 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
When considering empirical medical management (EMT) options for men with unexplained infertility (UI), clomiphene citrate (CC) has been shown to positively influence sperm parameters in hypogonadal men. Unfortunately, the optimal cut point for defining hypogonadism for this patient population has not been established. We hypothesized that hypogonadal men with UI having the lowest serum total testosterone (TT) (<265 ng/dL) would have a significant post-CC improvement in both TT and semen characteristics compared to those in the TT > 264 ng/dL group. We performed our study based on an IRB-approved retrospective chart review of 83 males with UI receiving more than 90 days of 50 mg daily CC. Serum TT and semen characteristics were studied in 83 patients before and in 23 patients after CC treatment. Median TT level increased from 256 ng/dL to 630 ng/dL (p < 0.001, n = 83) and SC increased from 6 (/ml) to 20 (
/ml) (p < 0.016, n = 23). Overall, our results demonstrated the following: (1) CC treatment at all currently used serum TT cut-points resulted in significant improvement in both TT (p < 0.001) and sperm concentration (p = 0.03). No significant change in post-CC sperm motility or morphology was noted. (2) Correlation and linear regression analyses demonstrated that CC treatment significantly increased TT in 96% (22 of 23) of patients, and (3) when grouped as two cohorts (≤264 and >264 ng/dL), sperm concentration and TT improved 2.3 to 2.6-fold (p < 0.001) and 1.45 to 2.5-fold (p < 0.01) respectively. Thus, for hypogonadal men with UI, CC significantly improved TT and sperm concentration regardless of pre-treatment, baseline serum TT level. For this reason, CC treatment should be considered in men with UI having a TT < 400 ng/dL.
Introduction
Worldwide, approximately 8–12% of couples experience infertility with male factor infertility representing the sole factor in up to one-third of these cases (Sigman and Jarow Citation1997; Centers for Disease Control and Prevention Citation2009). Particularly challenging are those presenting with idiopathic male infertility. These men have altered semen characteristics without an identifiable cause, making it a challenging condition to diagnose and treat (Chehab et al. Citation2015; Usadi and Merriam Citation2015; Agarwal et al. Citation2019).
The work-up for male factor infertility typically starts with a semen analysis using values defined by the World Health Organization (WHO) (Cooper et al. Citation2010). Semen characteristics below the 2010 WHO thresholds are defined by decreased sperm concentration (oligozoospermia; <15 106/ml), reduced sperm motility (asthenospermia; <40%), and abnormal sperm morphology (teratozoospermia; <4% strict morphology) (Kumar and Singh Citation2015). It is also well established that hypogonadism unfavorably impacts male fertility by negatively affecting testicular spermatogenesis (Dabaja and Schlegel Citation2014). Hypogonadism is generally defined as a decrease in serum TT with a variety of reference (cutoff) values being used to define hypogonadism: including 264 ng/dL, 300 ng/dL, and 400 ng/dL. There is no consensus in the literature regarding an optimal TT definition for hypogonadism for use with infertile men (Trussell et al. Citation2019). The 300 ng/dL cut point represents the American Urological Association (AUA) guideline definition for low TT (Mulhall et al. Citation2018) and is often utilized in the male infertility literature. Serum TT level less than 400 ng/dL was used by the endocrine society (Practice Committee for the American Society for Reproductive Medicine in collaboration with the Society for Male Reproduction and Urology Citation2008). And finally, a study investigating four cohorts of healthy non-obese European and American men between the ages of 19 and 39 highlighted a TT ranging from the 2.5th percentiles of 264 ng/dL and 916 ng/dL, where 264 was recommended as the definition for hypogonadism (Travison et al. Citation2017); which incidentally, also defines the lower limit of normal TT according to the current Endocrine Society Guidelines (Bhasin et al. Citation2018).
In men not pursuing fertility, symptoms of hypogonadism (for example, the ADAM questionnaire) (Mohamed et al. Citation2010), can be effectively treated using exogenous sources. However, use of testosterone replacement therapies (TRT) in the form of gels, pellets, injections, or patches will have a negative feedback on the hypothalamic-pituitary-axis, adversely impacting both spermatogenesis and intratesticular testosterone concentrations. For this reason, TRT should not be considered for treating infertile men with hypogonadism.
