ABSTRACT
The COVID-19 pandemic has led to a worldwide health emergency that has impacted 188 countries at last count. The rapid community transmission and relatively high mortality rates with COVID-19 in modern times are relatively unique features of this flu pandemic and have resulted in an unparalleled global health crisis. SARS-CoV-2, being a respiratory virus, mainly affects the lungs, but is capable of infecting other vital organs, such as brain, heart and kidney. Emerging evidence suggests that the virus also targets male and female reproductive organs that express its main receptor ACE2, although it is as yet unclear if this has any implications for human fertility. Furthermore, professional bodies have recommended discontinuing fertility services during the pandemic such that reproductive services have also been affected. Although increased safety measures have helped to mitigate the propagation of COVID-19 in a number of countries, it seems that there is no predictable timeline to containment of the virus, a goal likely to remain elusive until an effective vaccine becomes available and widely distributed across the globe. In parallel, research on reproduction has been postponed for obvious reasons, while diagnostic tests that detect the virus or antibodies against it are of vital importance to support public health policies, such as social distancing and our obligation to wear masks in public spaces. This review aims to provide an overview of critical research and ethics issues that have been continuously emerging in the field of reproductive medicine as the COVID-19 pandemic tragically unfolds.
Abbreviations: ACE2: angiotensin- converting enzyme 2; ART: Assisted reproductive technology; ASRM: American Society for Reproductive Medicine; CCR9: C-C Motif Chemokine Receptor 9; CDC: Centers for Disease Control and Prevention; COVID-19: Coronavirus disease 2019; Ct: Cycle threshold; CXCR6: C-X-C Motif Chemokine Receptor 6; ELISA: enzyme-linked immunosorbent assay; ESHRE: European Society of Human Reproduction and Embryology; ET: Embryo transfer; FSH: Follicle Stimulating Hormone; FFPE: formalin fixed paraffin embedded; FYCO1: FYVE And Coiled-Coil Domain Autophagy Adaptor 1; IFFS: International Federation of Fertility Societies; IUI: Intrauterine insemination; IVF: In vitro fertilization; LH: Luteinizing Hormone; LZTFL1: Leucine Zipper Transcription Factor Like 1; MAR: medically assisted reproduction services; MERS: Middle East Respiratory syndrome; NGS: Next Generation Sequencing; ORF: Open Reading Frame; PPE: personal protective equipment; RE: RNA Element; REDa: RNA Element Discovery algorithm; RT-PCR: Reverse=trascriptase transcriptase-polymerase chain reaction; SARS: Severe acute respiratory syndrome; SARS-CoV-2: Severe Acute Respiratory Syndrome Coronavirus 2; SLC6A20: Solute Carrier Family 6 Member 20; SMS: Single Molecule Sequencing; T: Testosterone; TMPRSS2: transmembrane serine protease 2; WHO: World Health Organization; XCR1: X-C Motif Chemokine Receptor
It is hard to believe that something as small as an RNA virus could turn our world upside down in a matter of months. The COVID-19 (SARS-CoV-2) virus is about 50 times smaller than a red blood cell and contains a single strand 26,000 base RNA (Zhou et al. Citation2020). Compare this with the 9 billion base pairs in the human genome! An apt demonstration is that in nature, powerful forces can come in very small packages. But how does COVID-19 stack up with other pandemics the world has known ()?
Figure 1. Schematic presentation of the most world pandemics during history. The COVID-19 pandemic has (at August 2020) already caused the death of 0,65 million people, although it is impressive that this pandemic is at the beginning of its impact (only six months ago)
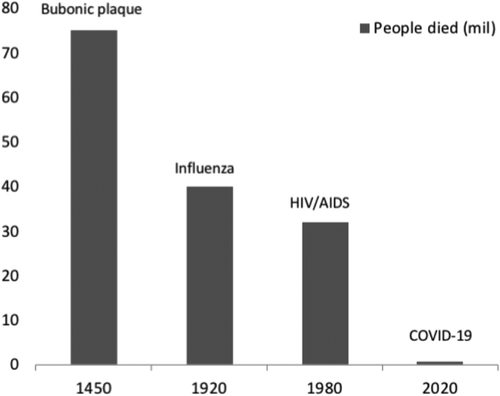
The COVID-19 virus has been called the modern plague. This is in reference to the bubonic plague or Black Death, which holds the record for being the most lethal pandemic in human history (Alchon Citation2003). An estimated 75 million people died during the outbreak in mid 14th century Eurasia and North Africa. Almost half of Europe’s population was wiped out, as the plague was lethal to 30 to 90% of those infected. The plague caused flu symptoms, hemorrhage and pneumonia, as well as painful swollen lymph nodes that form pus-filled boils called buboes. Unlike COVID-19, and according to modern genetic analyses, the plague was due to the bacterium Yersinia pestis. It spread through flea bites and contact with animals (especially rats) but was not spread widely between humans. Notably, the plague still exists today, with about 651 cases reported worldwide annually (https://www.cdc.gov/plague/maps/index.html). It is curable with common antibiotics.
