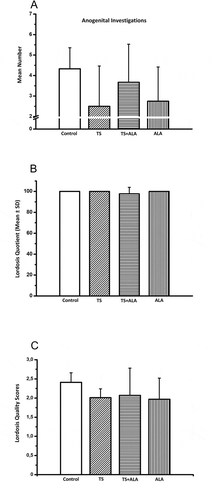
There was no significant change in all sexual behavior parameters including frequency of anogenital investigations, lordosis quotient and lordosis quality in all treatment groups compared to the control group. However, the mean number of anogenital investigations was slightly lower in TS, ALA and TS+ALA groups compared to control group (2.5 ± 1.9, 2.8 ± 1.6, 3.7 ± 1.8 and 4.3 ± 1, respectively, ). Although the lordosis quotient was similar in all groups (TS, ALA and control groups were 100%, TS+ALA group was 97.6 ± 6.3%), there was a tendency of decline in the lordosis quality scores in all treatment groups compared to control group (2 ± 0.2, 2 ± 0.5, 2.1 ± 0.7 and 2.4 ± 0.2 for TS, ALA, TS+ALA and control groups, respectively, ).
Figure 2. Effects of maternal tobacco smoke and/or ALA treatment on sexual motivation and receptivity in female offspring. (A) Frequency of anogenital investigations. (B) Lordosis quotient (lordoses/mounts x 100). (C) Lordosis quality scores. Each experimental group consisted of 7 rats. Data are given as mean ± SD. * p < 0.001 with control group (one-way ANOVA and post hoc Tukey test). ALA: Alfa lipoic acid, TS: Tobacco smoke.
Effects of TS and/or ALA on LH, FSH, total antioxidant status and total oxidant status levels
The LH levels were significantly increased in TS, TS+ALA and ALA and groups compared with the control group (2.27 ± 0.22 mIU/mL, 2.7 ± 0.33 mIU/mL, 2.67 ± 0.24 mIU/mL and 2.65 ± 0.32 mIU/mL, respectively, p < 0.05 ()). However, there was no significant change in serum FSH levels between groups (2.47 ± 0.32 mIU/mL, 2.74 ± 0.39 mIU/mL, 2.53 ± 0.16 mIU/mL and 2.67 ± 0.34 mIU/mL, respectively, p > 0.05 ()).
Table 2. Effects of TS and/or ALA on LH, FSH (mlU/ml), TAS and TOS levels (pg/mL) in female rats (n= 7 for each group).
Effects of TS and/or ALA on expression levels of apoptosis-related genes in rat ovary tissue
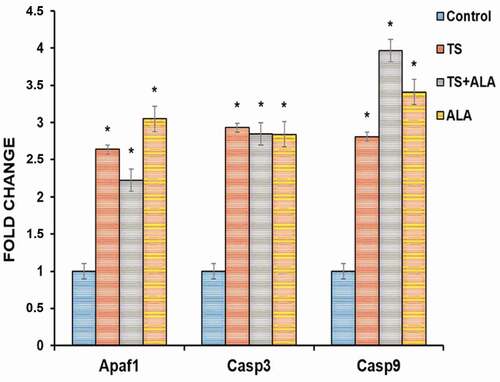
Apaf1, Casp3 and Casp9 mRNA levels showed a statistically significant increase in TS, TS+ALA and ALA groups compared with the control group (respectively, p = 0.025, 0.038, 0.016; p = 0.020, 0.020, 0.010; p = 0.022, 0.011 and 0.013) ().
Figure 3. Alteration of Apaf1, Casp 3 and Casp 9 mRNA expression levels in female offspring ovaries of tobacco smoke and/or ALA treatment. Compared to control group; Apaf1, Casp 3 and Casp 9 gene expressions significantly increased in TS, TS+ALA and ALA groups (p < 0.05). Each experimental group consisted of 7 rats. Data are given as mean ± SD. * p < 0.05 (one-way ANOVA and post hoc Tukey test). ALA: Alfa lipoic acid, TS: Tobacco smoke.
Histological effects of TS and/or ALA on rat ovary tissue
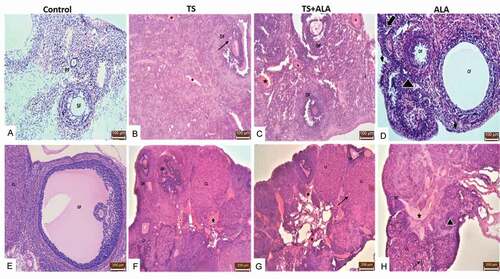
Histological examinations with a light microscope of ovary tissues demonstrated normal morphology in the control group ( A,E). When compared with the control group; degeneration of some follicle, vascular congestion, cyst follicles (CF), germinal epithelial degeneration, increased inflammatory cell number and vacuolization in granulosa and luteal cells were observed in TS, TS+ALA and ALA groups B,C,D,F,G,H). İn addition, atretic follicles were detected at different stages of follicular development in TS, TS+ALA and ALA groups. The results of the histological scoring are shown in .
Table 3. The histopathological scores of ovaries
Figure 4. Photomicrographs of the effects of maternal tobacco smoke and/or ALA treatment on offspring ovary tissue: (A,E) control groups; normal ovarian tissue and some follicles; primary follicle (PF), secondary follicle (SF), Graafian follicle (GF), corpus luteum (CL) (B,F) (x20, x10 respectively) TS groups; ovarian tissue showing degenerated follicle (DF), vascular congestion (star), atretic follicle (AF), cystic follicle (CF) vacuolization in granulosa cells (black arrow) (C,G) (x20, x10 respectively) TS+ALA groups; ovarian tissue showing vacuolization in corpus luteum (black arrow), vascular congestion (star), degenerated follicle (DF) (D,H) (x20, x10 respectively) ALA groups; ovarian tissue showing germinal epithelial degeneration (arrowhead), decreased primordial follicle (thick arrow), increased inflammatory cell (triangle) (x20, x10 respectively) (Hematoxylin&Eosin).
