ABSTRACT
The action of myo-inositol (MI), belonging to the inositol family, has been shown to improve sperm quality. To further elucidate the efficacy of this substance in male fertility, we investigated in vivo the effects of a nutraceuticals mix, containing mainly myo-inositol (MI) and in vitro the action of the MI on human male gamete performance. Sperm samples were evaluated from 51 men: 21 healthy normozoospermic and 30 oligoasthenoteratozoospermic (OAT). In the latter group, 15 patients were orally treated with the nutraceutical mix and in the remaining 15 patients only MI was used directly on their ejaculated sperm. Comparing the pathologic samples with respect to normal samples we observed that motility, viability, Bcl-2 phosphorylation, and cholesterol efflux increased after in vitro and in vivo treatments. Glucose-6-phosphate dehydrogenase activity as well as triglycerides level and lipase activity highlighted an enhancement of energy expenditure upon the treatment. Uncapacitated sperm is characterized by an anabolic metabolism, to generate an energy reservoir which will be spent during the capacitation, an energy-consuming process needed to acquire the competence for the fertilization. Intriguingly, our finding highlights that the treatment with these substances facilitated the switch from uncapacitated to capacitated sperm, promoting the acquisition of the male gamete fertilizing capacity. Our data suggested that these substances act both directly on sperm and on spermatogenesis, improving the performance of OAT sperm invitro and invivo. The positive effects of these treatments could be of great help for men and couples who have difficulty to conceive achild in anatural way and/or during medical-assisted reproduction.
Abbreviations: 30 OAT-untreated patients; B: 15 OAT patients treated in vivo; Bovine serum albumin (BSA); C: 15 OAT patients treated in vitro; cholesterol oxidase–peroxidase (CHOD–POD); H: Normozoospermic samples; HM: sperm from normospermic patients treated in vitro with MI; MI: Myoinositol: IM: Immobile motility; NP: Non-progressive motility; OAT: Oligoasthenoteratozoospermic; PPP: Pentose Phosphate Pathway; PR: Progressive motility; WHO: World Health Organization.
Introduction
The World Health Organization (WHO) and the American Fertility Society (AFS) define the infertility as a disease of the reproductive system when a couple is not able to conceive after 12–24 months of regular and unprotected sexual intercourse (Cooper et al. Citation2010). The incidence of infertility tends to increase due to various factors such as exposure to chemical agents, environmental pollution, smoking, eating habits, high age at the time of marriage, sexual habits with risk of sexually transmitted diseases, or psycho-emotional factors. Fertility or subfertility is a concern of the couple. In fact, infertility can be of female (40–55%) or male (25–40%) origin, of the couple (20%) or unexplained (10%) (Coco and Zarbo Citation2010). Male infertility has shown a significant increase, with a sperm count declined by 50 to 60% over the past decades (Kumar and Singh Citation2015). At least 30 million men worldwide are infertile, with incidence peaks in Central Europe and the Western world. These data indicate that male infertility is a global health problem (Agarwal et al. Citation2015). Furthermore, varicoceles, infections, hormonal deficiencies, cryptorchidism, prostatic pathologies, post-parotitic orchitis, torsions of the spermatic cord, traumas, and iatrogenic factors due to previous invasive surgeries of the inguinal region, endocrine disorders, drug intake, chromosomal abnormalities (i.e. Klinefelter syndrome) and genes (microdeletions of the Y chromosome) all negatively affect male reproductive ability (Coco and Zarbo Citation2010).
Oligoasthenoteratospermia is characterized by the reduction of number, motility, and morphology alterations of spermatozoa; several therapies are suggested to counteract such a disease, nevertheless, they are often inappropriate. In order to improve reproductive performance in humans, pharmacological research aims to identify both hormonal and non-hormonal molecules capable of ameliorating sperm parameters offering a means to intercede.
It has been shown that sperm capacitation involves a metabolic reprogramming in the human male gamete (Guido et al. Citation2011; De Amicis et al. Citation2011) and that spermatozoa are able to modulate their own metabolism independently by the systemic regulation (De Amicis et al. Citation2012a). Recently, the action of myo-inositol (MI), a C6 sugar alcohol stereoisomer, belonging to the inositol family, has been investigated in male reproduction. Several studies have highlighted the correlation between male infertility and positive effects based on use of inositol to induce spermatic functionality (Colone et al. Citation2010). It has been demonstrated that oral supplementation of MI improves some sperm features as well as the hormone and metabolic profiles in subfertile patients (Calogero et al. Citation2015; Gulino et al. Citation2016; Montanino Oliva et al. Citation2016). MI oral supplementation during controlled ovarian stimulation and ART reduces the total amount of gonadotropins used and the length of controlled ovarian hyperstimulation in both PCOS and non-PCOS women (Facchinetti et al. Citation2020c). In addition, MI in vitro treatment of human spermatozoa has been shown to improve total and progressive sperm motility (Colone et al. Citation2010, Citation2017; Condorelli et al. Citation2012, Citation2017; Bevilacqua et al. Citation2015; Scarselli et al. Citation2016; Artini et al. Citation2016). In the female, MI appears to be considered a therapeutic option for their infertility associated with insulin resistance (Condorelli et al. Citation2017). Indeed, the inositols MI and D-chiro-inositol (DIC) which are insulin second messengers, appear to be effective in treating PCOS, improving metabolic and hormonal state, and restoring spontaneous ovulation (Laganà et al. Citation2018a, Citation2018b). However, profound differences between the physiological functions of MI and DIC exist, for example their intestinal absorption, although these require further studies (Facchinetti et al. Citation2020a). Beyond the type and combination, inositols are insulin sensitizers. Attention must be paid on their use in assisted reproductive treatments, given the role of insulin (Aquila et al. Citation2005a) and insulin sensitizers in human spermatozoa as we showed (Aquila et al. Citation2006; Rago et al. Citation2020). Indeed, it has been reported that the 40:1 ratio supplementation of MI and DIC in the treatment of PCOS is effective (Facchinetti et al. Citation2016, Citation2020b), DIC has been shown to have a minor effect on spermatozoa in assisted reproductive treatment (ART) (Facchinetti et al. Citation2020c).
