 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
In this study, the level of serum anti-angiotensin II type 1 receptor autoantibodies (AT1-AA) was determined. It was found that the positive rate of AT1-AA in serum of infertile women is higher than that of healthy non-pregnant women. Spearman correlation analysis showed that AT1-AA was negatively correlated with oocyte maturation rate (r = −0.29, P < 0.01) and AT1-AA was positively correlated with IL-6 (r = 0.68, P < 0.01). Logistic regression analysis showed that age, BMI, type of infertility, years of infertility, history of poor pregnancy outcome, condition of fallopian tube, and polycystic ovary syndrome were not factors influencing the level of AT1-AA in the serum of infertile patients. The results indicated that AT1-AA was involved in the pathological changes of infertile women. AT1-AA may be related to oocyte maturation disorder, ovulation disorder. Interestingly it appears to induce an inflammatory reaction, although the specific mechanism is not clear. However, the level of AT1-AA is not affected by individual differences in infertile patients.
Abbreviations: E2:estradiol; P:progesterone; RAS:Renin-angiotensin system; Ovras:renin-angiotensin system; ACE1:angiotensin-converting enzyme-1; AngII:angiotensin II; AT1R:Angiotensin II type 1 Receptor; BMI:Body Mass Index; AT1-A:anti-angiotensin II type 1 receptor autoantibodies.
Introduction
Female-related infertility accounts for about 50% of couples’ infertility (Simopoulou et al. Citation2019), and its pathogenesis is complex, which has not been fully elucidated (Lindsay and Vitrikas Citation2015). With the development of reproductive immunology, immune factors have been shown to be one of the important causes of infertility (Deroux et al. Citation2017). Studies have found that reproductive system autoantibodies (including anti-human chorionic gonadotropin, anti-endometrial antibody, anti-ovarian antibody, anti-sperm antibody, etc.) can affect serum estradiol (E2) and progesterone (P) as well as other hormone levels (Yasin et al. Citation2016), that can also affect other processes like egg discharge, fertilization, embryo implantation. These can cause infertility. However, the known immune antibody screening cannot explain many cases of clinical infertility, so it is urgent to expand the scope of screening.
The renin angiotensin system (RAAS) is an important regulatory system to maintain blood pressure, water and electrolyte balance and cardiovascular homeostasis. Angiotensin II (AngII), as the main effector molecule in RAAS, has a strong vasoconstrictive effect, stimulates the central sympathetic nervous system, and increases the secretion of vasopressin and aldosterone (Gohlke et al. Citation1988). AngII type 1 receptor (AT1R) mainly mediates some physiological effects of AngII, such as increasing protein synthesis and participating in the growth and proliferation of blood vessels. Anti-Angiotensin II type 1 Receptor Autoantibodies (AT1-AA), which have the same affinity as Ang II and the ability to activate AT1R, can be used as a new biological target for the evaluation of the autoimmune disease progression.
RAAS exists not only in the circulatory system, but also in the ovary, heart, kidney, adrenal gland, and other tissues. The local concentration may exceed the concentration in the plasma, and it can independently affect organ function through autocrine, paracrine, and cellular endocrine pathways. A local RAAS signal, the ovarian renin-angiotensin system (OVRAS), has been found in the adult ovary. By immunostaining follicles, angiotensinogen, angiotensin and renin were identified (Palumbo et al. Citation2016). Renin is secreted by cultured follicles and granulosa cells, and angiotensin I and II receptors are found in the ovary (Palumbo et al. Citation2016).
OVRAS plays a key role in ovarian physiology and disease. AT1R mediates the effect of AngII on the local physiology of reproductive tissues, thereby regulating follicle maturation and angiogenesis. Ang II regulates ovarian steroid production and corpus luteum formation, and stimulates oocyte maturation and ovulation by AT1R on granulosa cells. OVRAS plays a role in regulating oocyte development, vascular tension and angiogenesis, as well as steroid hormone secretion (Cunningham et al. Citation2016). However, in women with infertility associated with non-ovarian factor, low ovarian reserve, and polycystic ovary syndrome, OVRAS is disordered, in which AT1R expressed on granulosa cells changes, suggesting that OVRAS will affect the conception process (Pan et al. Citation2013).
