ABSTRACT
For patients with recurrent implantation failure in IVF, histologic or transcriptomic testing of the endometrium during the mid-secretory phase is often considered. Histological dating of endometrial biopsies (Noyes criteria) can determine if endometrial morphology is consistent with the period of receptivity. Alternatively, endometrial tissue can be sent for a commercial Endometrial Receptivity Array (ERA) test which characterizes the gene expression of the endometrium using a panel of 238 genes that have been implicated in endometrial receptivity. This study aimed to compare the two tests to assess their concordance and to examine the ability of the ERA to successfully predict implantation and pregnancy in a subsequent personalized embryo transfer. A retrospective review was done of 97 patients with a history of implantation failure who underwent an ERA, 35 of whom had histologic dating on the same sample. ERA and histology were classified as ‘concordant’ when samples were receptive by both tests or non-receptive by both tests. The ERA result was then used to personalize the embryo transfer day, and pregnancy rates from the first subsequent frozen transfer cycle were analyzed. The results indicated that there is poor concordance between ERA and histological dating with only 40.0% agreement and a kappa (95%CI) = −0.18 (−0.50, 0.14). According to the ERA, 48.5% of biopsies were receptive, 47.4% were non-receptive and 2.01% were insufficient tissue for analysis. The clinical pregnancy rate in patients shown to be receptive by ERA was 26.7% and non-receptive was 22.5% following the subsequent personalized ET (p = 0.66). This study concludes that there is a high degree of discordance between histological dating of the endometrium and molecular analysis by ERA. There was no evidence of clinical benefit when embryo transfer was personalized according to ERA in patients with a history of implantation failure.
Introduction
While advancements in stimulation protocols and laboratory techniques have steadily improved in vitro fertilization (IVF) outcomes, implantation failure remains a clinical challenge. A leading problem in reproductive medicine is recurrent implantation failure (RIF), which refers to patients who fail to conceive after repeated transfers of high-quality embryos, although a consensus definition is still lacking with respect to the minimum number of failed transfers. An often-quoted definition proposed by Coughlan and colleagues defines RIF as the failure to achieve a clinical pregnancy after transfer of at least four ‘good-quality’ embryos, but proposed definitions have ranged from ≥3 failed ‘high-quality’ embryo transfers to ≥10 failed embryos (Stern et al. Citation2003; Simon and Laufer Citation2012; Coughlan et al. Citation2014). Successful implantation requires an intricate dialogue between a competent embryo and a receptive endometrium. While failed implantation is often attributed to embryonic factors, after multiple high-quality embryos have failed to implant, non-embryonic factors such as defects in endometrial receptivity must be considered (Revel Citation2012).
Our understanding of human endometrial receptivity is nascent, as is our ability to evaluate the endometrium for its readiness for implantation. It is known that the endometrial environment is not conducive to implantation throughout the menstrual cycle. Classic studies have established that the endometrium is only receptive during a narrow ‘window of implantation (WOI)’ that coincides with the development of a blastocyst. In physiologic cycles, this WOI starts in the mid-secretory phase, approximately 7 days after the LH surge and lasts for 4–5 days (Bergh and Navot Citation1992; Wilcox et al. Citation1999). In assisted reproduction, this receptive state is recapitulated by the supraphysiologic levels of estrogen and progesterone produced in fresh embryo transfer cycles or by exogenous hormones administered in the case of frozen embryo transfers.
Although the temporal boundaries of the physiologic WOI have been delineated during the natural menstrual cycle, accurate diagnostic tools to assess endometrial receptivity are lacking for patients with infertility or RIF. The standard of care for endometrial evaluation in IVF is ultrasonographic measurement of endometrial thickness during the proliferative phase. However, ultrasound measurements are prone to observer variability, have poor predictive value for implantation, and are often normal in the RIF population (Järvelä et al. Citation2005; Bonilla-Musoles et al. Citation2013). Since the 1950s, histological dating of secretory phase endometrial biopsies using Noyes’ criteria has been used in ‘mock’ cycles to assess whether the endometrium exhibits appropriate histological response to the duration of progesterone exposure (Noyes et al. Citation1950). However, the functional relevance of histological dating has been called into question, and its accuracy suffers from significant intra- and inter-observer variability (Smith et al. Citation1995; Murray et al. Citation2002).