Effects of TS and/or ALA on ovarian follicle and corpus luteum counts
In TS, TS + ALA and ALA groups, the number of ovarian follicles (primordial, primary, secondary and graafianfollicle) decreased compared to the control group (respectively, 9.8 ± 1.9, 5.4 ± 1.14, 5.8 ± 0.83 and 6.2 ± 0.83; 6.8 ± 0.83, 3.0 ± 0.70, 2.6 ± 0.54 and 3.2 ± 0.44; 8.0 ± 1.58, 3.6 ± 1.14, 3.4 ± 0.54 and 4.0 ± 0.7; 3.6 ± 1.14, 1.4 ± 0,54, 1.2 ± 0.83 and 1.6 ± 0.64 (p < 0.05) ()). In the ovaries of the TS and TS + ALA groups, the total number of follicles decreased by approximately 53% and 46% in ALA groups compared to the control group. When compared to the control group; the number of corpus luteum numbers also decreased in TS, TS + ALA and ALA groups (respectively, 12.63 ± 1.14, 7.0 ± 0.83, 6.92 ± 1.12 and 7.2 ± 0.92 (p < 0.05) ()).
Table 4. Effects of TS and/or ALA on ovarian follicle and corpus luteum count in female rats (n= 7 for each group).
Apoptotic cell and immunohistochemical findings
As a result of microscopic examination, 8-OHdG and 4-HNE, which are markers of oxidative stress, immunoreactivities were observed in regressed corpus luteum, theca cells and granulosa cells of follicles. 8-OHdG and 4-HNE indicated positivity in both nucleus and cytoplasm. Significantly increased 8-OHdG and 4-HNE immunoreactivities were observed in TS, TS+ALA and ALA groups compared with the control group (respectively, 0.14 ± 0.08, 1.68 ± 0.69, 1.74 ± 0.61 and 1.38 ± 0.4; 0.22 ± 0.17, 1.74 ± 0.61, 1.86 ± 0.53 and 1.56 ± 0.32 (p < 0.05) ()). The results of the 8-OHdG and 4-HNE immunoreactivity histoscores are shown in .
Table 5. Apoptotic index (%), 8-OHdG and 4-HNE staining histoscores in groups
Figure 5. Arrows indicate TUNEL positive cells and 8-OHdG and 4-HNE immunoreactivities of the groups. Compared to control group, TUNEL positive cells, 8-OHdG and 4-HNE immunoreactivities significantly increased in TS, TS+ALA and ALA groups (p < 0.05). The areas stained in red indicated 8-OHdG or 4-HNE immunopositive staining. Also, brown nuclei indicated TUNEL positive cells. All slides were counterstained with hematoxylin (x20). TUNEL: Terminal deoxynucleotidyl transferase-mediated deoxyuridine-biotin nick end labeling, 8-OHdG: 8-hydroxydeoxyguanosine, 4-HNE: 4-Hidroxynonenal.
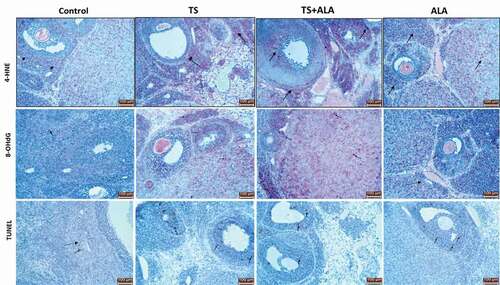
When TUNEL findings were examined, apoptotic cells were found to be significantly higher in TS, TS + ALA and ALA groups compared to control group (2.8 ± 0.83, 19.68 ± 2.32, 22.24 ± 1.51 and 19.28 ± 2.16, respectively (p < 0.05) ; ).
Discussion
This study represents the first comprehensive rat model of prenatal tobacco smoke and ALA administration on female offspring reproductive function. It reinforces existing literature commenting that maternal exposure affects female fertility yield, that is currently being discussed due to contradictory studies.
Exposure to fetal and neonatal nicotine causes a wide range of short- and long-term health problems, including postnatal reproductive dysfunction in offspring (Bruin et al. Citation2010; Behl et al. Citation2013). These reproductive disruptions may be connected to abnormal fetal gonadal development and/or impaired postnatal gonadal function (Hakonsen et al. Citation2014). It is known that smoking causes a direct toxic effect on tissues by causing an increase in reactive oxygen species (ROS) and this increase causes oxidative stress by changing oxidative balance in tissues (Sena and Chandel Citation2012). In a study with rats, nicotine exposure during intrauterine and/or lactation periods has been found to increase ROS levels in offspring (Camargo et al. Citation2014). In our study, in accordance with the aforementioned literature, it was observed that exposure to tobacco smoke increased TOS levels, which are ROS markers. Besides, compared to the control group, a statistically significant increase in the 8-OHdG and 4-HNE immunoreactivities, which is the oxidative stress marker induced by ROS, was detected in the TS groups.
Under long-term oxidative stress conditions, the unstable reactivity of ROS causes free radical damage in DNA, protein, carbohydrate and lipids and eventually mitochondrial-mediated apoptosis and cell death (Cao and Kaufman Citation2014). Studies in mice and rats have been reported to decrease levels of anti-apoptotic Bcl-2 in ovaries, increase proapoptotic markers such as Bax, Casp3 and DNA fragmentation, and decrease follicle survival and cell viability as a result of cigarette smoke or PAH exposure (Tsai-Turton and Luderer Citation2006; Neal et al. Citation2010). In our study, the increase in Apaf-1, Casp3 and Casp9 mRNA expression, which are the proapoptotic markers involved in apoptosis in the ovarian tissues of the TS groups, was one of the findings supporting the increase in apoptotic cells. Based on these findings, we can say that oxidative stress, which we think is associated with increased ROS in the groups, causes a rise in the number of apoptotic cells.
Excessive ROS production affects follicle development by increasing apoptosis in ovaries, and particularly primordial follicles are sensitive to cigarette smoke (Gannon et al. Citation2012). In addition, increased ROS has been reported to impair corpus luteal cell integrity and is associated with the function of the corpus luteum (Sawada and Carlson Citation1996). In our study, supporting prior studies, there was a significant decrease in the number of follicles belonging to the TS group compared to the control group. Since primordial follicle numbers are indicative of ovarian reserve and reproductive functions, we believe that the decrease in this follicle pool may lead to early ovarian failure and affect fertility of rats in puberty. Also, a decline in the number of corpus luteum was observed in the TS group and vacuolization in luteal cells was the finding supporting that ROS causes degeneration in the corpus luteum. This also revealed that tobacco-smoke exposure can have an endocrine-disrupting effect by inducing corpus luteum degeneration. In the light of all this information, it shows that the abnormal oxidative stress levels, increased cellular apoptosis, the abnormal relationship between oocyte and granulosa cells, impaired oocyte nuclear function and corpus luteal cell integrity may play a role in folliculogenesis and potential mechanisms underlying the impaired reproduction caused by tobacco smoke.