It has been reported that DCI has a beneficial action on sperm mitochondrial function in vitro. The mitochondrion represents a crucial point for sperm motility providing energy by glycolysis and oxidative phosphorylation, both in normozoospermic men and in patients with asthenozoospermia (Condorelli et al. Citation2020). The evaluation of mitochondrial membrane potential (MMP) gives information on sperm mitochondrial function, in fact, healthy mitochondrion is capable of fully supporting sperm energy demand (Condorelli et al. Citation2020). The beneficial effects of inositols on sperm motility and mitochondrial function can be due to their many actions: insulin-sensitizing properties, antioxidant and prokinetic activity, and hormonal regulatory effects. It has been shown that DCI acts to a lesser extent with respect MI (Facchinetti et al. Citation2020c), since it appears that MI plays a pivotal role in the physiology of reproduction, having beneficial actions on the oocytes development, spermatozoa, and embryos, while DCI has a smaller effect on spermatozoa between the two inositols. However, the beneficial effects of MI in both female and male reproduction are validated with their clinical use of MI in the ART.
Interestingly, the 2013 Florence International Consensus Conference on MI and DIC in obstetrics and gynecology discussed a number of research questions concerning the use of the two stereoisomers in ART. Particularly, the physiological involvement of MI-Ins in oocyte maturation and sperm cell function has been evaluated, since several studies indicate that the inositol plays a crucial role in oocyte and spermatozoa development by itself or through its derivatives. The cellular content of inositols is represented almost entirely by Myo-Ins (>99%) and for the remaining part by a second stereoisomer, DCI-Ins. An imbalance between Myo-Ins and DCI-Ins may lead to a reduction in insulin and FSH signaling, as observed in PCOS patients (Unfer et al. Citation2014: Heimark et al. Citation2014). The literature analyzed by the Conference Scientific Committee highlighted the beneficial effects of Myo-Ins treatment in ART, in particular at the level of ovarian response to exogenous gonadotropins as well as oocyte and embryo quality. In this regard, administration of Myo-Ins, alone or in combination with DCI-Ins (in the physiological plasma ratio of 40:1), could be a predictive factor in improving ART outcomes (Bevilacqua et al. Citation2015).
In our study, we evaluated in oligoasthenoteratozoospermic (OAT) sperm functionality: A group of OAT patients were treated orally with a nutraceutical mix, primarily containing MI and another OAT group was treated by combining MI directly with their ejaculated sperm.
Different sperm features were studied when treated with these substances, including motility, survival, and for the first time, capacitation, glucose, and lipid metabolism. Our data suggest that the effects obtained may be due particularly to the MI, highlighting a positive effect both directly on sperm and on spermatogenesis. However, the molecular mechanisms through which these actions occur need to be further investigated.
Results
Effect of nutraceuticals mix and MI on human sperm motility
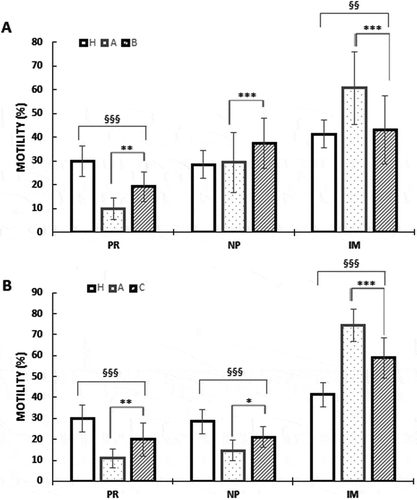
Increased sperm motility was observed in group B and C with respect to untreated OAT men group A (). OAT samples had a significant lower PR sperm motility and a higher percentage of IM spermatozoa compared to normozoospermic samples (group H) as shown in . In vitro treatment of sperm treatment with a solution of MI at a concentration of 2 mg/ml, B samples significantly improved sperm motility compared to the untreated A samples. Specifically, after treatment, the PR and NP motility in B samples significantly increased; while in the IM spermatozoa were significantly decreased (). In vivo treatment yielded similar results after the oral treatment of the patients with the nutraceuticals mix. In fact, in C samples, PR and NP motility were significantly improved compared to the untreated samples A, whereas, the IM spermatozoa motility significantly decreased (). This positive effect on motility was also evident when the B and C samples were compared with respect to H.
Figure 1. Effects of nutraceuticals mix and MI on motility (A) in vitro (B) in vivo. Motility is expressed as percentage in healthy volunteer’s donors (H), OAT untreated patients (A), sperm from OAT patients treated in vitro with a concentrated solution of MI (B) and OAT patients treated in vivo with a nutraceuticals mix, containing mainly MI (C). PR = progressive motility, NP = non-progressive motility, IM = immobile sperm). The columns represent the mean ± SD. Variables before and after treatment analyzed with Student’s paired t-test. p-value: * ≤ 0.05; ** < 0.01; *** < 0.001. One-way ANOVA between groups. p-value: § ≤ 0.05; §§ < 0.01; §§§ < 0.001.
Effect of nutraceuticals mix and MI on human sperm viability
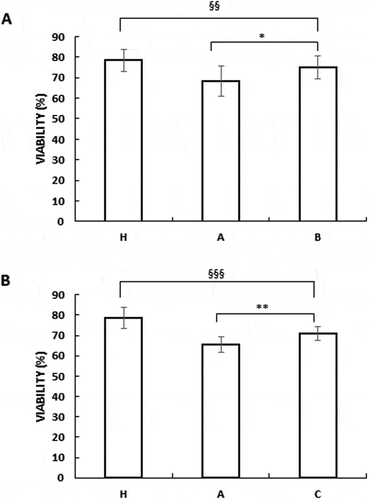
Increased sperm viability was observed in group B and C with respect to the untreated OAT men in group A (). OAT samples had a lower viability compared to normozoospermic samples (group H) as shown in . This positive effect on vitality was also evident when the B and C samples were compared with respect to H.
Figure 2. Nutraceuticals mix and MI improved sperm viability (A) in vitro (B) in vivo. Viability expressed as percentage in healthy volunteer’s donors (H), OAT untreated patients (A), sperm from OAT patients treated in vitro with a concentrated solution of MI (B) and OAT patients treated in vivo with a nutraceuticals mix, containing mainly MI (C).The results are presented as mean ± SD. Variables before and after treatment analyzed with Student’s paired -test. p-value: * ≤ 0.05; ** < 0.01. One-way ANOVA between groups. p-value: §§ < 0.01; §§§ < 0.001
Nutraceuticals mix and MI modulated Bcl2 phosphorylation
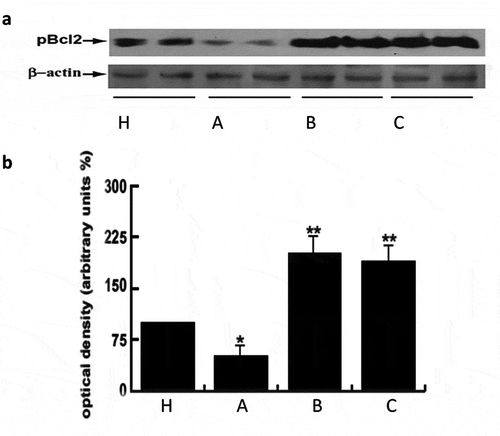
At molecular level as in human sperm, their survival is regulated by the phosphorylation level of key proteins such as Bcl-2(Aquila et al. Citation2007, Citation2010a). Bcl-2 phosphorylation at serine 70, is a physiologically relevant phosphorylation site for cell survival (Aquila et al. Citation2007, Citation2010a; De Amicis et al. Citation2012b). By Western blot analysis, the treatment with these substances, both in vitro and in vivo, significantly induced the phosphorylation of Bcl2 protein compared to untreated patients (). Collectively, these treatments effectively promote human sperm survival also with respect to normal subjects.