More recently, the Endometrial Receptivity Assay or ERA (Igenomix, Valencia, Spain) was developed as an objective transcriptomic test of endometrial receptivity, which characterizes the endometrium as ‘receptive’ or ‘non-receptive’ based on the expression of 238 genes (Díaz-Gimeno et al. Citation2011). The developers of this test have suggested that the window of implantation may be displaced in some patients with RIF, leading to the concept of personalized embryo transfer (pET) determined by an individual’s transcriptomic panel (Ruiz-Alonso et al. Citation2013). Like histological dating, the ERA requires an endometrial biopsy obtained in a mock cycle, but it is significantly more costly. Although this commercial test is purportedly superior to histological dating in fertile populations (Díaz-Gimeno et al. Citation2013), this has not been clearly demonstrated in the RIF population. While most of the available literature on ERA outcomes has been published by the ERA developers (Díaz-Gimeno et al. Citation2013; Ruiz-Alonso et al. Citation2013; Citation2014), several other independent research teams have started to evaluate the outcomes of pET using the ERA. These studies have been limited by small sample sizes (Hashimoto et al. Citation2017; Tan et al. Citation2018; Bassil et al. Citation2018), and skewed toward patients with more favorable prognoses (Tan et al. Citation2018; Bassil et al. Citation2018; Neves et al. Citation2019)
The objectives of this study we twofold: a) to compare the results of histological dating to ERA testing, and 2) to assess the ability of the ERA to predict implantation and pregnancy in a subsequent pET in patients with implantation failure. To our knowledge, this is the largest report of the validity of ERA as a diagnostic tool and of the outcomes following pET, exclusively in patients with a history of implantation failure.
Results
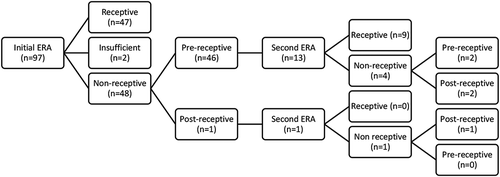
Results of the ERA were reported for 97 patients with a history of implantation failure, with 35 undergoing concurrent histological dating. There was a total of 151 personalized embryo transfer cycles from 97 patients included in the data set. Of these 97 patients, 65 (67%), 20 (20.6%), 7 (7.2%), 1(1%), 3 (3.1%) and 1 (1%) patients had 1,2,3,4,5 and 6 personalized embryo transfer cycles following ERA, respectively. Initial ERA results were R in 48.5% (47/97) of patients, NR in 47.4% (48/97) of patients and insufficient in 2.01% (2/97). These numbers are reflected in . Timing of non-receptive results varied from 12 hours pre- or post- WOI to 48 hours pre- or post-WOI. The patients with insufficient endometrial biopsies and those with missing personalized embryo transfers were excluded. The remaining 93 patients (146 cycles) formed the study population (NR = 46, R = 47).
Figure 1. Pathway of ERA testing.The following infographic depicts the diagnostic workup for patients, beginning with initial ERA. Patients who were receptive on the first ERA continued to embryo transfer without any further workup. Patients who were non-receptive had the option to continue to embryo transfer or were offered a second ERA with an adjusted mock-cycle. 14/48 patients chose to re-try their ERA with the results indicated above
shows the baseline demographics of these patients, with no significant differences identified between the R and NR groups in terms of age, BMI, or history of failed cycles. There was a statistical but not clinical difference in ovarian reserve (AFC, AMH) between the groups. Prior to ERA, the R group had failed to conceive after the consecutive transfer of a mean of 3.85 embryos and the NR group a mean of 3.78 embryos (p = 0.89). A similar proportion of women in both groups had undergone pre-implantation genetic testing – aneuploidy (PGT-A) (36.4% R vs 28.6% NR, p = 0.45) or egg donation (19.6% R vs 8.7% NR, p = 0.17). An average number of 1.17 embryos were transferred in the R group and 1.25 in the NR group (p = 0.70).