A major pathway in OVRAS is angiotensin-converting enzyme-1 (ACE1)/AngII/AT1R. The balance between this pathway is important in the process of follicular development, granulosa cell apoptosis and ovulation (Cavallo et al. Citation2017). Studies have shown that another potential mechanism of ACE1’s involvement in female fertility involves increased oxidative stress. It is worth noting that reactive oxygen species (ROS) can damage the pathophysiology of human reproduction (Agarwal et al. Citation2005). One of the most important consequences of increased oxidative stress is the development of an inflammatory response. It has been reported that excessive AngII can promote oxidative stress through activation of AT1R, which leads to an inflammatory effect (Benicky et al. Citation2009). Inflammatory cytokines play an important role in the regulation of follicular development and ovulation. The change of their expression or activity can lead to the prolonging the ovarian cycle and ovulation disorder (Deepika et al. Citation2013).
Therefore, the increased ACE1 level will produce excessive AngII, which may damage the reproductive ability and lead to infertility due to the increase of oxidative stress. However, captopril as an ACE1 inhibitor, does not affect ovulation in rats and rabbits (Xia et al. Citation2003), indicating that ACE1/AngII/AT1R is not the only way to regulate ovulation and induce inflammation. It is not clear whether other stimulating factors that affect AT1R eventually lead to infertility, like the regulation of ovarian blood flow and ovulation events by angiotensin receptor agonists.
As an autoantibody specific to AT1R in the RAS system, AT1-AA (Angiotensin II type 1 Receptor Autoantibody) was first found in patients with eclampsia (Xia and Kellems Citation2013), and later shown to have influence on other diseases, such as Graves’ disease, hypertension, myocardial fibrosis (Xu et al. Citation2014; Hao et al. Citation2018). As an active autoantibody, AT1-AA has attracted much attention because of its ability to activate AT1R continuously. Whether AT1-AA is distributed in infertility patients, and its pathological significance is not clear. Therefore, in order to determine the relationship between the level of AT1-AA and the oocyte development process or ovulation, this study detected the AT1-AA level in serum of female infertility patients. Statistical correlation analysis with AFC, the number of oocytes obtained, and the oocyte maturation rate to identify risk factors affecting the level of AT1-AA in infertility patients.
Results
High level of AT1-AA in female infertility
The serum AT1-AA level of 227 participants from Taiyuan central hospital was analyzed by ELISA and evaluated as a function of basic information related to infertility. There was no significant difference in age (24.89 ± 4.16 VS 25.97 ± 3.75, P > 0.05) and BMI (Body Mass Index) (23.61 ± 3.74 kg/m2 VS 23.84 ± 3.51 kg/m2, P > 0.05) between infertile and healthy non-pregnant women. There was no history of smoking in the two groups. The length of infertility was 4.01 ± 1.93 years. It shows that there is no difference in basic information between the infertile and healthy non-pregnant women, so they can participate in the experiment as an experimental group and a control group.
A total of 138 cases of infertility were collected, of which 35 cases were AT1-AA positive (P/N > 2.1), the positive rate was 25.36%; 89 healthy non-pregnant women, 8 cases were positive, the positive rate was 8.98%. The positive rate of AT1-AA in the serum of infertile patients was higher than that of normal women, and the difference between the two groups was statistically significant (χ2 = 9.45, P < 0.01), indicating that AT1-AA may play a role in infertility.
Parameter analysis of infertility patients
Difference analysis of clinical values and experimental data
Through the analysis of the difference between the positive and negative groups of AT1-AA in the ovulation induction program among infertile patients, it is found that there is no statistical significance between the two groups (χ2 = 0.50, P > 0.05), as shown in . The results showed that there was no difference between AT1-AA positive group and negative group due to different ovulation induction programs. Therefore, it is meaningful to compare the difference in AFC between the two groups. The correlation between the AT1-AA titer with AFC, the number of received oocytes, oocyte maturation rate (oocyte maturation rate = number of mature oocytes/number of oocytes retrieved × 100%) after the ovulation induction program was determined.
Table 1. Analysis of the difference between AT1-AA positive and negative groups in the ovulation induction program
shows that in the infertile patients, there was no significant difference between the number of positive or negative AT1-AA groups in terms of age, BMI and infertility years. There is no significant difference in the levels of sex hormones: FSH, LH, E2, PRL, T, PRG; thyroid function: TSH, FT3, FT4. Among the inflammatory factors, e.g., IL-6, there was a significant difference between the positive and negative of AT1-AA groups (t = 8.51, P < 0.05). This data suggested that AT1-AA may be able to trigger the inflammatory reaction, and the correlation between AT1-AA and IL-6 should be analyzed.