Figure 3. Nutraceuticals mix and MI induced Bcl2 phosphorylation. Panel A) 70 μg of sperm protein lysates were used for western blot analysis of Bcl2 phosphorylation. Healthy volunteer’s donors were used as positive control (H). A = OAT untreated patients, B = sperm from OAT patients treated in vitro with MI; C = OAT patients treated in vivo with the supplement mentioned above. For each group three different pooled samples were used. β-actin was used as loading control. Panel B) Densitometric evaluation of the bands
Nutraceuticals mix and MI induced cholesterol efflux
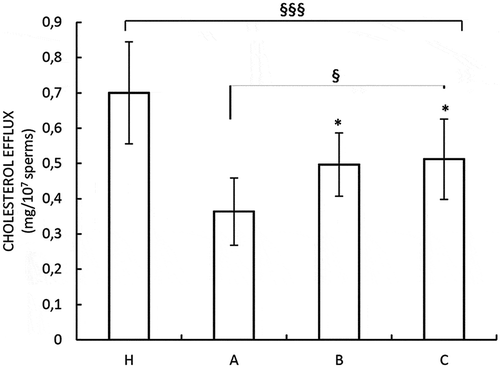
We investigated the influence of these substances on sperm cholesterol efflux, which is a hallmark of capacitation, in our samples (De Amicis et al. Citation2012a). Our data showed an increased cholesterol efflux in both in vitro and in vivo treated sperm (), although in the OAT samples treated with these substances the values were lower with respect to that observed in samples from normal subjects.
Figure 4. Effects of nutraceuticals mix and MI on capacitation. Spermatozoa from healthy volunteer’s donors were used as positive control (H). A = OAT untreated patients, B = sperm from OAT patients treated in vitro with MI; C = OAT patients treated in vivo with a nutraceuticals mix, containing mainly MI. The results are presented as mean ± SD. Variables before and after treatment analyzed with Student’s paired t-test. p-value: * ≤ 0.05. One-way ANOVA between groups. p-value: § p ≤ 0.05; §§§ < 0.001.
Nutraceuticals mix and MI influenced glucose metabolism through the pentose phosphate pathway (PPP)
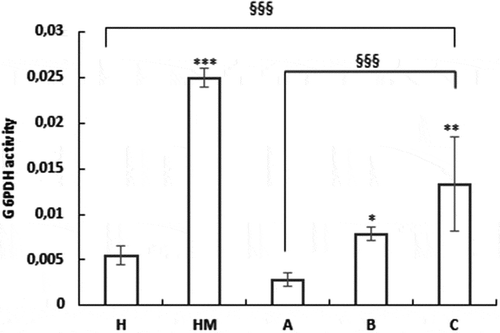
Our recent data suggested that sperm has the ability to modulate autonomously the energetic substrate availability on the basis of its energy needs independently from the systemic regulation (De Amicis et al. Citation2011; Guido et al. Citation2011). Our previous studies demonstrated metabolic reprogramming during the switch from uncapacitated to capacitated sperm. An Energy expenditure occurs during capacitation. Specifically, sperm glucose metabolism through the PPP is very important (Aquila et al. Citation2005a, Citation2005b). Therefore, we evaluated the action of these treatments on the activity of the G6PDH, the first enzyme of the PPP. Treatment with these substances either in vitro and in vivo was able to significantly induce the G6PDH activity on B and C samples with respect to the untreated A (). It seems that this enzymatic activity increased to a greater extent than normal subjects in vivo and in vitro treated sperm. We also evaluated the effect of MI on G6PDH activity in normospermic men (group named, HM). Interestingly, in healthy sperm samples, the G6PDH activity is significantly increased when MI was added in vitro. These results suggest that MI also improves sperm performance in normozoospermic patients. In OAT patients samples, the enzyme activity is positively affected by MI, although to lower extent when compared to normal patient samples.
Figure 5. Nutraceuticals mix and MI influenced glucose metabolism through the PPP. H = sperm from healthy volunteer’s donors; HM = sperm from normospermic patients treated in vitro with MI; A = OAT untreated patients; B = sperm from OAT patients treated in vitro with MI; C = OAT patients treated orally with a nutraceutical mix, containing prevalently MI. G6PDH activity has been performed as described in Materials and Methods. Data are expressed in nmol min-1/106 spermatozoa. The results are presented as mean ± SD. Variables before and after treatment analyzed with Student’s paired t-test. p-value: *<0.02; ** ≤ 0.001; *** < 0.0001. One-way ANOVA between groups. p-value: §§§ <0.001.
Effect of nutraceuticals mix and MI on lipid metabolism
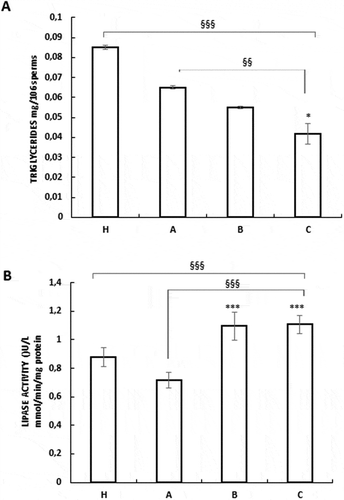
Metabolic reprogramming during the sperm functional maturation, also involves lipid metabolism. Thus, we investigated the effects of these substances on the intracellular triglyceride levels and the lipase activity. A reduction of the triglyceride content was observed after treatment with these compounds in vitro and in vivo of B and C samples with respect A untreated OAT sperm (). These results are well correlated with the data obtained from the lipase activity, which was significantly induced by the treatments (). Altogether, these data indicated a lipid lowering effect of our treatments both in vivo and in vitro in OAT samples.
Figure 6. Nutraceuticals mix and MI modulated lipid metabolism in human sperm. Assays of triglycerides content (A) and lipase activity (B) were performed as described in the Materials and Methods. The results are presented as mean ± SD. Variables before and after treatment analyzed with Student’s paired t-test. p-value: * ≤ 0.05; *** < 0.0001. One-way ANOVA between groups. p-value: §§ < 0.01; §§§ < 0.001.