Table 1. Comparison of the characteristics for all patients in the study for their first ERA
Outcomes following pET
represents the clinical outcomes for women who underwent a subsequent pET guided by their ERA results. For the R group, the implantation rate from pET was 25.0%, where 26.7% achieved clinical pregnancy. 10% of these women went on to have a live birth. In comparison, the implantation rate from pET for NR women was 35.5%, clinical pregnancy achieved in 22.5% and 0 patients went on to have a live birth.
Table 2. Comparison of outcomes for the first personalized embryo transfer cycle following ERA
We conducted a multiple logistic regression analysis to further compare the clinical pregnancy rate adjusted for the number of embryos transferred. For all first cycles following the ERA test, of 93 patients, the odds ratio (95%CI) of clinical pregnancy (receptive vs non-receptive) was 1.22 (0.45, 3.30). No statistical significance was observed.
Concordance
The endometrial receptivity results of 35 ERA biopsies were compared to histological dating of the same biopsies for concordance. As shown in , the two tests were only concordant in 14 cases (40%). Analysis revealed a Kappa score (95%CI) of −0.18 (−0.50, 0.14), indicating no agreement.
Table 3. Comparison of ERA and histology reports for their first ERA
Subgroup analyses
Patients who were initially NR were given the option to repeat their ERA, adjusting the mock-cycle in accordance with their ERA result (i.e., if results indicated 24 hours pre-receptive then repeat biopsy was done 24 hours later). Alternatively, patients could continue to pET with the same adjustments. In , we compared NR ERA patients who did and did not repeat their ERA prior to embryo transfer. Within the NR category, 95.8% of patients were pre-receptive and 4.2% were post-receptive on initial ERA. 30.4% (14/46) of NR patients underwent a second confirmatory ERA. Of these women, 64.3% (9/14) of patients were R on the second cycle while 35.7% (5/14) were still NR on their second ERA (3 pre-receptive; 2 post-receptive). Of patients with repeat biopsy, only 10 pursued further embryo transfer cycles (8 receptive, 2 post-receptive).
Table 4. Comparison of outcomes between those patients with repeated and without repeated ERA for all embryo transfer cycles
Clinical pregnancy rates were 33.3% for those who chose to repeat their ERA and 38.7% for those who did not (). The miscarriage rate was 5.0% for receptive and 11.76% for all non-receptive patients.
Although the results of histological dating were not used to inform embryo transfers, a subgroup analysis of outcomes was done for comparison (). Of the 35 biopsies, 27 women underwent further embryo transfers. In the histologically receptive patients, the mean implantation rate was 18.2%, clinical pregnancy was 27.3% and the live birth rate was 0%. For histologically non-receptive patients, the rates were 13.3%, 25.0%, and 7.14%, respectively. The miscarriage rate was 27.3% for receptive and 6.67% for non-receptive. There was also no statistical difference between groups.
Table 5. Comparison of outcomes controlling for initial histology result for all patient embryo transfer cycles
Discussion
We have presented the largest available series that compares ERA testing to histological dating in patients with implantation failure that follows the outcomes of their embryo transfers personalized to the transcriptomic result. We demonstrated poor concordance between the commercially available transcriptomic test and traditional histological dating by Noyes criteria performed by an expert gynecologic pathologist. Our study did not demonstrate significant differences in pregnancy outcomes in the subsequent frozen ET for patients who were receptive versus non-receptive by either ERA or histological assessment.
The first study to propose that ERA was superior to histology compared the ability of each test to predict the timing of biopsy from LH surge (Díaz-Gimeno et al. Citation2013). However, this study was performed on a population of healthy ovum donors. A study by Hashimoto et al. (Citation2017) on patients with RIF (defined as failure of ≥3 embryo transfers) demonstrated 80% concordance between ERA determination of receptivity and histological dating, but the pathologists were not blinded to the timing of biopsy therefore limiting the reliability of the results. In our larger series, with a blinded expert gynecologic pathologist, a significantly lower concordance (40%) between the two tests than previously reported.
Further complicating concordance, the proportion of receptive vs non-receptive ERA results in patients with RIF has also varied significantly in the literature. The reported rate of NR in RIF patients has ranged from 24% to 44% in previous studies (Ruiz-Alonso et al. Citation2013; Hashimoto et al. Citation2017; Tan et al. Citation2018; Neves et al. Citation2019). Our study reported an even higher rate of non-receptivity on ERA (49%) in our patient population than previously reported. A challenge with cross-study comparison is the heterogeneity of the patient population in different studies. As there is no consensus definition for RIF, each study selected its own unique definition of the condition.