There is no definitive information about the endocrine effects of smoking (Tweed et al. Citation2012). Studies have reported that nicotine exposure during pregnancy can affect the hypothalamic–pituitary–gonadal (HPG) axis, causing changes in gonad development, thereby, affecting the fertility of the offspring (Richburg and Dwyer Citation2010; Camlin et al. Citation2016). ROS plays a role in regulating gonadotropin-releasing hormone receptor (GnRHR) signals by taking part in the activation of the MAP kinase signal cascade in gonadotropes, inactivation of negative feedback mechanisms of the pituitary hormones, and the participation of hormones in the exocytosis process (Terasaka et al. Citation2017). Besides, it has been demonstrated that ROS increases calcium in the cell by means of micro-RNA modulation and Gaq/11 signaling of gonadotropins, while increased calcium increases ROS in the cell, thereby, regulating GnRHR activation by both inhibition of negative feedback mechanisms and stimulation of exocytosis. GnRHR induced by these routes will thus increase the production of gonadotropin from the pituitary (Nguyen et al. Citation2010; Lannes et al. Citation2015). In our study, based on TAS/TOS ratios obtained from the groups, we think that the increase in ROS occurred in the other three groups compared to the control group. We believe that there may be a link between increased ROS in the groups and the stimulation of GnRH receptors in the pituitary, production of GnRH in the hypothalamus and hormone exocytosis secreted by gonadotropins in the pituitary, and elevated serum LH levels. It was also elicited that high LH levels were connected with ovarian histopathology. Studies of transgenic mice in which FSH hormone levels were kept constant but serum LH levels were kept high, the presence of disrupted and thinning granulosa cells in the ovaries, abnormal preovulatory follicles filled with blood in the antrum, loss of primordial follicle and hemorrhagic cystic follicles were detected (Risma et al. Citation1997; Flaws et al. Citation1997). Studies have shown that increased LH binds to LH receptors on the surface of antral and primordial follicles induce apoptosis and causes follicle loss (Teerds and Dorrington Citation1995). In our study, it was observed that LH levels measured in the diestrus stage increased significantly in TS, TS+ALA and ALA groups, and this increase was thought to be related to increased apoptotic cell numbers in the follicles and histopathological findings.
Sexual dysfunction is more common in women who smoke, compared to nonsmokers (Çiftçi et al. Citation2015). However, the pathophysiological mechanisms related to smoking, the relationship between women’s sexual dysfunction and sexual behavior is still unclear. In one study, it was observed that there was a significant decrease in the number of female rats approaching male rats exposed to cigarette smoke (Beck and Biały Citation2000). The studies of Harte and Meston’s revealed that acute nicotine exposure reduced genital arousal by 30% and disrupted the normal sexual response (Harte and Meston Citation2008). The reason for this is that smoking, which is a free radical source, leads to endothelial dysfunction and reduces clitoral bleeding (Yalın and Mete Citation2011). In addition, smoking was reported to affect sexual activity by increasing androgen hormone levels and decreasing estrogen and progesterone levels (Cochran et al. Citation2008). In our study, it was determined that the degree of lordosis determining sexual performance and anogenital investigation that showed sexual motivation decreased in all groups, though it is not statistically significant. Based on all this information, it can be assumed that exposure to maternal tobacco smoke and ALA administrations may affect sexual motivation and sexual performance in females and cause sexual dysfunction through both changed hormone levels and increased oxidative stress. For this hypothesis to be definite, estrogen, progesterone and androgen hormone analysis should be measured.
Although high ROS levels are clearly associated with infertility by causing pathological results in female reproductive systems, the safe dose of oral antioxidants used to prevent this damage during pregnancy is unknown. In addition, the results of antioxidant treatments applied during pregnancy are highly controversial (Taylor Citation2001; Rahnama et al. Citation2013). In some studies with animal models, it has been reported that ALA positively affects follicle development in ovaries and inhibits apoptosis in cells by increasing total antioxidant capacity and decreasing ROS levels (Hatami et al. Citation2014a, Citation2014b). It has also been suggested that ALA induces apoptosis in cells by stimulating mitochondrial permeability and hydroxyl radical and lipid peroxidation formation (Moini et al. Citation2002; Aoyama et al. Citation2006). In addition, it has been revealed that ALA administration causes oxidative protein damage by increasing plasma lipid hydrogen peroxide levels in rats (Cakatay and Kayali Citation2005). Considering all these, follicular development disorders and histopathological findings observed in the ovaries of the groups undergoing ALA were associated with increased ROS. The increase in TOS/TAS levels in ALA treated groups also supports this toxicity. We think that the toxic effect of ALA may depend on the dose selected, duration of the application and/or time of administration (during pregnancy). In our study, increased apaf-1, Casp9, Casp3 mRNA levels, apoptotic cell numbers and 8-OHdG and 4-HNE immunoreactivities in the ovarian tissues belonging to the ALA groups, were also other findings consistent with ALA toxicity. On one hand, the data on the potential uses of antioxidants in supporting pregnancy are not clear and no study has been conducted on the ovarian tissues and reproductive functions of rats in puberty after maternal ALA use. Our study is the first in this field to address this gap. On the other hand, ALA has also been reported to be effective in the HPG axis by inhibiting AMP-activated protein kinase (AMPK), which plays a role in suppressing LH from the pituitary gland and GnRH from the hypothalamus (Andrade et al. Citation2013; Coyral-Castel et al. Citation2008). Based on this information, ALA is thought to be highly suppressing AMPK due to high dose toxicity and as a result preventing the effect of AMPK on LH, causing a malfunction in the HPG axis. We suggest that one of the mechanisms underlying increased LH levels in ALA applied groups may be via this route. The metabolic pathways between ALA and HPG axis need further research.