Discussion
Infertility affects an estimated 15% of couples globally, amounting to 48.5 million couples. Males considered to be responsible for 20–30% of infertility cases and contribute to 50% of cases overall. Male infertility had a significant increase over the past decades (Kumar and Singh Citation2015). In this study, we investigated in vitro the action of the MI and in vivo the effects of a nutraceuticals mix, primarily containing MI, on human male gamete performance.
The action of MI has been investigated in male reproduction and in female infertility. Several studies have highlighted the correlation between male infertility and positive effects based on use of inositol to induce spermatic functionality (Colone et al. Citation2010). It has been demonstrated that oral supplementation of MI improves some sperm features including total as well as progressive sperm motility (Colone et al. Citation2010, Citation2017; Condorelli et al. Citation2012, Citation2017; Bevilacqua et al. Citation2015; Scarselli et al. Citation2016; Artini et al. Citation2016) and metabolic profiles in subfertile patients (Calogero et al. Citation2015; Gulino et al. Citation2016; Montanino Oliva et al. Citation2016).
During the last decades, a substantial body of research has shown that MI plays an important role in female infertility. In recent years, several findings have focused their attention on the role of the MI in insulin-dependent processes, including polycystic ovary syndrome (PCOS), one of the most common female endocrine disorders. (Bevilacqua and Bizzarri Citation2016). Lately, Showell Marian et al. (Citation2018) have provided evidence of the effectiveness of oral supplementation of inositol for reproductive outcomes among subfertile women with PCOS who are trying to conceive.
These recent reports strongly suggest that the inositol can be positively associated with the management of PCOS patients enrolled in assisted reproduction procedures (Facchinetti et al. Citation2016). Several studies have also shown that MI has a physiological role in mammalian gametogenesis, embryonic development and a positive clinical impact on human medically assisted reproduction. Mouse embryo studies have shown that inclusion of MI in human embryo culture media is capable of producing an increase in embryo quality during IVF cycles (Colazingari et al. Citation2014).
Furthermore, the mouse embryo exposure to MI through in vitro preimplantation development increases proliferation activity and blastocyst production. The biochemical mechanisms elicited by MI in preimplantation embryos and the effects of MI on postimplantation/postnatal development were evaluated. The results of these studies have highlighted how the MI is possibly an important supplement for human preimplantation embryo culture in assisted reproduction technology (Kuşcu et al. Citation2016).
Sperm motility and viability are fundamental features for a successful fertilization and may represent relevant predictive values for spontaneous conception (Sripada et al. Citation2010). In our study, a beneficial effect of the substances used on sperm motility and viability, either in vitro and in vivo was observed. Intriguingly, viability after treatment increased similarly to that observed in samples from normal subjects. The data of group B and C were compared both to group A (untreated samples) and to group H (normal samples). Noteworthy, in the patient samples treated with MI we achieved a marked improvement in the parameters compared to group A, both in terms of motility and vitality. However, a lower effect on these parameters was observed compared to the control group H.
Inositols, a family of cyclic carbohydrates, include the MI which can be converted into DCI by a specific epimerase stimulated by insulin. The main pathway of insulin cascade begins from the inositol triphosphate (IP3), reaching Akt which regulates a wide range of proteins by phosphorylation. (Condorelli et al. Citation2020) have previously shown that MI increases sperm motility in patients with asthenozoospermia by increasing sperm MMP. This biofunctional sperm parameter is closely associated to sperm motility. Both MI and DCI are derived components of cell membrane phospholipids, which play a structural and functional role. Indeed, cell membrane phospholipids are also the source of inositol triphosphate (IP3). The latter, regulates the activity of different hormones, including follicle-stimulating hormone (FSH). Furthermore, IP3 binds receptors on mitochondria and endoplasmic reticulum, inducing calcium release from internal stores, activating protein kinase C. This process is also responsible for the acrosome reaction, essential for allowing spermatozoa to penetrate the zona pellucid (ZP), as necessary for fertilization (Larner et al. Citation2010). The increase in intracellular calcium i leads to a concomitant increase of calcium into the flagellum, thus prompting myotubule contraction and flagella movement (Yoshida and Yoshida Citation2011). Akt, which acts on numerous downstream proteins, negatively regulates pro-apoptotic proteins by direct phosphorylation. For example, phosphorylation of the Bcl-2 at Ser70 site, causes its translocation from the mitochondrial membrane to the cytosol; therefore, it does not act as an apoptotic factor but rather a marks survival. Indeed, the concomitant phosphorylation of AKT at S473 and T308 sites and that of the Bcl-2 at Ser70 site is used to confirm the sperm survival at molecular level (Aquila et al. Citation2007, Citation2010a). (Aquila et al. Citation2007, Citation2010a). In this study, the treatments induced the phosphorylation of the Bcl-2 at Ser70 both in vitro and in vivo, corroborating its action on human sperm survival also at molecular level.
For the first time, we investigated the effect of these compounds on cholesterol efflux, a hallmark of capacitation, showing an increased cholesterol efflux in sperm from OAT patients treated in vitro with MI and in vivo with a nutraceuticals mix, containing mainly MI. Sperm membrane cholesterol efflux contributes to the signaling pathway that controls sperm capacitation (Travis and Kopf Citation2002). It has been shown to initiate signaling events leading to tyrosine phosphorylation of sperm proteins, also representative of the capacitation status (Visconti et al. Citation1995). Capacitation is a complex process that makes male gamete responsive to the acrosome reaction allowing its interaction and fusion with the oocyte. Sperm, during its lifetime, passes through two different physiological stages: an uncapacitated steady state, during which the gamete economizes, and/or stores energy, and a state of functional maturation, during which the gamete becomes capacitated with considerable energy expenditure. Generally, it might be viewed that the uncapacitated gamete is representative of anabolic metabolism, while in the capacitated state is catabolic. Capacitated sperm display an increased metabolic rate and overall energy expenditure, presumably to effect the required multifaceted changes (Guido et al. Citation2014; Montanaro et al. Citation2015). Our previous studies demonstrated that during the switch from uncapacitated to capacitated sperm, a metabolic reprogramming occurs and it involves both lipid and glucose metabolism. In this study, for the first time the possible effect of our treatments on glucose and lipid metabolism in sperm were investigated. Our data showed an increase in G6PDH activity, indicating an augmented glucose metabolism through the PPP, both in normal and in OAT patients. Concomitantly, a significant decrease in triglyceride levels and an increase in the lipase activity occurred. Collectively, these data highlight that our treatments facilitate the switch from uncapacitated to capacitated sperm, promoting the acquisition of male gamete fertilizing ability.