Table 2. Difference analysis of AT1-AA positive and negative groups
AT1-AA is negatively correlated with oocyte maturation rate, while IL-6 is positively correlated
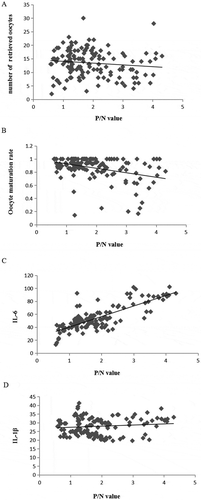
AT1-AA was assessed as a function of AFC, the number of oocytes received, oocyte maturation rate, by spearman correlation. The results showed that there was no correlation between AT1-AA and AFC (P > 0.05), and there was no statistically significant difference between AT1-AA and the number of received oocytes (P > 0.05, ), but there was a significant negative correlation between AT1-AA and the oocyte maturation rate (r = −0.29, P < 0.01, ). Spearman correlation analysis of AT1-AA with inflammatory factors IL-6 and IL-1β showed that AT1-AA was positively correlated with IL-6 (r = 0.68, P < 0.01, ). There was no significant correlation with IL-1β (r = 0.04, P > 0.05), ). The results show that AT1-AA is closely related to the oocyte maturation disorder, which may be related to triggering inflammation.
Figure 1. Correlation analysis of P/N value of AT1-AA in infertile women. (A) is a scatter plot of P/N value and the number of received oocytes in infertility, there is no statistical significance between AT1-AA and the number of received oocytes (P > 0.05). (B) is a scatter plot of P/N value and oocyte maturation rate in infertility, there is a significant negative correlation between AT1-AA and the oocyte maturation rate (r = −0.29, P < 0.01).The results show that AT1-AA is closely related to the oocyte maturation disorder. (C) is a scatter plot of P/N value and IL-6 in infertility, AT1-AA was positively correlated with IL-6 (r = 0.68, P < 0.01). (D) is a scatter plot of P/N value and IL-1β rate in infertility, AT1-AA was no significant correlation with IL-1β (P > 0.05). AT1-AA may be related to triggering inflammation
Analysis of epidemiological factors affecting AT1-AA
Age, BMI, type of infertility, years of infertility, history of adverse pregnancy events, condition of fallopian tube, and polycystic ovary syndrome were included in logistic regression equation. After adjusting for other factors through multinomial logistic regression, the results showed that: advanced age, obesity, primary Infertility, years of infertility, poor pregnancy history, unobstructed fallopian tubes, and polycystic ovary syndrome did not affect the levels of AT1-AA in the serum of infertile patients. See .
Table 3. Logistic regression analysis of influencing factors of AT1-AA
Discussion
As a new component of OVRAS, AT1-AA may play an important role in the treatment and regulation of infertility (Medenica et al. Citation2018). Our results showed that the positive rate of AT1-AA in infertile women was 25.36% higher than that in healthy non-pregnant women (8.98%) (χ2 = 9.45, P < 0.01). It showed that AT1-AA may play a role in infertility. AT1-AA has the same affinity and ability to activate AT1R as AngII. However, our early research found that it has its own unique way of functioning (Lei et al. Citation2018). It is suggested that in the new autoimmune OVRAS pathway, AT1-AA can be used as a new biomarker in the progress of infertility, which will help us to reinterpret the pathogenesis of OVRAS in infertility and predict disease.
Our study addressed whether the changes in AT1-AA directly caused the difference in hormone levels, when there is no significant difference between the positive and negative groups of AT1-AA in basic clinical parameters, such as age, BMI, infertility years. There were also no significant differences in sex hormone levels: FSH, LH, E2, PRL, T, PRG; and thyroid function: TSH, FT3, FT4; AFC. This suggested that AT1-AA does not directly affect the basic hormone level of ovarian function and ovarian reserve. However, OVRAS activity is regulated by gonadotropins, which directly regulates the production of sex hormones. In turn, the effect of AngII on the production of steroid hormones has been confirmed in animal experiments (Palumbo et al. Citation2016), indicating that OVRAS and sex hormones interact and regulate each other throughout the cycle. Since AT1-AA cannot directly affect hormone levels, it is likely to activate the downstream target and act on the ovary.