It is known that genetic and environmental factors and endogenous signals play a role in the onset of puberty (Navarro et al. Citation2007). Since the onset of puberty is a risk factor for sexual performance, alcohol and drug use, depression and some chronic diseases, it is important to determine the factors affecting pubertal development at the population level (Golub et al. Citation2008; Hayatbakhsh et al. Citation2009). In addition, it has been reported that the age of menarche may be an early marker of endocrine and reproductive dysfunction (Opdahl et al. Citation2011). In the literature, it was observed that the number of studies examining the relationship between tobacco and puberty was limited and that these studies were performed mostly on people. However, in studies with people, the results were quite contradictory. In one study, prenatal cigarette exposure was reported to affect puberty early in girls while another study found no relationship between maternal cigarette smoke exposure and girls’ follicle count, cycle length, FSH, LH levels and early puberty (Windham et al. Citation2008; Ferris et al. Citation2010; Gollenberg et al. Citation2015). Another contradictory result was on the effect of smoking on the menstrual cycle. In a few studies, it was revealed that smoking leads to menstrual cycle irregularity, decreases fertility in women and brings menopausal age early, while another study did not find a significant difference in cycle order (Thomas et al. Citation1993; Kato et al. Citation1999). In our study, no significant change in the total estrous cycle was detected among the groups. However, it was determined that advanced the onset of puberty in the female offspring of the TS group.
As discussed above we provide evidence that oxidative stress caused by prenatal ALA and tobacco-smoke administration affects reproductive function in female rats in the pubertal period. Our biochemical, immunohistochemical and molecular findings revealed that oxidative stress caused by prenatal tobacco smoke and ALA administration caused some long-term adverse effects on the reproductive health of female rat offspring by affecting folliculogenesis, HPG axis, hormone secretion and sexual development. This may affect the intrauterine hormonal environment during pregnancy, and this early fetal exposure can have harmful effects on the future condition of reproductive health. We strongly recommend that women of reproductive age should not smoke or/be exposed to, but also encourage their smoking cessation methods to protect their fertility and prevent reproductive dysfunction in their children. Current research by others is addressing whether the properties of ALA are suitable for use in diabetes, vascular disease, hypertension and inflammation. However, use of maternal ALA in these doses we used in the study showed a prooxidant effect in the offspring, and therefore, optimizing conditions for effective safe dose is required. Current use is not wise.
Materials and methods
Experimental design
Twenty-eight Sprague-Dawley female rats (170 ± 10 g), eight-weeks old were obtained from the Firat University Experimental Research Unit (FUDAM). Rats were fed standard rat food, tap water and housed under hygienic standard laboratory conditions.12-hour light-dark cycles, 21 ± 1°C standard temperature and humidity (55 ± 5%) were provided. Rats were randomly divided into four groups (n = 7): control, tobacco smoke (TS), tobacco smoke + alpha-lipoic acid (TS+ALA) and alpha-lipoic acid (ALA). Tobacco smoke and ALA administrations were performed for a total of 11 weeks, 8 weeks before and during pregnancy. At the end of the 8th-week female rats in all groups were mated with sexually experienced twenty-eight Sprague-Dawley male rats (420 ± 10 g and 14–16 weeks old). All of twenty-eight female rats became pregnant. The date on which sperm was observed in the vaginal smear was accepted as gestational day 0. Administrations were continued during pregnancy with female adult rats. Rats in the TS and TS+ALA groups were exposed to tobacco smoke twice a day for one hour starting from the eighth week before mating and during pregnancy. Tobacco used in the assay was provided from Adiyaman tobacco store (Elazig, Turkey). Rats exposed to tobacco smoke were placed in designed cages separately. Rats were placed into whole-body exposure chambers (150x50x50 cm3) made from glass and the smoke obtained by burning 10 grams of tobacco at a temperature of approximately 350°C was introduced into the glass cage between 09:00–10:00 and 14:00–15:00 through the air pump (AP-001 Aquarium Air Pump Xilong, China) (provide 250 mL of air every 10 s). The total amount of tobacco used per day was 20 g. These tobacco contained 1.5 mg/g of nicotine and carbon monoxide (CO) level of tobacco smoke was 15 mg/g. During tobacco-smoke exposure, 23–25°C temperature was provided. After the tobacco-smoke exposure, rats were received fresh air until their next exposure. The rats in control group were exposed fresh air by the same apparatus. ALA (cat: 29,862 Lot: 002241–20,161,019, DL-a-Lipoic acid, Chem-Impex Int’l Inc, USA) was dissolved in saline and administered 20 mg/kg by oral gavage. This treatment was conducted to the ALA and TS+ALA groups every other day from the eighth week before mating and during pregnancy (Al Ghafli et al. Citation2004). In addition, the control group received only saline as a vehicle by oral gavage. All treatments ended with birth. It was demonstrated that standardization of litter sizes contributes to pup’s survival and growth (Venturelli et al. Citation2019). Hence, we determined to standardize litter sizes from five to nine pups. Pups and dams were housed all together to PND 21 within litters. Pups were weaned on PND 21. After weaning, the offsprings were placed in separate cages as females and males. Total number of 42 male offspring in the groups were removed from the experiment. It was determined that dams in all groups had at least one female offspring. For this reason, one pup was chosen randomly from each dam and the experiment was continued with seven female pups for each group (n = 7). Puberty onset was monitored by examination of the vaginal opening in female rat pups. Subsequently, the estrous cycle was conducted daily for 15 days and determined by examination of the vaginal smear cytology. After sexual behavior tests were completed, female animals in all groups were rapidly decapitated with guillotine in diestrus phase. The ovaries were rapidly removed and used for histological, biochemical and quantitative real-time RT-PCR analyses. Blood was sampled from the trunk of decapitated rats and centrifuged at 4000 rpm for 5 minutes at +4°C. Then, the serum samples obtained were stored at – 20°C until the next analyses.
Determination of puberty onset and estrous cycle in offspring
To determine the onset of puberty in female rat offspring, the vaginal opening (complete canalization of vagina) was daily examined at 10:00–11:00 from postnatal day (PND) 30 for each group (Ojeda and Urbanski Citation1988). Also, the body weight at the time of vaginal opening was recorded in all groups. Afterward, the daily vaginal smear was examined to monitor the estrous cyclicity at 10:00–12:00 for 15 consecutive days (average 3 estrous cycles) after completion of the vaginal opening in all groups (starting at PND 58). The vaginal smear of each rat was obtained with a micropipette filled with 200 µL of saline. The smear samples were then placed on a glass slide and immediately examined unstained in the light microscope at 10X and 40X magnification. For defining the estrous cycle phases (metestrus, diestrus, proestrus and estrus), the proportion of leukocytes, cornified epithelial cells, and nucleated epithelial cells was taken into account in microscopic examination (Marcondes et al. Citation2002). Determination of estrous cycle phases was made by a histologist who was blinded to the experimental groups.