In comparison, CC, an orally active non-steroidal fertility drug (Patankar et al. Citation2007; Travison et al. Citation2017), acts as an estrogen agonist/antagonist which exists as a racemic mixture of two isoforms (En-clomiphene and Zu-clomiphene) (Patankar et al. Citation2007; Chehab et al. Citation2015). CC is a selective estrogen receptor modulator (SERM) that blocks the negative feedback of estrogen on the pituitary gland. Through this interaction, increased levels of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) are released by the anterior pituitary. As such, the use of CC has been shown to positively influence both sperm concentration and serum TT in those hypogonadal men with UI (Chehab et al. Citation2015; Usadi and Merriam Citation2015).
Of note, although the Food and Drug Administration (FDA) has approved CC for the treatment of female infertility, there is wide-spread, off-label use of CC to treat infertile males with UI (Chehab et al. Citation2015; Usadi and Merriam Citation2015; Agarwal et al. Citation2019). Such use of empirical medical therapy (EMT) for infertile men is confounded by the lack of both FDA approval and professional medical organization guidelines (Chehab et al. Citation2015). Nonetheless, selective estrogen receptor modulators (SERMs) use is prevalent with infertile males with a meta-analysis showing: (1) significant increase in both sperm concentration and pregnancy rates, (2) significantly improved fertility rates in hypogonadal men, and (3) improved semen characteristics (Chua et al. Citation2013).
Ultimately, treating hypogonadal infertile men with UI is a vexing problem with limited options. Although there are several studies supporting EMT use for treating UI, results have been both varied and seemingly unreliable (Patankar et al. Citation2007; Chehab et al. Citation2015; Usadi and Merriam Citation2015; Agarwal et al. Citation2019).
Herein, we test a hypothesis that in hypogonadal men with UI, those with the lowest serum TT would yield a significant improvement in post-CC TT level and semen characteristics.
Results
This is a retrospective IRB-approved chart review of 83 consecutive hypogonadal men with UI. Serum TT level and semen characteristics were studied in 83 patients before and in 23 patients after CC treatment. Descriptive statistics demonstrated that after CC treatment (),
Table 1. Summary of statistics
serum TT level increased from 256 ng/dL to 601 ng/dL (, . Mann–Whitney U-test demonstrated that the difference between the frequency distributions was significant at p < 0.001. Seventy-five percent quantile intervals were (199, 316) and (408, 803) respectively. Fold change (FC) is the ratio of two values providing a measure of how much the estimates of a random variable differ; FC provides rigor and better quantifies a treatment effect. FC increment (defined as post-CC TT to pre-CC TT values ratio) of the post-treatment mean and median values were 2.3 and 2.4, respectively. Similar results were obtained after applying t-testing (p < 0.001). TT level mean value was significantly increased from 250±83.6 ng/dL (n = 81) to 589±265.5 ng/dL (n = 23) at p < 0.001 ().
Figure 1. Box and Whisker plots of testosterone (TT) and semen concentrations. In men with unexplained infertility these variables are increased after treatment with clomiphene citrate (CC). Upper panel: TT concentration, lower panel: semen concentration
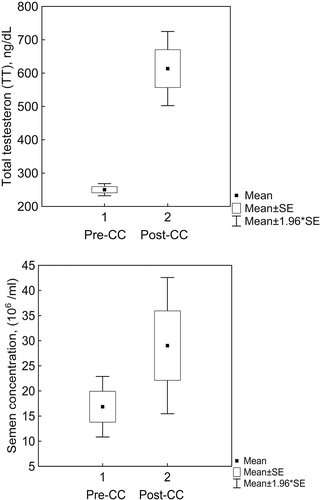
We also carried out a similar analysis for sperm characteristics including volume, concentration, morphology and motility. After treatment, mean values of SC increased from a baseline of 16±26.0 (/ml (n = 80) to 28±32.3 (
/ml) (n = 23) in the post-CC treatment group. Median values were 5.8 (
/ml) and 20.5 (
/ml) respectively; 75% quantiles (0.4, 17.5) and (4.2, 34,0)
/ml, respectively (). FC increments of the post-treatment mean and median values were 1.72 and 3.5, respectively. Mann–Whitney U-test demonstrated that the difference between the pre CC and post-CC treatment SC distributions was significant at p < 0.016. The t-test also showed significance (p < 0.01). Again, variables in the groups were treated as independent samples. The other sperm parameters (motility and morphology) did not show a significant response to CC treatment.