The results obtained in this study are in agreement with our previous findings, where substances which induced the capacitation, showed a reduction of the glucose as well as the triglyceride content, concomitantly with increased energy expenditure (Guido et al. Citation2011; De Amicis et al. Citation2012a). Of note, we presume that the effects induced by our treatments are probably mostly due to the MI. From a clinical point of view, our results showed a positive action of the treatments both directly on the male gamete and on spermatogenesis. An important biologic issue must be considered, the MI action in the two different conditions is exerted on different processes. The in vivo treatment for three months acts on the entire spermatogenetic program increasing sperm functionality. During the treatment directly on the ejaculated sperm, a short time is needed to produce similar effects. Indeed, in the latter case, several sperm features studied upon MI, including motility, survival, and for the first time, capacitation, glucose, and lipid metabolism, occur in a rapid way, suggesting that this nutraceutical mix is able to directly interact with sperm molecules/kinases involved in the above-mentioned functions.
Oral supplementation with the nutraceutical mix seems to be an easy handling method, without any risks and side effects, to improve the performance of OAT sperm and its positive effects could be of great help for all those men and couples who have difficulty conceiving a child. In addition, sperm treatment in vitro could also be effective and beneficial during semen preparation for in vitro fertilization procedures. Further studies are needed to enhance our understanding of the effects of our treatments on medical-assisted reproduction as well as the mechanism/s of action through which this substance acts.
Materials and methods
Chemicals
Bovine serum albumin (BSA) protein standard, Laemmli sample buffer, pre-stained molecular weight markers, phosphate-buffered saline (PBS) and all other chemicals were purchased from Sigma Chemical (Milan, Italy). Earle’s balanced-buffered solution (EBBS) was obtained from Genaxxon Bioscience (Milan, Italy). Acrylamide bisacrylamide was purchased from Labtek Eurobio (Milan, Italy). Eosin Y was from Farmitalia Carlo Erba (Milan, Italy). A gel band purification kit, the ECL Plus Western blotting detection system, HybondTM ECL TM and Hepes sodium salt were purchased from Amersham Pharmacia Biotech (Buckinghamshire, UK). Cholesteroloxidase–peroxidase (CHOD–POD) enzymatic colorimetric, triglyceride, lipase activity, and glucose-6-phosphate dehydrogenase (G6PDH) assays were from Inter-Medical (Biogemina Italia, Catania, Italy). Rabbit anti-p-Bcl2 antibody (Ab), the anti-rabbit peroxidase-conjugated IgG were purchased from Cell Signaling (Milan, Italy). Mouse anti-b-actin Ab was from Santa Cruz (Milan, Italy). Nutraceuticals mix is a supplement named Andrositol® (1 g MI, the main compound, 30 mg L-carnitine, L-arginine, vitamin E, 55 μg selenium and 200 μg folic acid) was a gift from Lo.Li. Pharma srl (Rome, Italy).
A concentrated solution consisting of MI (Andrositol® Lab) at a concentration of 2 mg/ml was a gift from Lo.Li. Pharma srl (Rome, Italy).
Patients recruitment
This is a prospective longitudinal study of OAT patients, undergoing fertility problems to the Family Counseling Center and to the Human Anatomy and Reproductive Biology Laboratory, located at the Health Center, University of Calabria, Italy. Couples were first observed for a period of 12–18 months and subsequently for another 6–12 months, for a total time of 24 months, during which they had regular, unprotected sexual intercourse and were unable to conceive. The inclusion criteria were subjects aged over 18 years, normozooospermic or OAT patients. The exclusion criteria were presence of varicocele, cryptorchidism, or prostatitis. As control group, fertile normozoospermic men were included: patients must not have had a history of infertility in the past. The human semen was collected, according to the World Health Organization (Citation2010) manual, from healthy and fertile volunteer donors (21 = group H, control) and from OAT patients (30 = group A, experimental). The OAT patients were subsequently divided into two groups depending on the treatment of choice: in 15 patients (group B) only MI was used directly on their ejaculated sperm and the remaining 15 patients (group C) were orally treated with the nutraceutical mix and after 3 months the semen samples were examined.
In vitro analysis: semen samples after collection were subjected to the swim-up purification, washed twice with unsupplemented EBSS medium and were incubated in the same medium for 30 minutes (min) at 37°C and 5% CO2, without (A) or with a solution of MI (Andrositol® Lab) at a concentration of 2 mg/ml (Colone et al. Citation2010; Condorelli et al. Citation2012), for 30 min at 37°C and 5% of CO2, according to the manufacturer instructions (group B).
In vivo analysis: patients (group C) were orally treated once a day with the nutraceuticals mix above mentioned, containing mainly MI for 3 months. Thereafter, the semen of these patients after collection, was subjected to the swim-up purification and washed two times with unsupplemented EBSS medium prior to be used in the different assays. All participants signed an informed consent form.
Sample collection and analysis
The semen was collected by masturbation, according to the WHO-recommended procedure (WHO Citation2010), after 3–5 days of sexual abstinence for each participant in the study and analyzed immediately after complete liquefaction. Each sample in our study is formed by three different pooled ejaculates to avoid individual differences, both for normal and OAT samples. Then, the samples were subjected to the Swim-up to select only sperms with greater motility and to eliminate other cell types (round cells and dead sperm) (World Health Organization Citation2010). The same number of swim-up purified sperm, 10 × 106 in each tube, both for normal and OAT samples were used in all experimental conditions considered in the study. Swim-up purified sperm, washed two times with unsupplemented EBSS medium, were treated as indicated above. It is important to point out that the semen samples from the patients of group C were evaluated before and at the end of treatment. The semen profiles before the treatment with these substances were included in the group A. Specifically, since as we reported we used 21 healthy normozoospermic, therefore we presented the mean values of 7 samples for the group H. As it concerns the OAT, by using 30 ejaculates, we presented the mean values of 10 samples for group A, of which 5 samples were treated in vivo and the other five samples in vitro.
The data were analyzed by Student’s paired t-test and each column represents the mean values of three different samples ± SD, as reported in the statistical analysis.
Sperm motility and viability
Sperm motility and viability were assessed by means of light microscopy examining an aliquot of each sperm sample which had been incubated as above mentioned. Motility and viability pre- and post-treatment were determined. Sperm motility were assessed by conventional method using Makler counting chamber (World Health Organization Citation2010), at least 200 spermatozoa and expressed as percentage of motile sperm (progressive = PR, non-progressive = NP, immobile = IM).