Studies have shown that in the natural cycle and the cycle stimulated by exogenous gonadotropin, OVRAS will play a role in oocyte maturation under the influence of gonadotropin. This has been confirmed in a number of animal studies (Lightman et al. Citation1987; Kim et al. Citation2017). Therefore, the study was carried out after exogenous stimulation, that is, we analyzed the correlation between AT1-AA and the number of oocytes received and oocyte maturation rate by spearman correlation, respectively. The results showed that along with no difference between the positive and negative AT1-AA groups in the ovulation induction program (χ2 = 0.50, P > 0.05), there was no correlation between AT1-AA and oocytes. However, there was a significant negative correlation between AT1-AA and oocyte maturation rate (r = −0.29, P < 0.01), suggesting that AT1-AA may be related to oocyte maturation disorders. This is consistent with the above study. It is suggested that AT1-AA can act on the ovary and affect the development and maturation of oocytes.
Some key studies indicate that AT1-AA play an important role in the inflammatory process, while ovulation itself is an inflammatory process (Dragun et al. Citation2005; Duffy et al. Citation2018). Our results show that among infertility patients, the level of IL-6 in the positive group of AT1-AA was significantly higher than that in the negative group (t = 8.51, P < 0.01). The results showed that AT1-AA was positively correlated with IL-6 (r = 0.68, P < 0.01). There was no significant correlation between IL-1β and AT1-AA (r = 0.04, P > 0.05). IL-1β is mainly expressed by innate immune cells in inflammatory injury. IL-1β regulates the expression of granulosa cell ovulation-related genes is the. IL-6 is known as an important inflammatory factor in human body. It is expressed in ovarian stromal cells, epithelial cells and follicles. It has various biological effects on immune and inflammatory response. Studies have found that ovulation disorders were associated with increased inflammation markers (Duffy et al. Citation2018). This is consistent with our results. AT1-AA is involved in the process of ovulation dysfunction and participates in the regulation of inflammatory factors in this process, but has little effect on the inflammatory factors produced by innate immune cells.
AT1-AA plays a role in infertility. Prevention and treatment of its pathological role may improve the outcome of infertility, e.g., the use of AT1R blocker. Studies have shown that the use of AT1R antagonist losartan can improve follicular development in obese women (Wallukat et al. Citation1999), but other studies have suggested that the use of AT1R antagonist in late pregnancy puts offspring at risk of kidney damage (Griendling et al. Citation2000), which may cause side effects during use. It is necessary to develop effective and safe blockers of AT1-AA. However, by examining epidemiological factors leading to high levels of AT1-AA in infertile women their reduction may effectively prevent and improve the level of AT1-AA in women of childbearing age. Logistic regression analysis was conducted to further analyze such factors as age, BMI, type of infertility, infertile years, adverse pregnancy history, fallopian tube condition, and polycystic ovary syndrome. The results showed that none of the above factors affected the in vivo level of AT1-AA in infertile patients. It is suggested that the production of AT1-AA and the possible effect of oocyte maturation disorder are closely related to individual differences. However, the response of age to ovarian function is an important factor, and the age of the subjects in this study is relatively concentrated, so it does not reflect this phenomenon very well.
The application of these new AT1-AA to the ovaries may help us reinterpret the function of OVRAS (Sagae et al. Citation2015), filling in the knowledge gap of the best pharmacological operation in reproductive medicine and family planning. This study found that the level of AT1-AA in infertile patients is higher than that of healthy non-pregnant women, and the high level of AT1-AA in infertility may be related to oocyte maturation disorders and ovulation disorders. It is confirmed that AT1-AA plays an important role in the inflammation process. Our pilot study points to the downstream signaling pathway activated by AT1-AA and AT1R in infertile women, which may lead to ovulation disorder and inflammatory responses. It is suggested that AT1-AA should be explored in human reproduction and assisted reproductive technology. However, this study is limited to the study of serum components, and the lack of in vitro experiments that would independently confirm the effect of AT1-AA on follicles. This would help clarify the physiological mechanism of AT1-AA.