Next, we tested a hypothesis that CC treatment increased TT and sperm concentration regardless of which TT cut point was used. Wilcoxon matched pairs test yielded high confidence differences for both TT and SC (p = 0.0003 and p = 0.028 for TT respectively). Median TT after CC treatment increased from 265 ng/dL to 630 ng/dL. Median post-treatment sperm concentrations increased from 4.3 (/ml) to 20.5 (
/ml).
The Spearman rank correlation coefficient between TT post-CC and TT pre-CC levels was (r = 0.70, p = 0.001) in the studied group. The linear regression model displayed a significant positive increment in post-CC TT level regardless of the TT cut points (). This indicates a significant positive increment in post-CC TT level. This was further confirmed by the mean value ratio, which improved 2.5-fold (613/272) after treatment. Importantly, shows that CC treatment effect was realized in 22 of the 23 patients. This result is highly significant (p = 0.000003 by Binomial distribution test, Toh et al. Citation2011). We observed similar results for SC in the same patients. Spearman rank correlation analysis between SC post-CC and SC pre-CC levels was r = 0.80 (p = 0.002, ANOVA Fisher test). Interestingly, that shift parameter indicates positive increment of the SC equals 13 (/ml) with a standard deviation of 5.8 (
/ml) (p = 0.038).
Figure 2. Paired samples testosterone (TT) – sperm concentration at clomiphene citrate (CC) pre-treatment (Pre-CC) and post-treatment (Post-CC). A: TT levels. B: sperm concentration. C: Correlation analysis and linear regression model, CC treatment effect at different baseline TT. D: Changes of TT after breakdown of lower/higher TT concentration at 264 ng/dL (n = 23)
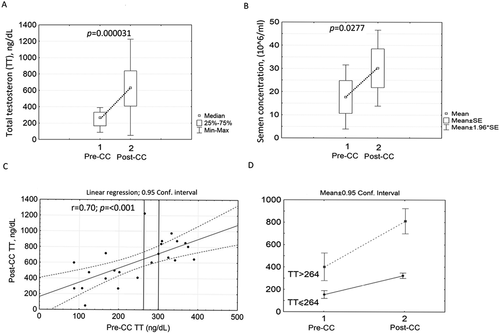
We initially hypothesized that patients with a TT<264 would have a greater improvement in post-CC TT values. In order to do this, we tested our hypothesis that worsening (lower scalability) TT would predict a more robust treatment response. To that end, we split the 23 patients into two groups based on a pre-CC treatment TT cutoff value of 264 ng/dL. Eleven patients were in the TT ≤ 264 group, and 12 were in the TT > 264 ng/dL group. This finding was discordant with our initial hypothesis as the treatment response for TT was improved in both groups. We used Wilcoxon Matched Pairs test to compare TT level before and subsequent to CC treatment and found that TT level was significantly increased in both groups (p = 0.004 in TT ≤ 264 group and p = 0.002 in TT > 264 group). We also observed a 2.6-fold median increment in the TT≤ 264 group and a 2.3-fold median increment in the TT > 264 group (). Furthermore, SC median was increased by 1.45-fold in the TT≤ 264 group and 2.5-fold increment in the TT>264 group (p < 0.03). Of note, a similar analysis using the t-test confirmed the above results.
The independent groups data analysis will increase the confidence and robustness of this paired study because with a larger pre-treatment group size (81 samples), the power of tests increases. Making the result more rigorous, we: (1) excluded from the Pre-treatment group the 23 patients havening post-treatment outcome, (2) randomized TT data of other patients, and (3) splinted that subsets on the mutually independent groups with size 23 samples in each group. Using all pre-treated groups and the 23 samples of post-treated group, we carried out multiple comparisons using Kruskal–Wallis ANOVA and median tests. Both tests showed that only the post-treated 23 sample group was statistically different vs the pre-CC treatment subgroups at p < 0.001 and there were no significant differences between any pre-treatment subgroups.