At the time of the plague in medieval Europe, the world was naïve to a key, disease-fighting concept: hygiene and public health. This life-saving idea actually took hold in the mid-19th century after wide acceptance of the germ theory of disease (Walker et al. Citation2006). However, the medieval plague first introduced the idea of quarantine to limit the spread of disease, a powerful weapon still used today in response to epidemic diseases (https://www.cdc.gov/quarantine/historyquarantine.html).
Although still an emerging global health issue, the COVID pandemic also does not yet compare in severity to the second largest pandemic in history: the influenza epidemic of 1918–20 (Crosby Citation2003), commonly referred to as the Spanish Flu. With an estimated death toll of 40 million lives and a death rate of 10–20%, it tore across the globe and infected over 1/3 of the world’s population. It is theorized that the main reason that this epidemic reached all corners of the world was because it was the first to happen during the modern age of global travel. Also notable about this flu was that it was lethal to healthy individuals in addition to the elderly and immunocompromised, a brutal combination leading to a world-wide pandemic (Gagnon et al. Citation2013).
The third largest pandemic the world has seen is HIV-AIDS. Since its discovery in humans in 1976, it has killed 32 million people (https://www.amfar.org/worldwide-aids-stats/). This virus is one of particularly subtle design, spreading through body fluids via sex, saliva and blood, and weakening healthy immune systems such that normally benign infections or diseases become lethal. Although no HIV vaccine is currently available, modern medicine has developed sophisticated antiretroviral medicines that allows for near normal life expectancy. It is into this history of worldwide human pandemics that the COVID-19 virus now takes its place. We should also bear in mind that we are still early in this pandemic and our current understanding might have to be completely rethought as further information becomes available.
Impact of COVID-19 on spermatogenesis and male fertility
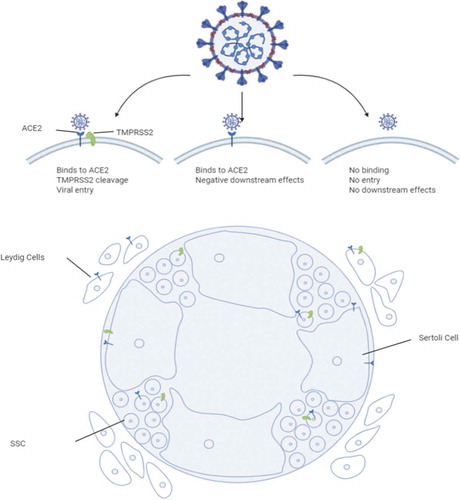
The novel SARS-CoV-2 virus has spread across the globe and induced a worldwide pandemic since its initial outbreak in Wuhan province, China, during late 2019. Many studies have focused on the impact of SARS-CoV-2 infection on the lungs but little is known about whether this virus affects male fertility. There is, however, rapidly accumulating evidence that SARS-CoV-2 infects tissues of the male urogenital tract (Illiano et al. Citation2020). The infection of the cells involves the presence of ACE2receptors and priming and cleavage of the virus spike proteins by TMPRSS2 (Hoffmann et al. Citation2020; Wrapp et al. Citation2020). Specifically, TMPRSS2 primes and cleaves the S1/S2 site on the SARS-CoV-2 spike protein and this S1 protein then binds to ACE2 to facilitate entry into the cell (Hoffmann et al. Citation2020; Wrapp et al. Citation2020) (). Therefore, cells that express high levels of ACE2 may be highly susceptible to viral entry. The virus’ major receptor ACE2 is also expressed in adult spermatogonia, Sertoli cells and Leydig cells, and consistently the virus has been detected in semen samples (Fu et al. Citation2020; Hikmet et al. Citation2020; Li et al. Citation2020c). It is currently unclear, which cellular reservoir might be at the origin of this phenomenon. In Leydig and Sertoli cells, ACE2 expression is around 5% (about 3x the expression of ACE2 in alveolar type 2 cells). In contrast, TMPRSS2 expression is very low, which means that while viral entry is very low the possibility of adverse downstream effects through the binding of ACE2 is still possible. Approximately 1.3% of SSCs express ACE2, while 2% express TMPRSS2 (). ACE2 expression levels appear to be age-dependent, peaking during adulthood and diminishing in aged individuals (Abobaker and Raba Citation2020). However, little attention is currently being given to possible short- and long term effects on gonocyte differentiation during early stages of spermatogenesis in infected pre-pubertal children. Given that ACE2 mRNA is detectable in testicular biopsies of infant patients diagnosed with undescended testis, it is conceivable that severe forms of COVID-19 in children may negatively affect gonad development (www.germonline.org; Hadziselimovic et al. Citation2009; Lardenois et al. Citation2010; Chalmel et al. Citation2012).