The inclusion criteria to have ERA at our clinic and be part of this study was the failure of ≥2 consecutive embryo transfers with morphologically high-quality blastocysts. However, the mean number of failed embryos transferred in our study population was 3.8 embryos. Therefore, our study findings represent a population quite likely to have a true endometrial receptivity dysfunction. What remains fairly consistent in our study and others is that majority of ERA-tested NR samples are displaced toward a pre-receptive signature rather than a post-receptive one. Our study indicated that 96% of NR samples are pre-receptive in the RIF population, and this figure has ranged from 67% to 84% in previous studies (Ruiz-Alonso et al. Citation2013; Hashimoto et al. Citation2017; Tan et al. Citation2018). This finding is interesting as historic histological studies of stimulated IVF cycles have identified the opposite trend of advanced rather than delayed endometrial maturation, yet most RIF patients in hormone-replaced mock cycles are found to be pre-receptive by ERA. This phenomenon certainly warrants further mechanistic investigation.
Our study also highlights a potential issue with ERA test reproducibility. Of the 14 patients with an initial NR ERA who chose to have a second ‘confirmatory’ ERA on the day recommended by the test result, 5/14 (36%) still had an NR signature on the repeat biopsy. This finding either reflects poor reproducibility of the ERA test or suggests that the NR signature may not always be ‘fixed’ by more exposure to progesterone. This finding is divergent from a previous report from the ERA test developers that showed a 100% reproducibility of the ERA result when repeated on the same cycle day within 29–40 months from the initial biopsy (Díaz-Gimeno et al. Citation2013). However, this report was limited in sample size as only seven healthy fertile control patients were studied. In general, it should be acknowledged that the gene expression of any subsequent endometrial biopsy may be altered by previous sampling, as demonstrated by a previous paper by Kalma et al. (Citation2009).
Previous observational studies have reported a wide range of pregnancy outcomes after pET in patients with RIF. Ruiz-Alonso et al. (Citation2013) demonstrated an implantation rate of 33.9% in a subsequent pET in patients who were R on initial ERA, and 38.5% in patients who were NR (Ruiz-Alonso et al. Citation2013). However, this study consisted of low numbers, with only 37 patients analyzed (29 in the R group and eight in the NR group).
More recently, two other groups have contrasted the outcomes of pET in RIF patients with R versus NR ERA results. Hashimoto et al. (Citation2017) reviewed 50 patients with RIF (defined as failure of ≥3 embryo transfers) who had endometrial sampling for ERA and found no significant difference between pET outcomes (implantation rate 32.8% in R group vs. 31.6% in NR group, p = 0.92). A study by Tan et al. (Citation2018) of 30 patients undergoing pET with PGT-A screened euploid embryos also showed no significant difference in implantation rates (44.4% in R group vs. 66.7% in NR group, p = 0.28). This study included patients that chose to have an ERA prior to euploid embryo transfer, some of whom had a history of RIF defined as failure of ≥2 embryo transfers, and some of whom did not have a history of implantation failure at all. A related study by Neves et al. (Citation2019) on PGT-A and donor oocyte cycles compared post-ERA transfer rates to embryo transfers in the same period without performing an ERA. The patients in this study had failed ≥1 previous euploid embryo transfer or ≥2 previous donor oocyte-embryo transfers. They found no difference in implantation rates in the PGT-A euploid embryo transfer arm whether or not ERA was performed (55.6% post-ERA vs. 65.0% control). However, there was an unexpected finding of a significant reduction in implantation rate in the donor oocyte-embryo transfer arm when ERA was performed (26.8% post-ERA vs. 57.2% control). The pregnancy rates in the latter two studies were higher than our study because the patients selected were of more favorable prognosis than those in previous studies. They had failed fewer previous transfers on average (some with no history of RIF) and had chosen to do PGT-A to screen for euploid embryos or use donor oocytes.