Thus, the un-paired pre-treatment data comparison provides fully complementary results to our pre- and post-treatment 23 paired data. These datum comparisons with post-treatment data increase the power and robustness of our data analysis and strongly support our working hypothesis of significance of treatment effect in pre-CC treatment TT<265 group.
Discussion
It has been established that SERMs, such as CC, should be considered as a treatment option for hypogonadal, infertile men. Such treatment has been found to be effective, have minimal side effects/complications, and low cost (Patankar et al. Citation2007; Chua et al. Citation2013; Chehab et al. Citation2015; Usadi and Merriam Citation2015; El Meliegy et al. Citation2017; Wheeler et al. Citation2019). Notably, an optimal CC dose has not been established, with dosing recommendations ranging from 12.5 to 400 mg per day (Chehab et al. Citation2015). Contemporary CC dosing starts at either 25 or 50 mg daily, with this cohort starting at 50 mg daily. It is worth noting that this study used serum TT as the indication for treating men with UI (required to have a normal hypothalamic-pituitary-gonadal axis) with CC, and not an effort to treat men with symptoms of hypogonadism/andropause.
Hypogonadism is generally defined as a low serum TT with several definitions (or cut points) used by practitioners. The 300 ng/dL represents the AUA guideline definition for hypogonadism and is the most common definition utilized by male infertility literature (Menke et al. Citation2010). Alternatively, TT levels less than 400 ng/dL are suggested by the endocrine society (Practice Committee for the American Society for Reproductive Medicine in collaboration with the Society for Male Reproduction and Urology Citation2008). Finally, 264 ng/dL represents the result from a contemporary study which investigated 4 cohorts of healthy non-obese European and American men between the ages of 19 and 39, highlighted by a TT which ranged from the 2.5th percentiles of 264 ng/dL and 916 ng/dL (Travison et al. Citation2017). Given the varied definitions for hypogonadism and the lack of guidelines for treating men with UI, it is necessary to reevaluate the effect of CC on TT and semen characteristics at all three literature-based TT cut points.
In this study, hypogonadal men with UI demonstrated improved TT and sperm concentration after CC treatment, regardless of the serum TT cut point used. Our findings suggest that pre-treatment TT level is scalable for treatment benefit starting with lowest TT cut point used to define hypogonadism. For this reason, men with baseline serum TT levels below 400 ng/dL should be considered for CC (or SERM) treatment as this resulted in post-treatment improvement in both TT and sperm concentration. Again, as there was no significant deviation from the linear regression pattern at any of the three TT cut-points, CC should be considered a valuable option for treating men with UI presenting with baseline serum TT < 400 ng/dL (and possibly higher).
Limitations of this study include the fact that it was a retrospective analysis of only 23 paired pre- and post-CC – lacking strict control for serum TT collection time. This may have affected some TT measurements as males with blood draws later than 11:00 AM could have a false low TT due to the diurnal variation in male testosterone production. In addition, surveys were not used to assess symptoms of hypogonadism. Finally, neither free testosterone, calculated bioavailable testosterone, nor post-CC estradiol levels were available for review.
While one meta-analysis of randomized controlled trials demonstrated that SERM treatment is associated with statistically significant increases in sperm concentration, motility, and pregnancy rates (Chua et al. Citation2013), not all similar studies are able to confirm such a desirous outcome (Willets et al. Citation2013). It seems necessary to further investigate if the positive effects of CC treatment on TT level and sperm concentration associates with improved pregnancy and live birth outcomes. Future studies should include a larger cohort size, post-CC pregnancy and live birth rates, and a study designed with the opportunity to evaluate personalized/multifactorial variables. Lastly, future studies may need to consider a higher serum TT cut point (>400 ng/dL) for prompting EMT in hypogonadal men with UI.
In conclusion, CC improved TT and sperm concentration regardless of which baseline serum TT cut point was used to define hypogonadism. Therefore, all men with UI presenting with any TT less than 400 ng/dL should consider CC treatment as a cost-effective EMT infertility treatment option. Ultimately, to optimize pregnancy and live birth rates for such men, a multi-variant, longitudinal study of clinically relevant prognostic parameters should be considered.