Materials and methods
Research materials
The serum and medical records of healthy non-pregnant women who did not diagnose infertility and of infertile women who underwent invitro fertilization embryo transfer (IVF-ET or ICSI) from July 1, 2018 to December 31, 2019 were collected at Taiyuan Central Hospital. The healthy non-pregnant women all had a history of pregnancy. The common characteristic of infertile women is infertility. The causes of these women’s infertility include primary infertility and secondary infertility. The protocol was reviewed and approved by the ethics committee of Taiyuan central hospital. All the subjects understood the content of the experiment and signed the informed consent, and carried out according to the declaration of Helsinki principles. Inclusion criteria: no major heart, lung, kidney, liver, and other diseases. Exclusion criteria: suffering from major heart, lung, kidney, liver and other diseases, the cause of infertility comes from male infertility (sperm abnormality, etc.) and females have positive infertility-related immune antibodies. Serum and medical records of 89 healthy non-pregnant women and 138 infertile women in reproductive center were collected.
Research methods
AT1-AA detection method
The titer of AT1-AA in serum was detected by SA-ELISA. In brief, menstrual serum was obtained from patients with IVF/ICSI, and SA-ELISA was used to detect the titer of AT1-AA in serum. The synthesized AT1-AA peptide was dissolved in 100 mM Na2CO3 (pH 11.0) at a concentration of 10 μg/ml, coated with a blank ELISA plate at 100 μl/well, and frozen overnight at 4°C; The supernatant was discarded from the plate, and 200 μL/well of PBST (Phosphate Buffered Saline+Tween-20) diluted milk was added. After air drying, and discard the supernatant after 1 h of water bath at 37°C in thermostat water bath cauldron, and wash three times with PBST; The positive control, negative control and the sample to be tested were diluted in the milk according to 1:100, 100 μL/well were added to the peptide-coated and non-peptide-coated holes, respectively. Discard the supernatant after 1 h of water bath at 37°C, and wash three times with PBST; Dilute the corresponding secondary antibody labeled with horseradish peroxidase in milk, add 100 μl/well to the sample, discard the supernatant after 1 h of water bath at 37°C, and wash three times with PBST; The substrate ABTS and the substrate buffer are configured according to 1:1.1 (the formulation of substrate buffer: Na2HPO4•12H2O 18.41 g, citric acid C6H8O7•H2O 5.10 g are dissolved in 1 L distilled water, and citric acid regulates the pH to 5.0). When in use, add 0.5 µl H2O2,100 μl/well, and take a water bath at 37°C for 30 min. After the reaction is stable, use the enzyme scale to measure the OD value of each pore at λ = 405 nm; P/N ≥ 2.1 is positive, P/N ≤ 1.5 is negative, P/N = (sample OD value – blank control OD value)/(negative control OD value – blank control OD value).
Detection of sex hormone level
In the morning of blood sampling, the subjects were uniformly tested by enzymelinked immunofluorescent assay in the male laboratory of the reproductive center of Taiyuan Central Hospital.
Calculation formula
Oocyte maturation rate = number of mature oocytes number of oocytes retrieved times 100%
Detection of IL-6 and IL-1β by ELISA
The ELISA kits (Wuhan Boster Bioengineering Co., Ltd.) of IL-6 and IL-1β were used. According to the experimental instructions, the standard substance with certain concentration gradient was prepared, which was (pg/ml): 10,000, 2000, 1000, 500, 250, 125, 62.5, 31.2, 0. A total of 9 EP tubes. The sample to be tested is diluted at 1:2. The standard substance and the sample to be tested were added into the enzyme plate with 100 µl/well in turn, covered with the plate sealing membrane, incubated at 37°C for 90 min. After shaking off the blocking solution in the enzyme plate without washing, the prepared biotin antibody against rat IL-6, IL-1β was directly added, 100ul/well, covered with the sealing plate membrane, incubated at 37°C for 60 min. The plates were washed with PBST for 3 times, 1 min/time, then the ABC (avidin peroxidase complex) working solution of 100 µl/well was added, covered with sealing membrane, incubated at 37°C for 30 min. The plates were washed 5 times with PBST for 1 min each time. Then TMB was added to 90 µl/well for 20–25 min at 37°C. Blue could be seen in some high-concentration wells. When TMB stop solution was added into 100 µl/well, the blue color changed to yellow immediately. Read the OD value at 450 nm.A standard curve according to the OD value of the standard sample. From the standard curve, it can be observed that there is a positive correlation between the OD value and the concentration of IL-6, IL-1β. Bring the OD value of the measured sample into the standard curve to calculate the concentration of the sample.