Sexual behavior test
After determining the effects of TS and/or ALA treatments on estrous cycle, taking of vaginal smears was daily continued in order to perform sexual behavior test in all female rats on estrus phase. All sexual behavior tests were conducted in the standard mating cage (40 x 40 × 40 cm) during the dark phase between 13:00 and 15:00. All rats in the estrus phase were tested for sexual behavior with sexually experienced male rats. On the experiment day, each experimental female rat stayed in the mating cage for 15 min for adaptation. After adaptation, the test female was placed individually in the mating cage on together with a sexually active male rat for 10 min or until the test female received 10 mounts (Uphouse et al. Citation2014). Proceptive (anogenital investigation) and receptive (lordosis; the dorsiflexion reflex posture) sexual behaviors were scored. Briefly, the number of anogenital investigation, sniffing the genital area of the stimulus male, was measured as an index of female sexual motivation (Afonso et al. Citation2007). Additionally, both the lordosis quotient (number of lordoses/number of mounts x 100) and lordosis quality were calculated as sexual receptivity of the female rats. The lordosis quality was computed according to the intensity of the lordosis responses scored on a 4-point scale (0–3) (Hardy and DeBold Citation1971; Van de Poll and Van Dis Citation1977). After completing the sexual behavior tests, all cyclic female rats were decapitated on first diestrus phase.
ELISA assays
Blood serum samples were assayed using a plate reader (Multiskan FC, Thermo Scientific). Serum luteinizing hormone (LH), follicle-stimulating hormone (FSH), total antioxidant status (TAS) and total oxidant status (TOS) levels were measured using rat enzyme-linked immunosorbent assay (ELISA) kits in compliance with the kit instruction manuals. LH and FSH kits were purchased from the same company (Shanghai Sunred Biological Technology Co., Ltd., China). In addition, TAS and TOS kits were purchased from the other company (Beijing Andy Huatai Technology Co., Ltd., China). The test’s results were expressed as mIU/mL, IU/L, U/mL and pg/mL for LH, FSH, TAS and TOS kits, respectively. The assay range was 0.3–60 mIU/mL, 0.25–60 IU/L, 0.2–8 U/mL and 3.75–120 pg/mL for LH, FSH, TAS and TOS kits, respectively. Intra-assay coefficient variation was below 9% and inter-assay coefficient variation was below 11% and sensitivities were 0.206 mIU/mL and 0.202 IU/L for LH and FSH kits, respectively. Intra-assay coefficient variation was below 8% and inter-assay coefficient variation was below 10% and the sensitivities were 0.1 U/mL and 0.7 pg/mL for TAS and TOS kits, respectively.
Total RNA purification, complementary DNA (cDNA) synthesis and quantitative real-time PCR (qPCR) analysis
Total RNA was purified by using TransZol Up reagent (Transgen Biotech, China) following the producer’s directions. First-strand cDNA was synthesized using a High-Capacity cDNA Reverse Transcription Kit (applied biosystems by Thermo Fisher Scientific, Lithuania). cDNA synthesis was performed using a thermal cycler (Veriti, Applied Biosystems, Singapore) at 25°C for 10 min, 37°C for 120 min and 85°C for 5 min.
mRNA expression levels of the apoptotic protease-activating factor 1 (Apaf1), caspase 3 (Casp3) and caspase 9 (Casp9) in ovary tissues of rats were analyzed by quantitative Real Time-PCR (qPCR) system (7500 Real Time-PCR, Applied Biosystems, Singapore) using 5x HOT FIREPol EvaGreen qPCR Mix Plus reagent (Solis BioDyne, Estonia). Amplification conditions were as follows: 12 min at 95°C, followed by 15 s at 95°C, 20 s at 60–65°C and 20 s at 72°C for 40 cycles. mRNA expression levels of the genes were determined as the relative fold change compared to the control and normalized to the Glyceraldehyde-3-Phosphate Dehydrogenase (GADPH) expression. Analyzed primer pairs () were designed and synthesized by Sentegen Biotech custom oligosynthesis service (Ankara, Turkey). 2-∆∆Ct method was used to calculate the differences in the gene expressions.
Table 6. Characteristics of the primers used for qPCR analysis
Histological evaluation
Ovaries were fixed in 10%-buffered formaldehyde, and then embedded in paraffin. Samples were cut at 5 µm thicknesses and stained with hematoxylin and eosin (H&E) by a light microscope (NovelN-800 M, Ningbo, China). Follicles were identified as healthy if they had a solid oocyte and contained organized layers of granulose and thecal cells. The follicles were considered atretic if they had a degenerate oocyte and granulosa cells with pycnotic nucleus poured into the lumen around the oocyte. Follicular cysts (FC) were identified fluid-filled pockets of ovaries that formation within a secondary follicle. The surrounding zona granulosa was attenuated. The cyst cavity was lined by a simple squamous epithelium. The severity of damage in the ovarian tissue was evaluated semiquantitatively in terms of the specified changes; inflammatory cell number, vascular congestion, germinal epithelial degeneration, follicular degeneration (follicle cell loss + follicular fluid increase), atretic and cystic follicle, vacuolization in granulosa cells and corpus luteum. Histoscores were given as 0 = absent, 1 = weak, 2 = moderate and 3 = strong for each parameter (Mazaud et al. Citation2002). All histological evaluations were performed by two blinded investigators.
Ovarian follicle and corpus luteum counts
All types of follicles were identified according to Pedersen and Peters’ classification system (Pedersen and Peters Citation1968). Shortly, if the oocyte is surrounded by a squamous cell layer, it is called primordial, if it is surrounded by a cuboidal granulosa cells layer, it is called primary, if it is surrounded by multi-layer granulose cells, it is called secondary, if it is surrounded by multi-layer granulose cells and large antrum around the oocyte, it is called graafian follicle. Corpus luteum and the total follicle counts in ten random sections of the ovarian tissue were counted under light microscopy (NovelN-800 M, Ningbo, China) at a magnification of ×10.