Materials and methods
Study sample
We studied an IRB-approved chart review of 83 consecutive male patients with UI treated at our Upstate New York clinic. They filled out a standardized questionnaire – assessing differences in lifestyle, medication use, and prior utilization of assisted reproduction [in-vitro fertilization (IVF) and intrauterine insemination (IUI)]. The men had a mean age of 35 years compared to 32 years for their partners. The initial office visit was the first exposure to a male infertility expert for 89% of the men with some couples even reporting prior failed attempts at IUI and IVF (7.6% and 6.1%, respectively) without prior male partner evaluation.
We excluded the following patients: (1) those with a baseline TT > 400 ng/dL (as they were not offered CC at our facility), (2) those received letrozole, (3) those with azoospermia or not meeting criteria for UI (such as genetic findings or, evidence for primary/secondary hypogonadism), or (4) those missing pre- or post-treatment semen analysis values. Ultimately, 23 patients had their pre- and three-month-post-CC treatment sperm characteristics and TT levels analyzed. Semen analysis was performed using 2010 WHO guidelines on ejaculated specimens with a 3–5 day abstinence period. After liquefaction for 30 minutes, semen samples were evaluated for sperm count, motility and strict Kruger morphology. Serum TT values were tested using serum radioimmunoassay analysis.
Statistical analysis
We used the following statistical analyses: one-way ANOVA, t-test, Mann–Whitney U-test, Wilcoxon matched pairs test, Spearman correlation coefficient and linear regression analyses. Statistical significance was defined by a p-value <0.05. For data analysis, we used Statistica-7 and IBM-SPSS-25 software. Binomial distribution test was calculated by (Toh et al. Citation2011). These methods were used in order to evaluate the differences and correlations in paired pre- and post-CC treatment serum TT level and SC.
Ethics approval
An IRB-approved chart review of 83 consecutive male patients with UI treated at our Upstate New York clinic.
Author contributions
Data collection: JT, AD, RK; manuscript preparation, analysis and interpretation: JT, VK; manuscript discussion and review: JT, AD, RK, VK; statistical setup and final version of manuscript writing: VK.
Acknowledgments
We thank Patrick Curtin MD and Joseph Hartnett MD for their work on this project and Callum Newton for assistance with database maintenance.
Disclosure statement
Trussell, Delu, Kiltz, and Kuznetsov have no conflict of interest.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
References
- Agarwal A, Parekh N, Panner Selvam MK, Henkel R, Shah R, Homa ST, Ramasamy R, Ko E, Tremellen K, Esteves S, et al. 2019. Male oxidative stress infertility (MOSI): proposed terminology and clinical practice guidelines for management of idiopathic male infertility. World J Men’s Health. 37(3):296–312. doi:https://doi.org/10.5534/wjmh.190055
- Bhasin S, Brito JP, Cunningham GR, Hayes FJ, Hodis HN, Matsumoto AM, Snyder PJ, Swerdloff RS, Wu FC, Yialamas MA. 2018. Testosterone therapy in men with hypogonadism: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 103(5):1715–1744.
- Centers for Disease Control and Prevention (2009). Infertility FAQs. [accessed 2012 Jun 11]. http://www.cdc.gov/reproductivehealth/infertility.
- Chehab M, Madala A, Trussell JC. 2015. On-label and off-label drugs used in the treatment of male infertility. Fertil Steril. 103(3):595–604. doi:https://doi.org/10.1016/j.fertnstert.2014.12.122.
- Chua ME, Escusa KG, Luna S, Tapia LC, Dofitas B, Morales M. 2013. Revisiting oestrogen antagonists (clomiphene or tamoxifen) as medical empiric therapy for idiopathic male infertility: a meta-analysis. Andrology. 1(5):749–757. doi:https://doi.org/10.1111/j.2047-2927.2013.00107.x.
- Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HWG, Behre HM, Haugen TB, Kruger T, Wang C, Mbizvo MT, et al. 2010. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 16:231–245. doi:https://doi.org/10.1093/humupd/dmp048.