Sperm viability was expressed as percentage on total mobile spermatozoa by Eosin-Nigrosin stain. An independent observer scored 200 cells for stain uptake (dead cells) or exclusion (live cells). Sperm motility and viability were evaluated by at least two examiners for any parameter in the different experimental conditions. Viability was evaluated before and after pooling the samples and no adverse effects were obtained among the treatment on human sperm viability (data not shown) (De Amicis et al. Citation2012a; Aquila et al. Citation2013).
Western blot analysis
Swim-up purified sperm samples, washed twice with unsupplemented EBSS medium, were treated as indicated and centrifuged for 5 min at 5000 rpm. The pellet was re-suspended in lysis buffer (62.5 mmol/liter Tris-HCl, pH 6.8; 150 mM NaCl; 2% SDS; 1% Triton X100; 10% glycerol; 1 mM phenylmethylsulfonylfluoride; 10 μg/ml leupeptin; 10 μg/ml aprotinin; 2 μg/ml pepstatin) as previously described (Aquila et al. Citation2002; De Amicis et al. Citation2013). Equal amounts of protein (70 µg) were boiled for 5 min, separated by 11% polyacrylamide gel electrophoresis, transferred to nitrocellulose sheets for 75 min at 100 V. Membranes were incubated for 60 min with 5% nonfat dry milk in 0.2% Tween 20 in Tris-buffered saline (TBST) at room temperature and subsequently probed overnight at room temperature with an appropriate dilution of the indicated primary and secondary antibody (De Amicis et al. Citation2012b; Aquila et al. Citation2015). Blots were developed using the ECL Plus Western blotting detection system according to the manufacturer’s instructions. As loading control, all membranes were subsequently stripped (glycine 0.2 M, pH 2.6 for 30 min at room temperature) of the first Ab and reprobed with anti-βactin. The protein bands were quantified by scanning densitometry (Imaging Densitometer GS-700 Bio-Rad laboratories Inc., CA, USA).
Measurement of cholesterol in the sperm culture medium
Cholesterol was measured in the incubation medium from human spermatozoa by a cholesterol oxidase–peroxidase (CHOD–POD) enzymatic colorimetric method accordingly to the manufacturer’s instructions (Guido et al. Citation2011). Sample culture media were recovered by centrifugation, lyophilized, and subsequently dissolved in 1 mL of reaction buffer. The samples were incubated for 10 min at room temperature, then the cholesterol content was measured with the spectrophotometer at 505 nm (Aquila et al. Citation2005a, Citation2010a). The sensitivity limit for the assay was 0.05 mg/dl. The inter- and intra-assay variations were 0.04% and 0.03%, respectively. Cholesterol results are shown as mg x 107 spermatozoa.
G6PDH activity
The samples were incubated for 30 min at 37°C and 5% CO2 in the presence or absence of the indicated treatments. The G6PDH activity was measured at a wavelength of 340 nm as previously described (Aquila et al. Citation2005a, Citation2010a; Guido et al. Citation2011; Malivindi et al. Citation2018). The results of the assay are presented as nmol min−1/106 spermatozoa.
Triglyceride assay
Triglycerides were measured with the enzymatic colorimetric glycerol-3-phosphate oxidase-POD method according to the manufacturer’s instructions (Inter-Medical) (Aquila et al. Citation2005a, Citation2010a; Guido et al. Citation2011). 15 µg of sperm protein extracts were loaded into individual cuvettes containing buffer for spectrophotometric determination. Data are presented as μg/106 spermatozoa.
Lipase activity assay
Lipase activity was evaluated by the method of Panteghini et al. (Citation2001) based on the use of 1,2-odilauryl-rac-glycero-3-glutaric acid-(6-methylresorufin) ester (DGGR) as substrate; 50 µg of sperm protein extracts were loaded into individual cuvettes containing buffer for spectrophotometric determination. DGGR is cleaved by lipase, resulting in an unstable dicarbonic acid ester, which is spontaneously hydrolyzed to yield glutaric acid and methylresorufin, a bluish-purple chromophore with peak absorption at 580 nm. The absorbance of samples was read every 20 s for 1.5 min. The rate of methylresorufin formation is directly proportional to the lipase activity in the sample. Data are presented as nmol/min/mg protein (Aquila et al. Citation2005a, Citation2010a; Guido et al. Citation2011).
Statistical analysis
The data obtained from motility, viability, cholesterol efflux assay, triglycerides assay, lipase activity, G6PDH activity, and glucose quantification (six replicate experiments using duplicate determinations), were presented as mean ± SD. The Western blotting analysis was performed in at least four independent experiments and the band intensities, evaluated in terms of arbitrary densitometric units were presented as columns representing the mean ± SD. The differences in variables before and after treatment were statistically analyzed with Student’s paired t-test (GraphPad Software 2018, Inc., La Jolla, CA, USA). The analysis of variance between groups was carried out using one-way ANOVA. P-value ≤0.05 was considered statistically significant.
Ethics approval
The study has been approved by the local medical-ethical committee with protocol n. 22,160 of 11/10/2017. The approval of the ethics committee was obtained by following the procedures governing people experimentation. All patients were trained on the project and signed informed consent before taking the exam.
Acknowledgments
We would like to thank Lo.Li Pharma srl for the gift of Andrositol®Lab. We would like to thank also Serena Gervasi and Maria Clelia Gervasi for the revision of the English language.
Disclosure statement
The authors declare that there is no conflict of interest that would prejudice the impartiality of this scientific work.
Additional information
Funding
References
- Agarwal A, Mulgund A, Hamada A, Chyatte MR. 2015. A unique view on male infertility around the globe. Reprod Biol Endocrinol. 13:37.
- Aquila S, Bonofiglio D, Gentile M, Middea E, Gabriele S, Belmonte M, Catalano S, Pellegrino M, Andò S. 2006. Peroxisome proliferator- activated receptor (PPAR)gamma is expressed by human spermatozoa: its potential role on the sperm physiology. J Cell Physiol. 209:977–986. doi:10.1002/jcp.20807.
- Aquila S, Gentile M, Middea E, Catalano S, Ando S. 2005a. Autocrine regulation of insulin secretion in human ejaculated spermatozoa. Endocrinol. 146:552–557. doi:10.1210/en.2004-1252.
- Aquila S, Gentile M, Middea E, Catalano S, Morelli C, Pezzi V, Ando S. 2005b. Leptin secretion by human ejaculated spermatozoa. J Clin Endocrinol Metab. 90:4753–4761. doi:10.1210/jc.2004-2233.
- Aquila S, Guido C, Santoro A, Gazzerro P, Laezza C, Baffa MF, Andò S, Bifulco M. 2010a. Rimonabant (SR141716) induces metabolism and acquisition of fertilizing ability in human sperm. Br J Pharmacol. 159(4):831–841. doi:10.1111/j.1476-5381.2009.00570.x.