TUNEL assay
Terminal deoxynucleotidyl transferase-mediated deoxyuridine-biotin nick end labeling (TUNEL) assay was used to identify apoptotic cells. ApopTagPlus Peroxidase in Situ Apoptosis Detection Kit (Chemicon, cat no: S7101, USA) was utilized to visualize the apoptotic cells. In the evaluation of TUNEL staining, the cells with blue nucleus were considered healthy, while the nuclei of apoptotic cells were brown. Two hundred and fifty cells were counted in 10 randomly selected fields. The apoptotic index (%) was calculated as a ratio of the TUNEL positive cell number to the total cell number (Lipponen et al. Citation1994).
Immunohistochemical evaluation
The avidin-biotin-peroxidase complex method was used to determine 8-hydroxydeoxyguanosine (8-OHdG) and 4-Hidroxynonenal (4-HNE) immunoreactivities (Polyclonal Antibody Rabbit 8-OHdG, AP06721, Bioassay Technology Laboratory, Shanghai, China; Polyclonal Antibody Rabbit 4-Hidroxynonenal, ab46545, Abcam, Cambridge, UK) in ovary tissue. Slides were counterstained with hematoxylin. The immunohistochemical histoscores were created on the foundation of immunoreactivity prevalence (0.1: <25%, 0.4: 26–50%, 0.6: 51–75%, 0.9: 76–100%) and severity (0: no, +0.5: very little, +1: little, +2: medium, +3: severe). (Histoscore = prevalence × severity) (Mallenby et al. Citation1991).
Statistical analysis
All values were expressed as mean ± standard deviation (SD) Statistical analyses and graphs were as follows: SPSS 21.0 and Origin 6.0. One‐way ANOVA post hoc Tukey’s HSD was used for evaluation of the data. In all analyses, p < 0.05 was considered statistically significant. The qPCR data were evaluated by using the ΔΔCt module at the Qiagen Gene Globe Data Analysis Center portal: https://geneglobe.qiagen.com/tr/analyze/. The qPCR module transformed threshold cycle (Ct) values to calculate results for gene expression. p < 0.05 was considered as significant.
Ethical approval
The study was approved by the local ethics committee of the Firat University in Elazığ (28.07.2016, 2016/14) adhered to European Union guidelines (Directive 2010/63/EU for animal experiments). All the animal experiments were performed according to the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines.
Authors contributions
Designed the study, analyzed data, revised the paper, and approved the final version: EEG; performed the research, analyzed data, drafted the article, and approved the final version: NKT, AT, NU, AY, RFA, SC, İEO.
Disclosure statement
The authors declare that there is no conflict of interest regarding the publication of this paper.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Afonso VM, Sison M, Lovic V, Fleming AS. 2007. Medial prefrontal cortex lesions in the female rat affect sexual and maternal behavior and their sequential organization. Behav Neurosci. 121(3):515–526.
- Al Ghafli MH, Padmanabhan R, Kataya HH, Berg B. 2004. Effects of α-lipoic acid supplementation on maternal diabetes-ınduced growth retardation and congenital anomalies in rat fetuses. Mol Cell Biochem. 261(1):123–135.
- Andrade J, Quinn J, Becker RZ, Shupnik MA. 2013. Amp-activated protein kinase is a key intermediary in gnrh-stimulated lhβ gene transcription. Mol Endocrinol. 27(5):828–839.
- Aoyama S, Okimura Y, Fujita H, E F S, Umegaki T, Abe K, Inoue M, Utsumi K, Sasaki J. 2006. Stimulation of membrane permeability transition by alpha-lipoic acid and its biochemical characteristics. Physiol Chem Phys Med NMR. 38(1):1–20.
- Beck J, Biały M. 2000. The role of mounts and intromissions in triggering ejaculation in rats. Acta Neurobiol Exp (Wars). 60(1):29–33.
- Behl M, Rao D, Aagaard K, Davidson TL, Levin ED, Slotkin TA, Srinivasan S, Wallinga D, White MF, Walker VR, et al. 2013. Evaluation of the association between maternal smoking, childhood obesity, and metabolic disorders: a national toxicology program workshop review. Environ Health Perspect. 121(2):170–180.
- Bruin JE, Gerstein HC, Holloway AC. 2010. Long-term consequences of fetal and neonatal nicotine exposure: a critical review. Toxicol Sci. 116(2):364–374.
- Cakatay U, Kayali R. 2005. Plasma protein oxidation in aging rats after alpha-lipoic acid administration. Biogerontology 6(2):87–93.
- Camargo I, Leite GA, Pinto T, Ribeiro-Paes JT. 2014. Histopathological findings in the ovaries and uterus of albino female rats promoted by co-administration of synthetic steroids and nicotine. Exp Toxicol Pathol. 66(4):195–202.
- Camlin NJ, Sobinoff AP, Sutherland JM, Beckett EL, Jarnicki AG, Vanders RL, Hansbro PM, McLaughlin EA, Holt JE. 2016. Maternal smoke exposure impairs the long-term fertility of female offspring in a murine model. Biol Reprod. 39:1–12.
- Cao SS, Kaufman RJ. 2014. Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxid Redox Signal. 21(3):396–413.
- Çiftçi H, Akın Y, Gülüm M. 2015. Sigaranın kadın cinselliğine etkileri. J Agent. 17(1):60–63.
- Coccini T, Gornati R, Rossi F, Signoretto E, Vanetti I, Bernardini G, Manzo L. 2014. Gene expression changes in rat liver and testes after lung instillation of a low dose of silver nanoparticles. J Nanomed Nanotechnol. 5(5):2–12.
- Cochran CJ, Gallicchio L, Miller SR, Zacur H, Flaws JA. 2008. Cigarette smoking, androgen levels, and hot flushes in midlife women. Obstet Gynecol. 112(5):1037–1044.
- Coyral-Castel S, Tosca L, Ferreira G, Jeanpierre E, Rame C, Lomet D, Caraty A, Monget P, Chabrolle C, Dupont J. 2008. The effect of amp-activated kinase activation on gonadotrophin- releasing hormone secretion in gt1-7 cells and ıts potential role in hypothalamic regulation of the oestrous cyclicity in rats. J Neuroendocrinol. 20(3):335–346.
- Doğanlar ZB, Uzun M, Ovali MA, Dogan A, Ongoren G, Doğanlar O. 2018. Melatonin attenuates caspase-dependent apoptosis in the thoracic aorta by regulating element balance and oxidative stress in pinealectomised rats. Appl Physiol Nutr Metabol. 44(2):153–163.