- Dabaja A, Schlegel PN. 2014. Medical treatment of male infertility. Transl Androl Urol. 3(1):2223–4683. doi:0.3978/j.issn.2223-4683.2014.01.06
- El Meliegy AE, Motawi A, El Salam MAA. Systematic review of hormone replacement therapy in the infertile man. Arab J Urol. 2017;16(1):140–147. doi:https://doi.org/10.1016/j.aju.2017.11.011
- Kumar N, Singh AK. 2015. Trends of male factor infertility, an important cause of infertility: a review of literature. J Hum Reprod Sci. 8(4):191–196. doi:https://doi.org/10.4103/0974-1208.170370.
- Menke A, Guallar E, Rohrmann S, Nelson WG, Rifai N, Kanarek N, Feinleib M, Michos ED, Dobs A, Platz EA, et al. 2010. Sex steroid hormone concentrations and risk of death in US men. AM J Epidemiol. 171:583–592. doi:https://doi.org/10.1093/aje/kwp415.
- Mohamed O, Freundlich RE, Dakik HK, Grober ED, Najari B, Lipshultz LI, Khera M. 2010. The quantitative ADAM questionnaire: a new tool in quantifying the severity of hypogonadism. Int J Impot Res. 22(1):20–24. doi:https://doi.org/10.1038/ijir.2009.35.
- Mulhall JP, Trost LW, Brannigan RE, Kurtz EG, Redmon JB, Chiles KA, Lightner DJ, Miner MM, Murad MH, Nelson CJ, et al. 2018. Evaluation and management of testosterone deficiency: AUA guideline. J Urol. 200:423–432. doi:https://doi.org/10.1016/j.juro.2018.03.115.
- Patankar SS, Kaore SB, Sawane MV, Mishra NV, Deshkar AM. 2007. Effect of clomiphene citrate on sperm density in male partners of infertile couples. Indian J Physiol Pharmacol. 51(2):195–198.
- Practice Committee for the American Society for Reproductive Medicine in collaboration with the Society for Male Reproduction and Urology. 2008. Androgen deficiency in the aging male. Fertil Steril. 90(suppl 3):S83–S87. doi:https://doi.org/10.1016/j.fertnstert.2008.08.094.
- Sigman M, Jarow JP. 1997. Endocrine evaluation of infertile men. Urology. 50:659–664. doi:https://doi.org/10.1016/S0090-4295(97)00340-3.
- Toh SH, Prathipati P, Motakis E, Kwoh CK, Yenamandra SP, Kuznetsov VA. 2011. A robust tool for discriminative analysis and feature selection in paired samples impacts the identification of the genes essential for reprogramming lung tissue to adenocarcinoma. BMC Genomics. 12(Suppl 3\(Suppl 3)):S24. doi:https://doi.org/10.1186/1471-2164-12-S3-S24.
- Travison TG, Vesper HW, Orwoll E, Wu F, Kaufman JM, Wang Y, Lapauw B, Fiers T, Matsumoto AM, Bhasin S, et al. 2017. Harmonized reference ranges for circulating testosterone levels in men of four cohort studies in the United States and Europe. J Clin Endocrinol Metab. 102(4):1161–1173. doi:https://doi.org/10.1210/jc.2016-2935
- Trussell JC, Coward RM, Santoro N, Stetter C, Kunselman A, Diamond MP, Hansen KR, Krawetz SA, Legro RS, Heisenleder D, et al. 2019. Association of low testosterone with semen parameters and live birth in men with unexplained infertility. Fertil Steril. 111:1129–1134. doi:https://doi.org/10.1016/j.fertnstert.2019.01.034.
- Usadi RS, Merriam KS. 2015. On-label and off-label drug use in the treatment of female infertility. Fertil Steril. 103(3):583–594. doi:https://doi.org/10.1016/j.fertnstert.2015.01.011.
- Wheeler KM, Sharma D, Kavoussi PK, Smith RP, Costabile R. 2019. Clomiphene citrate for the treatment of hypogonadism. Sex Med Rev. 7:272–276. doi:https://doi.org/10.1016/j.sxmr.2018.10.001.
- Willets AE, Crobo JM, Brown JN. 2013. Clomiphene for the treatment of male infertility. Reprod Sci. 20(7):739–744. doi:https://doi.org/10.1177/1933719112466304.