Our study builds on the existing research as it looks more closely at a larger series of patients with a history of implantation failure. As with previous studies, we found that pregnancy outcomes are still relatively poor for these patients, despite ERA sampling and personalized ET. We demonstrated an implantation rate of 25.0% in the R group vs. 35.5% in the NR group, p = 0.43. Interestingly, Bassil et al. (Citation2018) found similarly low clinical pregnancy rates in their study following ERA in a normal, non-RIF population (Bassil et al. Citation2018). The study concluded that there was no improvement in ongoing pregnancy rates after performing ERA testing and very little concordance between histology and ERA. These results call into question the validity of the ERA as a prognostic indicator and the effectiveness of personalizing embryo transfer decisions based on the results of ERA testing.
There are a few limitations to this study and its retrospective design. We lacked a control group of RIF patients who did not undergo ERA but rather had standard FET on P + 5. The ideal RIF control group would have had a sham endometrial biopsy in a mock cycle to control for possible effects of the biopsy itself, but it was not possible to find such a group retrospectively. Additionally, our RIF patients were heterogeneous in their decision to do PGT-A, so our pET outcomes were not optimized for embryonic factors. Moreover, the timing of pET in our clinic was based exclusively on the ERA result rather than histological dating. To our knowledge, there are no studies that personalize the day of embryo transfer based on histological analysis alone. It is important to recognize that ERA is a relatively new commercial test. As such, we are currently underpowered to detect significant differences in pregnancy outcomes between groups due to sample size limitations. To rigorously compare the predictive value of ERA testing to histological dating, further prospective studies comparing personalization of ET by ERA versus personalization by histological dating are necessary.
Materials and methods
Design and study population
In this retrospective cohort study, data were reviewed from 97 women aged 29–42 years old who underwent endometrial sampling for ERA and histological dating at Mount Sinai Fertility, Toronto, Canada, from May 2014-March 2019. The inclusion criteria for ERA testing at our clinic were the failure of at least two consecutive embryo transfers with morphologically high-quality blastocysts. The decision to have ERA testing was made by the patient in consultation with her physician. Patients were excluded if they did not have a structurally normal uterine cavity, as assessed by sonohysterogram or hysteroscopy, a BMI > 40, or were not considered RIF by our clinic's definition.
Endometrial biopsy
Each patient underwent a mock endometrial preparation cycle based on our typical endometrial preparation for frozen embryo transfer. This consisted of micronized 17 beta-estradiol (Estrace, Acerus Pharmaceuticals, Canada) 4 mg twice daily per vagina starting on day 2 of the menstrual cycle and continued for a minimum of 12 days until the endometrial lining measured ≥8 mm by transvaginal ultrasound. Micronized progesterone (Prometrium, Merk, Canada) 200 mg three times daily per vagina was then started. After five full days of progesterone treatment (P + 5), an endometrial biopsy was performed at the time a frozen-thawed embryo transfer would normally occur. An ‘ENDOCELL’ pipelle (Wallach Surgical Devices, Connecticut, USA) was used to perform the endometrial biopsy in standard aseptic fashion. The main portion of each biopsy was processed and sent for ERA testing according to the manufacturer’s protocol. In 35 women who had sufficient tissue collected and who consented, a portion of the biopsy was also sent to the Mount Sinai Hospital pathology lab in Toronto, Canada, for histological dating based on Noyes’ criteria (Noyes et al. Citation1950). The samples were analyzed by an expert gynecologic pathologist blinded to the ERA result or the day of biopsy.
The ERA designation of ‘receptive’ (R) vs. ‘non-receptive’ (NR) was provided by Igenomix, based on their proprietary analysis of expression of 238 genes. R samples expressed a transcriptomic signature on P + 5 that was deemed by the algorithm to be receptive to implantation. NR samples were further divided into ‘pre-receptive’ and ‘post-receptive,’ both expressing a transcriptomic signature deemed to be non-receptive to implantation on P + 5. Insufficient samples were characterized by a lack of or too little endometrial tissue sample to be analyzed by histopathological means or by RNA analysis for ERA.
In keeping consistence with previous studies, we defined receptive (R) histologic samples as those that exhibited the expected histological appearance of post-ovulatory day 5 (POD 5), consistent with the number of days (P + 5) of progesterone exposure at the time of endometrial biopsy (Hashimoto et al. Citation2017). Non-receptive (NR) histology samples were defined as those histologically dated earlier or later than POD 5. An insufficient result for both ERA and histological dating was given when there was not enough sample tissue to be analyzed.