- Ernst A, Kristensen SL, Toft G, Thulstrup AM, Hakonsen LB, Olsen SF, Ramlau-Hansen CH. 2012. Maternal smoking during pregnancy and reproductive health of daughters: a follow-up study spanning two decades. Hum Reprod. 27(12):3593–3600.
- Ferris JS, Flom JD, Tehranifar P, Mayne ST, Terry MB. 2010. Prenatal and childhood environmental tobacco smoke exposure and age at menarche. Paediatr Perinat Epidemiol. 24(6):515–523.
- Flaws JA, Abbud R, Mann RJ, Nilson JH, Hirshfield AN. 1997. Chronically elevated luteinizing hormone depletes primordial follicles in the mouse ovary. Biol Reprod. 57(5):1233–1237.
- Gannon AM, Stämpfli MR, Foster WG. 2012. Cigarette smoke exposure leads to follicle loss via an alternative ovarian cell death pathway in a mouse model. Toxicol Sci. 125(1):274–284.
- Gollenberg AL, Addo Y, Zhang Z, Hediger ML, Himes JH, Lee PA. 2015. In utero exposure to cigarette smoking, environmental tobacco smoke and reproductive hormones in US girls approaching puberty. Horm Res Paediatr. 83(1):36–44.
- Golub MS, Collman GW, Foster PM, Kimmel CA, Rajpert-De Meyts E, Reiter EO, Sharpe RM, Skakkebaek NE, Toppari J. 2008. Public health implications of altered puberty timing. Pediatrics 121(Supplement 3):218–230.
- Grasselli E, Voci A, Demori I, Vecchione G, Compalati AD, Gallo G, Goglia F, De Matteis R, Silvestri E, Vergani L. 2016. Triglyceride mobilization from lipid droplets sustains the anti-steatotic action of iodothyronines in cultured rat hepatocytes. Front Physiol. 6:418. doi:https://doi.org/10.3389/fphys.2015.00418.
- Habek D, Habek JC, Ivanisevic M, Djelmis J, Ivanisevic M, Djelmis J. 2002. Fetal tobacco syndrome and perinatal outcome. Fetal Diagn Ther. 17(6):367–371.
- Hakonsen LB, Ernst A, Ramlau-Hansen CH. 2014. Maternal cigarette smoking during pregnancy and reproductive health in children: a review of epidemiological studies. Asian J Androl. 16(1):39–49.
- Hardy DF, DeBold JF. 1971. The relationship between levels of exogenous hormones and the display of lordosis by the female rat. Horm Behav. 2(4):287–297.
- Harte CB, Meston CM. 2008. The inhibitory effects of nicotine on physiological sexual arousal in nonsmoking women: results from a randomized, double-blind, placebo-controlled, cross-over trial. The Journal of Sexual Medicine. 5(5):1184–1197.
- Hatami S, Zavareh S, Salehnia M, Lashkarbolouki T, Ghorbanian MT, Karimi I. 2014a. Total oxidative status of mouse vitrified pre-antral follicles with pre- treatment of alpha lipoic acid. Iran Biomed J. 18:180–187.
- Hatami S, Zavareh S, Salehnia M, Lashkarbolouki T, Ghorbanian MT, Karimi I. 2014b. The impact of alpha lipoic acid on developmental competence of mouse vitrified pre-antral follicles in comparison to those isolated from vitrified ovaries. Iran J Reprod Med. 12:57–64.
- Hayatbakhsh MR, Najman JM, McGee TR, Bor W, O’Callaghan MJ. 2009. Early pubertal maturation in the prediction of early adult substance use: a prospective study. Addiction. 104(1):59–66.
- Kato I, Toniolo P, Koenig KL, Shore RE, Zeleniuch-Jacquotte A, Akhmedkhanov A, Riboli E. 1999. Epidemiologic correlates with menstrual cycle length in middle aged women. Eur J Epidemiol. 15(9):809–814.
- Koga T, Ishida T, Takeda T, Ishii Y, Uchi H, Tsukimori K, Yamamoto M, Himeno M, Furue M, Yamada H. 2012. Restoration of dioxin-induced damage to fetal steroidogenesis and gonadotropin formation by maternal co-treatment with alpha-lipoic acid. PLoS One. 7(7):e40322. doi:https://doi.org/10.1371/journal.pone.0040322.
- Lannes J, L’Hote D, Garrel G, Laverriere JN, Cohen-Tannoudji B, Querat B. 2015. Rapid communication: a microRNA-132/212 pathway mediates GnRH activation of FSH expression. Mol Endocrinol. 29(3):364–372.
- Lipponen P, Aaltomaa S, Kosma VM, Syrjanen K. 1994. Apoptosis in breast cancer as related to histopathological characteristics and prognosis. Eur J Cancer. 30(14):2068–2073.
- Mallenby J, Dunyer J, Hawkins C, Hitchen C. 1991. Effects of experimental limbic on the estrus cycle and reproductive success in rats. Epilepsia. 34(2):220–227.
- Marcondes FK, Bianchi FJ, Tanno AP. 2002. Determination of the estrous cycle phases of rats: some helpful considerations. Braz J Biol. 62(4a):609–614.
- Mazaud S, Guigon CJ, Lozach A, Coudouel N, Forest MG, Coffigny H, Magre S. 2002. Establishment of the reproductive function and transient fertility of female rats lacking primordial follicle stock after fetal gamma-irradiation. Endocrinology. 143:4775–4787. doi:https://doi.org/10.1210/en.2002-220464.
- Moini H, Packer L, Saris NE. 2002. Antioxidant and prooxidant activities of alpha-lipoic acid and dihydrolipoic acid. Toxicol Appl Pharmacol. 182(1):84–90.
- Navarro VM, Castellano JM, Garcia-Galiano D, Tena-Sempere M. 2007. Neuroendocrine factors in the initiation of puberty: the emergent role of kisspeptin. Rev Endocr Metab Disord. 8(1):11–20.
- Neal MS, Mulligan Tuttle AM, Casper RF, Lagunov A, Foster WG. 2010. Aryl hydrocarbon receptor antagonists attenuate the deleterious effects of benzo[a]pyrene on isolated rat follicle development. Reprod Biomed Online. 21(1):100–108.
- Nguyen KA, Intriago RE, Upadhyay HC, Santos SJ, Webster NJ, Lawson MA. 2010. Modulation of gonadotropin-releasing hormone-induced extracellular signal-regulated kinase activation by dual-specificity protein phosphatase 1 in LbetaT2 gonadotropes. Endocrinology. 151(10):4882–4893.