Statistical methods
SPSS22.0 software was used for data cleaning and statistical analysis. Chi-square test was used for single factor analysis. Pearson correlation analysis or Spearman correlation analysis was used for correlation analysis, and multinomial logistic regression analysis was used for Multivariate analysis, P < 0.05 was statistically significant.
The multinomial logistic regression model was as follows:
The dependent variable Y is a multi-categorical variable, including g categories (Y = 1,2,3, …,g), m independent variables X1, X2, …, Xm. Where j = 1,2, …, g-1, β0j are the constant terms of the jth regression equation, β1j, β2j, … βmj are the regression coefficients of the independent variables X1, X2, …, Xm of the jth regression equation.
Ethics approval
The study was approved by the local ethics committees in Taiyuan, Shanxi, Taiyuan Central Hospital of Shanxi Medical University (2018LL207). All the subjects understood the content of the experiment and signed the informed consent, and carried out according to the declaration of Helsinki principles.
Authors’ contributions
Contributed to the concept and design of the study: FL, GY, XY; Performed the research and analyzed the data: RG, LX; Carried out the bioinformatics analysis: DI, PN;Prepared the original manuscript: LW, JG, FL; Undertook the clinical assessment of patients and sample collection: GY. Contributed to the supervision of the project, revised the final manuscript and gave the final approval: XY. All authors have read and approved the manuscript for submission.
Acknowledgments
FLand GY thanks the others in their lab for their help. Thanks for the support of Taiyuan Central Hospital.
Disclosure statement
The authors declare that they have no conflict of interest.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Agarwal A, Gupta S, Sharma RK. 2005. Role of oxidative stress in female reproduction. Reprod Biol Endocrinol. 3:1–21. doi:10.1186/1477-7827-3-28.
- Benicky J, Sánchez-Lemus E, Pavel J, Saavedra JM. 2009. Anti-inflammatory effects of angiotensin receptor blockers in the brain and the periphery. Cell Mol Neurobiol. 29:781–792. doi:10.1007/s10571-009-9368-4.
- Cavallo IK, Dela Cruz C, Oliveira ML, Del Puerto HL, Dias JA, Lobach VN, Casalechi M, Camargos MG, Reis AM, Santos RA, et al. 2017. Angiotensin-(1-7) in human follicular fluid correlates with oocyte maturation. Human Reproduction. 32(6):1–7. doi:10.1093/humrep/dex072.
- Cunningham MW Jr, Williams JM, Usry N, Wallukat G, Dechend R, LaMarca B, Amaral L. 2016. Agonistic autoantibodies to the angiotensin II Type 1 receptor enhance angiotensin II-induced renal vascular sensitivity and reduce renal function during pregnancy. Hypertension. 68(5):1308–1313. doi:10.1161/HYPERTENSIONAHA.116.07971.
- Deepika ML, Reddy KR, Yashwanth A, Rani VU, Latha KP, Jahan P. 2013. TNF-a haplotype association with polycystic ovary syndrome-a South Indian study. Assist Reprod Genet. 30(11):1493–1503. doi:10.1007/s10815-013-0080-4
- Deroux A, Dumestre-Perard C, Dunand-Faure C, Dunand-Faure C, Bouillet L, Hoffmann P. 2017. Female infertility and serum auto-antibodies: a systematic review. Clin Rev Allergy Immunol. 53(1):78–86. doi:10.1007/s12016-016-8586-z.
- Dragun D, Muller DN, Brasen JH, Fritsche L, Nieminen-Kelhä M, Dechend R, Kintscher U, Rudolph B, Hoebeke J, Eckert D, et al. 2005. Angiotensin II type 1-receptor activating antibodies in renal-allograft rejection. N Eng J Med. 352:558–569. doi:10.1056/NEJMoa035717.
- Duffy DM, Ko CM, Jo M, Brannstrom M, Curry TE. 2018. Ovulation: parallels with inflammatory processes. Endocr Rev. 40(2):55–67.
- Gohlke P, Ganten D, Lang RE, Unger T. 1988. The renin-angiotensin system: systemic and local function. Zeitschrift für Kardiologie. 77:1–12.
- Griendling KK, Sorescu D, Lassegue B, Ushio-Fukai M. 2000. Modulation of protein kinase activity and gene expression by reactive oxygen species and their role in vascular physiology and pathophysiology. Arterioscler Thromb Vasc Biol. 20:2175–2183. doi:10.1161/01.ATV.20.10.2175.