Personalized embryo transfer
Patients with an R ERA result would undergo a pET in a subsequent cycle using the same protocol and on the same cycle day as their initial ERA. Patients with an NR ERA result chose either to undergo another mock cycle with repeat ERA testing on the altered day recommended by their ERA result, or to proceed with pET on the recommended day without a second confirmatory biopsy (). All personalized embryo transfer decisions were based on the ERA result, not histological testing. All embryos were transferred at the blastocyst stage.
Statistical analysis
The study population was summarized using descriptive statistical methods. Patient characteristics were compared between two groups: first ERA receptive and non-receptive using Chi-square test for categorical variables and Student T-test or Wilcoxon rank-sum test, as appropriate, for continuous variables. To examine the impact of the first ERA on the primary outcome of clinical pregnancy, the primary outcomes were compared using Fisher exact test. We further conducted a multiple logistic regression to determine the impact of ERA on the primary outcome adjusted for the number of embryos transferred. Similar methods were used to examine the association between the first ERA and the other secondary outcomes. We used kappa statistics to assess the degree of the agreement between the ERA and histologic dating samples. The concordance and disconcordance between the two tests were also reported. Due to a smaller sample size in the histology group, the association between the histologic dating and outcomes of embryo transfer were examined descriptively. The data management and all statistical analyses were performed using SAS 9.4 (SAS institute Inc., Cary, NC). A two-sided p-value of <0.05 was considered to be statistically significant.
Ethics Approval
This study was approved by the Institutional Review Board of Mount Sinai Hospital, Toronto, Canada. Informed consent was obtained from all participants.
Author’s contributions
All authors contributed significantly to the study design, acquisition of data, analysis or interpretation, revisions, and final approval of the paper as outlined below. Study design, acquisition of data and interpretation of variables, final approval, student investigator, and primary author: AMC; statistical analysis, revisions and final approval: XYY; interpretation of all histologic dating, revisions and final approval: TJC; study design, selection and interpretation of variables, revisions, final approval, and co-primary investigator: EMG, CC.
Disclosure statement
The authors of this paper have no financial, personal, or professional conflicts of interest to disclose.
Additional information
Funding
References
- Bassil R, Casper R, Samara N, Hsieh T, Barzilay E, Orvieto R, Haas J. 2018. Does the endometrial receptivity array really provide personalized embryo transfer. J Assist Reprod Genet. 35(7):1–5. doi:https://doi.org/10.1007/s10815-018-1190-9.
- Bergh PA, Navot D. 1992. The impact of embryonic development and endometrial maturity on the timing of implantation. Fertil Steril. 58(3):537–542. doi:https://doi.org/10.1016/S0015-0282(16)55259-5.
- Bonilla-Musoles F, Raga F, Osborne NG, Castillo JC, Bonilla F Jr. 2013. Endometrial receptivity: evaluation with ultrasound. Ultrasound Q. 29(1):3–20. doi:https://doi.org/10.1097/RUQ.0b013e318281b60a.
- Coughlan C, Ledger W, Wang Q, Liu F, Demirol A, Gurgan T, Cutting R, Ong K, Sallam H, Li TC. 2014. Recurrent implantation failure: definition and management. Reprod Biomed Online. 28(1):14–38. doi:https://doi.org/10.1016/j.rbmo.2013.08.011.
- Díaz-Gimeno P, Horcajadas JA, Martínez-Conejero JA, Esteban FJ, Alamá P, Pellicer A, Simón C. 2011. A genomic diagnostic tool for human endometrial receptivity based on the transcriptomic signature. Fertil Steril. 95(1):50–60. doi:https://doi.org/10.1016/j.fertnstert.2010.04.063.
- Díaz-Gimeno P, Ruiz-Alonso M, Blesa D, Bosch N, Martínez-Conejero JA, Alamá P, Garrido N, Pellicer A, Simón C. 2013. The accuracy and reproducibility of the endometrial receptivity array is superior to histology as a diagnostic method for endometrial receptivity. Fertil Steril. 99(2):508–517.