- Aquila S, Middea E, Catalano S, Marsico S, Lanzino M, Casaburi I, Barone I, Bruno R, Zupo S, Andò S. 2007. Human sperm express a functional androgen receptor: effects on PI3K/AKT pathway. Hum Reprod. 22:2594–2605. doi:10.1093/humrep/dem243.
- Aquila S, Montanaro D, Guido C, Santoro M, Perrotta I, Gervasi S, De Amicis F, Lanzino M. 2015. Human sperm molecular anatomy: the enzyme 5alpha-reductase (SRD5A) is present in the sperm and may be involved in the varicocele-related infertility. Histochem Cell Biol. 144:67–76. doi:10.1007/s00418-015-1320-8.
- Aquila S, Santoro M, De Amicis F, Guido C, Bonofiglio D, Lanzino M, Cesario Maria G, Perrotta I, Sisci D, Morelli C. 2013. Red wine consumption may affect sperm biology: the effects of different concentrations of the phytoestrogen myricetin on human male gamete function. Mol Reprod Dev. 80:155–165. doi:10.1002/mrd.22145.
- Aquila S, Sisci D, Gentile M, Middea E, Siciliano L, Andò S. 2002. Human ejaculated spermatozoa contain active P450 aromatase. J Clin Endocrinol Metab. 87:3385–3390. doi:10.1210/jcem.87.7.8633.
- Artini PG, Casarosa E, Carletti E, Monteleone P, Di Noia A, Di Berardino OM. 2016. In vitro effect of myo-inositol on sperm motility in normal and oligoasthenospermia patients undergoing in vitro fertilization. Gynecol Endocrinol. 33:109–112.
- Bevilacqua A, Bizzarri M. 2016. Physiological role and clinical utility of inositols in polycystic ovary syndrome. Best Pract Res Clin Obstet Gynaecol. 37:129–139 review. doi:10.1016/j.bpobgyn.2016.03.007.
- Bevilacqua A, Carlomagno G, Gerli S, Montanino Oliva M, Devroey P, Lanzone A, Soulange C, Facchinetti F, Carlo Di Renzo G, Bizzarri M, et al. 2015. Results from the international consensus conference on myo-inositol and d-chiro-inositol in obstetrics and gynecology–assisted reproduction technology. Gynecol Endocrinol. 31::441–446. doi:10.3109/09513590.2015.1006616.
- Calogero AE, Gullo G, La Vignera S, Condorelli RA, Vaiarelli A. 2015. Myoinositol improves sperm parameters and serum reproductive hormones in patients with idiopathic infertility: a prospective double-blind randomized placebo-controlled study. Androl. 3(3):491–495. doi:10.1111/andr.12025.
- Coco LSS, Zarbo G 2010. Diagnosi e terapia dell’infertilità di coppia. Giornale Italiano di ostetricia e ginecologia XXXII.
- Colazingari S, Fiorenza MT, Carlomagno G, Najjar R, Bevilacqua A. 2014. Improvement of mouse embryo quality by myo-inositol supplementation of IVF media. J Assist Reprod Genet. 31:463–469. doi:10.1007/s10815-014-0188-1.
- Colone M, Calcabrini A, Unfer V, Stringaro A. 2017. Contribution of electron microscopy to study in vitro inositol effects on human spermatozoa. Eur Rev Med Pharmacol Sci. 21:4–9.
- Colone M, Marelli G, Unfer V, Bozzuto G, Molinari A, Stringaro A. 2010. Inositol activity in oligoasthenoteratospermia–an in vitro study. Eur Rev Med Pharmacol Sci. 14:891–896.
- Condorelli RA, Barbagallo F, Calogero AE, Cannarella R, Crafa A, La Vignera S. 2020. D-chiro-inositol improves sperm mitochondrial membrane potential: in vitro evidence. J Clin Med. 9:1373. doi:10.3390/jcm9051373.
- Condorelli RA, La Vignera S, Bellanca S, Vicari E, Calogero AE. 2012. Myoinositol: does it improve sperm mitochondrial function and sperm motility? Urology. 79(6):1290–1295. doi:10.1016/j.urology.2012.03.005.
- Condorelli RA, La Vignera S, Mongioi LM, Vitale SG, Lagana AS, Cimino L, Calogero AE. 2017. Myo-inositol as a male fertility molecule: speed them up! Eur Rev Med Pharmacol Sci. 21:30–35.
- Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HW, Behre HM, Haugen TB, Kruger T, Wang C, Mbizvo MT, et al. 2010. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 16:231–245. doi:10.1093/humupd/dmp048.
- De Amicis F, Guido C, Perrotta I, Avena P, Panza S, Ando S, Aquila S. 2011. Conventional progesterone receptors (PR) B and PRA are expressed in human spermatozoa and may be involved in the pathophysiology of varicocoele: a role for progesterone in metabolism. Int J Androl. 34:430–445. doi:10.1111/j.1365-2605.2010.01111.x.
- De Amicis F, Perrotta I, Santoro M, Guido C, Morelli C, Cesario MG, Bruno R, Aquila S. 2013. Human sperm anatomy: different expression and localization of phosphatidylinositol 3-kinase in normal and varicocele human spermatozoa. Ultrastruct Pathol. 37:176–182. doi:10.3109/01913123.2013.763881.
- De Amicis F, Santoro M, Guido C, Russo A, Aquila S. 2012a. Epigallocatechin gallate affects survival and metabolism of human sperm. Mol Nutr Food Res. 56:1655–1664. doi:10.1002/mnfr.201200190.
- De Amicis F, Santoro M, Guido C, Sisci D, Bruno R, Carpino A, Aquila S. 2012b. Progesterone through progesterone receptors affects survival and metabolism of pig sperm. Anim Reprod Sci. 135:75–84. doi:10.1016/j.anireprosci.2012.09.004.
- Facchinetti F, Dante G, Neri I. 2016. The ratio of MI to DCI and its impact in the treatment of polycystic ovary syndrome: experimental and literature evidences. In: Genazzani And AR, Tarlatzis BC, editors. Frontiers in gynecological endocrinology. Springer, Cham. ISGE Series; p. 103–109. https://doi.org/10.1007/978-3-319-23865-4_13.
- Facchinetti F, Appetecchia M, Aragona C, Bevilacqua A, Bezerra Espinola MS, Bizzarri M, D’Anna R, Dewailly D, Diamanti-Kandarakis E, Hernández Marín I, et al. 2020b. Experts’ opinion on inositols in treating polycystic ovary syndrome and non-insulin dependent diabetes mellitus: a further help for human reproduction and beyond. Expert Opin Drug Metab Toxicol. 16:255–274. doi:10.1080/17425255.2020.1737675.