- Hao WW, Zhang SL, Sun Y, Bai LN, Bian JW, Yu HC, Yin XC, Wang PL, Liu HR. 2018. Angiotensin II type 1 receptor autoantibody induces myocardial fibrosis by activating cardiac fibroblasts. Sheng Li Xue Bao. 70(4):343–353.
- Kim YJ, Kim YY, Kang BC, Kim MS, Ko IK, Liu HC, Rosenwaks Z, Ku SY. 2017. Induction of multiple ovulation via modulation of angiotensin II receptors in in vitro ovarian follicle culture models. J Tissue Eng Regen Med. 11(11):3100–3110. doi:10.1002/term.2214.
- Lei J, Zhang S, Wang P, Liao Y, Bian J, Yin X, Wu Y, Bai L, Wang F, Yang X, et al. 2018. Long-term presence of angiotensin II type 1 receptor autoantibody reduces aldosterone production by triggering Ca2+ overload in H295R cells. Immunol Res. 66(1):44–51. doi:10.1007/s12026-017-8963-6.
- Lightman A, Tarlatzis BC, Rzasa PJ. 1987. The ovarian renin angiotensin system: renin-like activity and angiotensin II/III immunoreactivity in gonadotropin-stimulated and unstimulated human follicular fluid. Am J Obstet Gynecol. 156(4):808–816. doi:10.1016/0002-9378(87)90336-X.
- Lindsay TJ, Vitrikas KR. 2015. Evaluation and treatment of infertility. Am Fam Physician. 91(5):308–314.
- Medenica S, Garalejic E, Arsic B, Medjo B, Bojovic Jovic D, Abazovic D, Vukovic R, Zarkovic M. 2018. Follicular fluid thyroid autoantibodies, thyrotropin, free thyroxine levels and assisted reproductive technology outcome. PLoS One. 13(10):e0206652. doi:10.1371/journal.pone.0206652.
- Palumbo A, Avila J, Naftolin F. 2016. The ovarian renin-angiotensin system (OVRAS): a major factor in ovarian function and disease. Reprod Sci. 23:1644–1655. doi:10.1177/1933719116672588.
- Pan PP, Zhan QT, Le F, Zheng YM, Jin F. 2013. Angiotensin-converting enzymes play a dominant role in fertility. Int J Mol Sci. 14(10):21071–21086. doi:10.3390/ijms141021071.
- Sagae SC, Gobo CG, Paz ED, Menegotto JB, Yamashita PK, Franci CR, Balbo SL. 2015. Blockade of AT1 receptor of angiotensin II reduces AFC in female rats with obesity induced by cafeteria diet. Rev Bras Ginecol Obstet. 37(7):302–307. doi:10.1590/S0100-720320150005352.
- Simopoulou M, Sfakianoudis K, Maziotis E, Grigoriadis S, Giannelou P, Rapani A, Tsioulou P, Pantou A, Kalampokas T, Vlahos N, et al. 2019. The impact of autoantibodies on IVF treatment and outcome: a systematic review. Int J Mol Sci. 20(4):892–896. doi:10.3390/ijms20040892.
- Wallukat G, Homuth V, Fischer T, Lindschau C, Horstkamp B, Jüpner A, Baur E, Nissen E, Vetter K, Neichel D, et al. 1999. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT(1) receptor. J Clin Investig. 103:945–952. doi:10.1172/JCI4106.
- Xia Y, Kellems RE. 2013. Angiotensin receptor agonistic autoantibodies and hypertension: preeclampsia and beyond. Circ Res. 113(1):78–87. doi:10.1161/CIRCRESAHA.113.300752.
- Xia Y, Wen H, Bobst S, Day MC, Kellems RE. 2003. Maternal autoantibodies from preeclamptic patients activate angiotensin receptors on human trophoblast cells. J Soc Gynecol Investig. 10(2):82–93. doi:10.1016/S1071-5576(02)00259-9.
- Xu J, Zhao L, Xiang G, Day MC. 2014. Relationship between autoantibody to the angiotensin II-1 receptor and cardiovascular manifestations of Graves’ disease. Exp Clin Endocrinol Diabetes. 122(4):254–258. doi:10.1055/s-0034-1370920.
- Yasin AL, Yasin AL, Basha WS. 2016. The epidemiology of anti-sperm antibodies among couples with unexplained infertility in North West Bank, Palestine. J Clin Diagn Res. 10(3):QC01–3.