- Ojeda SR, Urbanski HF. 1988. Puberty in the rat In: Knobil, E., Neill, J. (Eds.) The physiology of Reproduction. New York: Raven Press; pp. 1699–1737.
- Opdahl S, Alsaker MD, Janszky I, Romundstad PR, Vatten LJ. 2011. Joint effects of nulliparity and other breast cancer risk factors. Br J Cancer. 105(5):731–736.
- Packer L, Witt EH, Tritschler HJ. 1995. Alpha-Lipoic acid as a biological antioxidant. Free Radic Biol Med. 19(2):227–250.
- Parente E, Colannino G, Picconi O, Monastra G. 2017. Safety of oral alpha-lipoic acid treatment in pregnant women: a retrospective observational study. Eur Rev Med Pharmacol Sci. 21(18):4219–4227.
- Park MG, Ko KW, Oh MM, Bae JH, Kim JJ, Moon Du G. 2011. Effects of smoking on plasma testosterone level and erectile function in rats. J Sexual Med. 9:(2):472–481.
- Pedersen T, Peters H. 1968. Proposal for a classification of oocytes and follicles in the mouse ovary. J Reprod Fertil. 17(3):555–557.
- Rahnama A, Zavareh S, Ghorbanian MT, Karimi I. 2013. The effects of camp-elevating agents and alpha lipoic acid on in vitro maturation of mouse germinal vesicle oocytes. J Reprod Infertility. 14:173–183.
- Richburg JH, Dwyer JL. 2010. The sertoli cell as a target for toxicants. Compr Toxicol. 11:97–114.
- Risma KA, Hirshfield AN, Nilson JH. 1997. Elevated luteinizing hormone in prepubertal transgenic mice causes hyperandrogenemia, precocious puberty, and substantial ovarian pathology 1. Endocrinology. 138(8):3540–3547.
- Rogers JM. 2008. Tobacco and pregnancy: overview of exposures and effects. Birth Defects Res Part C Embryo Today Rev. 84(1): 1–15.
- Sawada M, Carlson JC. 1996. Intracellular regulation of progesterone secretion by the superoxide radical in the rat corpus luteum. Endocrinology. 137(5):1580–1584.
- Sena LA, Chandel NS. 2012. Physiological roles of mitochondrial reactive oxygen species. Mol Cell. 48(2):158–167.
- Sugimura Y, Murase T, Kobayashi K, Oyama K, Hayasaka S, Kanou Y, Oiso Y, Murata Y. 2009. α-Lipoic acid reduces congenital malformations in the offspring of diabetic mice. Diabetes Metab Res Rev. 25(3):287–294.
- Talebi A, Zavareh S, Kashani MH, Lashgarbluki T, Karimi I. 2012. The effect of alpha lipoic acid on the developmental competence of mouse isolated preantral follicles. J Assist Reprod Genet. 29(2):175–183.
- Taylor CT. 2001. Antioxidants and reactive oxygen species in human fertility. Environ Toxicol Pharmacol. 10(4):189–198.
- Teerds KJ, Dorrington JH. 1995. Immunolocalization of transforming growth factor alpha and luteinizing hormone receptor in healthy and atretic follicles of the adult rat ovary. Biol Reprod. 52(3):500–508.
- Terasaka T, Adakama ME, Li S, Kim T, Terasaka E, Li D, Lawson MA. 2017. Reactive oxygen species link gonadotropin-releasing hormone receptor signaling cascades in the gonadotrope. Front Endocrinol (Lausanne). 286:1–8.
- Thomas EJ, Edridge W, Weddell A, McGill A, McGarrigle HH. 1993. Endocrinology: the impact of cigarette smoking on the plasma concentrations of gonadotrophins, ovarian steroids and androgens and upon the metabolism of oestrogens in the human female. Hum Reprod. 8(8):1187–1193.
- Tomo PD, Silvestre SD, Cordone VGP, Giardinelli A, Faricelli B, Pipino C, Lanuti P, Peng T, Formoso G, Yang D. 2015. Centella Asiatica and Lipoic Acid, or a combination thereof, inhibit monocyte adhesion to endothelial cells from umbilical cords of gestational diabetic women. Nutr Metabol Cardiovasc Dis. 25(7):659–666. doi:https://doi.org/10.1016/j.numecd.2015.04.002.
- Tsai-Turton M, Luderer U. 2006. Opposing effects of glutathione depletion and follicle-stimulating hormone on reactive oxygen species and apoptosis in cultured preovulatory rat follicles. Endocrinology. 147(3):1224–1236.
- Tweed JO, Hsia SH, Lutfy K, Friedman TC. 2012. The endocrine effects of nicotine and cigarette smoke. Trends Endocrinol Metabol. 23(7):334–342.
- Uphouse L, Hiegel C, Adams S, Murillo V, Martinez M. 2014. Prior hormonal treatment, but not sexual experience, reduces the negative effects of restraint on female sexual behavior. Behav Brain Res. 259:35–40. doi:https://doi.org/10.1016/j.bbr.2013.10.031.
- Van de Poll NE, Van Dis H. 1977. Hormone induced lordosis and its relation to masculine sexual activity in male rats. Horm Behav. 8(1):1–7.
- Venturelli AC, Meyer KB, Fischer SV, Kita DH, Philipsen RA, Morais RN, Andrade AJM. 2019. Effects of in utero and lactational exposure to phthalates on reproductive development and glycemic homeostasis in rats. Toxicology. 421:30–40. doi:https://doi.org/10.1016/j.tox.2019.03.008.
- Windham GC, Zhang L, Longnecker MP, Klebanoff M. 2008. Maternal smoking, demographic and lifestyle factors in relation to daughter’s age at menarche. Paediatr Perinat Epidemiol. 22(6):551–561.
- Yalın T, Mete K. 2011. Endotel disfonksiyonu. Pamukkale Tip Dergisi. 4:152–157.
- Zhang JM, Wang HC, Wang HX, Ruan LH, Zhang YM, Li JT, Tian S, Zhang YC. 2013. Oxidative stress and activities of caspase-8, −9, and −3 are involved in cryopreservation-induced apoptosis in granulosa cells. Eur J Obstetrics Gynecology Reprod Biol. 166:52–55. doi:https://doi.org/10.1016/j.ejogrb.2012.09.011.