- Hashimoto T, Koizumi M, Doshida M, Toya M, Sagara E, Oka N, Nakajo Y, Aono N, Igarashi H, Kyono K. 2017. Efficacy of the endometrial receptivity array for repeated implantation failure in Japan: A retrospective, two‐centers study. Repro Med Biol. 16(3):290–296. doi:https://doi.org/10.1002/rmb2.12041.
- Järvelä IY, Sladkevicius P, Kelly S, Ojha K, Campbell S, Nargund G. 2005. Evaluation of endometrial receptivity during in‐vitro fertilization using three‐dimensional power Doppler ultrasound. Ultrasound Obstet Gynecol. 26(7):765–769. doi:https://doi.org/10.1002/uog.2628.
- Kalma Y, Granot I, Gnainsky Y, Or Y, Czernobilsky B, Dekel N, Barash A. 2009. Endometrial biopsy-induced gene modulation: first evidence for the expression of bladder-transmembranal uroplakin Ib in human endometrium. Fertil Steril. 91(4):1042–1049. doi:https://doi.org/10.1016/j.fertnstert.2008.01.043.
- Murray MJ, Meyer WR, Lessey BA, Zaino RJ, Novotny DB, Fritz MA. 2002. Endometrial dating revisited: a randomized systematic study of secretory phase histologic characteristics in normally cycling fertile women. Fertil Steril. 78:S67. doi:https://doi.org/10.1016/S0015-0282(02)03555-0.
- Neves AR, Devesa M, Martínez F, Garcia-Martinez S, Rodriguez I, Polyzos NP, Coroleu B. 2019. What is the clinical impact of the endometrial receptivity array in PGT-A and oocyte donation cycles? J Assist Reprod Genet. 36(9):1901–1908. doi:https://doi.org/10.1007/s10815-019-01535-5.
- Noyes RW, Hertig AI, Rock J. 1950. Dating the endometrial biopsy. Fertil Steril. 1:3–25. doi:https://doi.org/10.1016/S0015-0282(16)30062-0.
- Revel A. 2012. Defective endometrial receptivity. Fertil Steril. 97(5):1028–1032. doi:https://doi.org/10.1016/j.fertnstert.2012.03.039.
- Ruiz-Alonso M, Blesa D, Díaz-Gimeno P, Gómez E, Fernández-Sánchez M, Carranza F, Carrera J, Vilella F, Pellicer A, Simón C. 2013. The endometrial receptivity array for diagnosis and personalized embryo transfer as a treatment for patients with repeated implantation failure. Fertil Steril. 100(3):818–824. doi:https://doi.org/10.1016/j.fertnstert.2013.05.004.
- Ruiz-Alonso M, Galindo N, Pellicer A, Simon C. 2014. What a difference two days make: “personalized” embryo transfer (pET) paradigm: a case report and pilot study. Hum Reprod. 29(6):1244–1247. doi:https://doi.org/10.1093/humrep/deu070.
- Simon A, Laufer N. 2012. Assessment and treatment of repeated implantation failure (RIF). J Assist Reprod Genet. 29(11):1227–1239. doi:https://doi.org/10.1007/s10815-012-9861-4.
- Smith S, Hosid S, Scott L. 1995. Endometrial biopsy dating. Interobserver variation and its impact on clinical practice. J Reprod Med. 40(1):1–3.
- Stern C, Chamley L, Norris H, Hale L, Baker HW. 2003. A randomized, double-blind, placebo-controlled trial of heparin and aspirin for women with in vitro fertilization implantation failure and antiphospholipid or antinuclear antibodies. Fertil Steril. 80(2):376–383. doi:https://doi.org/10.1016/S0015-0282(03)00610-1.
- Tan J, Kan A, Hitkari J, Taylor B, Tallon N, Warraich G, Yuzpe A, Nakhuda G. 2018. The role of the endometrial receptivity array (ERA) in patients who have failed euploid embryo transfers. J Assist Reprod Genet. 35(4):683–692. doi:https://doi.org/10.1007/s10815-017-1112-2.
- Wilcox AJ, Baird DD, Weinberg CR. 1999. Time of implantation of the conceptus and loss of pregnancy. N Engl J Med. 340(23):1796–1799. doi:https://doi.org/10.1056/NEJM199906103402304.