- Facchinetti F, Bezerra Espinola MS, Dewailly D, Ozay AC, Prapas N, Vazquez-Levin M, Wdowiak A, Unfer V. 2020c. Breakthroughs in the use of inositols for assisted reproductive treatment (ART). Trends Endocrinol Metab. 31:570–579. doi:10.1016/j.tem.2020.04.003.
- Facchinetti F, Unfer V, Dewailly D, Kamenov ZA, Diamanti-Kandarakis E, Laganà AS, Nestler JE, Soulage CO. 2020a. Inositols in polycystic ovary syndrome: an overview on the advances. Trends Endocrinol Metab. 31:435–447. doi:10.1016/j.tem.2020.02.002.
- Guido C, Perrotta I, Panza S, Middea E, Avena P, Santoro M, Marsico S, Imbrogno P, Ando S, Aquila S. 2011. Human sperm physiology: estrogen receptor alpha (ERalpha) and estrogen receptor beta (ERbeta) influence sperm metabolism and may be involved in the pathophysiology of varicocele-associated male infertility. J Cell Physiol. 226:3403–3412. doi:10.1002/jcp.22703.
- Guido C, Santoro M, De Amicis F, Perrotta I, Panza S, Rago V, Cesario MG, Lanzino M, Aquila S. 2014. Human sperm anatomy and endocrinology in varicocele: role of androgen receptor. Reprod. 147:589–598. doi:10.1530/REP-13-0542.
- Gulino FA, Leonardi E, Marilli I, Musmeci G, Vitale SG, Leanza V, Palumbo MA. 2016. Effect of treatment with myo-inositol on semen parameters of patients undergoing an IVF cycle: in vivo study. Gynecol Endocrinol. 32:65–68. doi:10.3109/09513590.2015.1080680.
- Heimark D, McAllister J, Larner J. 2014. Decreased myo-inositol to chiro-inositol (M/C) ratios and increased M/C epimerase activity in PCOS theca cells demonstrate increased insulin sensitivity compared to controls. Endocrine Journal. 61(2):111–117. doi:10.1507/endocrj.EJ13-0423.
- Kumar N, Singh AK. 2015. Trends of male factor infertility, an important cause of infertility: A review of literature. J Hum Reprod Sci. 8:191–196. doi:10.4103/0974-1208.170370.
- Kuşcu N, Bizzarri M, Bevilacqua A. 2016. Myo-inositol safety in pregnancy: from preimplantation development to newborn animals. Int J Endocrinol. 2016:2413857. doi:10.1155/2016/2413857.
- Laganà AS, Garzon S, Casarin J, Franchi M, Ghezzi F. 2018a. Inositol in polycystic ovary syndrome: restoring fertility through a pathophysiology-based approach. Trends Endocrinol Metab. 29:768–780. doi:10.1016/j.tem.2018.09.001.
- Laganà AS, Vitagliano A, Noventa M, Ambrosini G, D’Anna R. 2018b. Myo-inositol supplementation reduces the amount of gonadotropins and length of ovarian stimulation in women undergoing IVF: a systematic review and meta-analysis of randomized controlled trials. Arch Gynecol Obstet. 298:675–684. doi:10.1007/s00404-018-4861-y.
- Larner J, Brautigan DL, Thorner MO. 2010. D-chiro-inositol glycans in insulin signaling and insulin resistance. Mol Med. 16:543–551. doi:10.2119/molmed.2010.00107.
- Malivindi R, Santoro M, De Rose D, Panza S, Gervasi S, Rago V, Aquila S. 2018. Activated-farnesoid X receptor (FXR) expressed in human sperm alters its fertilising ability. Reprod. 156:249–259.
- Montanaro D, Santoro M, Carpino A, Perrotta I, Amicis FD, Sirianni R, Rago V, Gervasi S, Aquila S. 2015. Human sperm liver receptor homolog-1 (LRH-1) acts as a downstream target of the estrogen signaling pathway. J Anat. 227(4):541–549. doi:10.1111/joa.12352.
- Montanino Oliva M, Minutolo E, Lippa A, Iaconianni P, Vaiarelli A. 2016. Effect of myoinositol and antioxidants on sperm quality in men with metabolic syndrome. Int J Endocrinol. 2016:1674950. doi:10.1155/2016/1674950.
- Panteghini M, Bonora R, Pagani F. 2001. Measurement of pancreatic lipase activity in serum by a kinetic colorimetric assay using a new chromogenic substrate. Ann Clin Biochem. 38:365–370. doi:10.1258/0004563011900876.
- Rago V, De Rose D, Santoro M, Panza S, Malivindi R, Andò S, D’Agata R, Aquila S. 2020. Human sperm express the receptor for glucagon-like peptide-1 (GLP-1) which affects sperm function and metabolism. Endocrinol. 161(4):bqaa031. doi:10.1210/endocr/bqaa031
- Scarselli F, Lobascio AM, Terribile M, Casciani V, Greco P, Franco G, Minasi MG, Greco E. 2016. Analysis of MYO-Inositol effect on spermatozoa motility, in hyper viscous ejaculates and in patients with grades II and III varicocele. Arch Ital Urol Androl. 88:279–283. doi:10.4081/aiua.2016.4.279.
- Showell Marian G, Mackenzie‐Proctor R, Jordan V, Hodgson R, Cindy F. 2018. Inositol for subfertile women with polycystic ovary syndrome. Cochrane gynaecology and fertility group. Cochrane Database Syst Rev. 2018:CD012378.
- Sripada S, Townend J, Campbell D, Murdoch L, Mathers E, Bhattacharya S. 2010. Relationship between semen parameters and spontaneous pregnancy. Fertil Steril. 94:624–630.
- Travis AJ, Kopf GS. 2002. The role of cholesterol efflux in regulating the fertilization potential of mammalian spermatozoa. J Clin Invest. 110:731–736.
- Unfer V, Carlomagno G, Papaleo E, Vailati MDS, Candiani MDM, Baillargeon MDJ. 2014. Hyperinsulinemia alters myoinositol to D-chiroinositol ratio in the follicular fluid of patients with PCOS. Reprod Sci. 21:854–858.
- Visconti PE, Baley JL, Moore GD, Pan D, Olds-Clarke P, Kopf GS. 1995. Capacitation in mouse spermatozoa I. Correlation between the capacitation state and protein phosphorylation. Dev. 121:1129–1137.
- World Health Organization. 2010. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 16:231–245.
- Yoshida M, Yoshida K. 2011. Sperm chemotaxis and regulation of flagellar movement by Ca2+. Mol Hum Reprod. 17:457–465.