Figure 2. Infection of testicular cells by SARS-CoV-2. Different entry mechanisms and effects depending on ACE2 and TMPRSS2 are given at the top. A schematic shows interstitial Leydig cells, Sertoli cells and spermatogonial stem cells as indicated (SSC). Symbols in blue and green represent ACE2 and TMPRSS2, respectively. The image was created with BioRender licensed to CA Easley
The field of male reproductive biology is in dire need of molecular leads to gain insight into the testicular infection mechanism and its potential consequences on spermatogenesis. In this context it is noteworthy that a recent interactome study has identified interactions between 26 structural and non-structural SARS-CoV-2 proteins with 332 human proteins (Gordon et al. Citation2020). It is possible that during infection such complexes may alter or impair the functions of specific testicular proteins, which in turn could result in immediate and/or long-term effects on spermatogenesis. Interesting candidates include proteins that are involved in proliferation of spermatogonial stem cells (SBNO1, which is bound by viral protein nsp12) (Shen et al. Citation2019), receptor transport from the endoplasmic reticulum to the cell surface and sperm morphology (REEP6, bound by M) (Devlin et al. Citation2020), PKA signaling (PRKAR2A bound by nsp13) (Burton and McKnight Citation2007), proteolysis important for sperm maturation (TYSND1, bound by nsp12) (Mizuno et al. Citation2013) and RNA splicing/translation during germ cell development (PTBP2, bound by orf9b) (Zagore et al. Citation2015).
Given that mammalian target proteins are present in ACE2-expressing testicular cells it is possible that their interactions with respective viral proteins are of biological and clinical importance (www.proteinatlas.org; Djureinovic et al. Citation2014; Uhlen et al. Citation2015). For example, nsp12 is the catalytic subunit of SARS-CoV-2’s RNA dependent RNA polymerase (Peng et al. Citation2020). The nsp12’s abnormal presence in mammalian cells might alter the activities of SBNO1 and TYSND1 that are involved in WNT signaling and peroxisome function, respectively. The former is relevant for spermatogonial stem cell differentiation (Yoshida Citation2018) and the latter is critical for sperm maturation (Mizuno et al. Citation2013). Also, nsp13 is an RNA helicase that via its interaction with PRKAR2A, might interfere with cAMP-dependent signaling important for lipid and glucose metabolism and hormonal regulation of Sertoli cells (Ni et al. Citation2020; Shu et al. Citation2020). The orf9b is a negative regulator of mitochondrial function and may also interfere with germ cell/Sertoli cell communication by recruiting PTBP2 into a bipartite protein complex (Shi et al. Citation2014; Hannigan et al. Citation2017). Finally, M is a structural component of the viral envelope that, by binding REEP6 could alter protein transport pathways important for spermiogenesis and oocyte fertilization (Devlin et al. Citation2020; Satarker and Nampoothiri Citation2020). In summary, it seems that viral proteins interact with human protein-targets that could exert their action at any time during the entire male reproductive cycle from spermatogonial stem cell differentiation to a spermatozoa’s ability to fertilize an oocyte. Notably, in a recent study, it was proposed that SARS-CoV-2 may also be a sexually transmitted virus as other viruses, like Ebola and Zika (Fei et al. Citation2020).
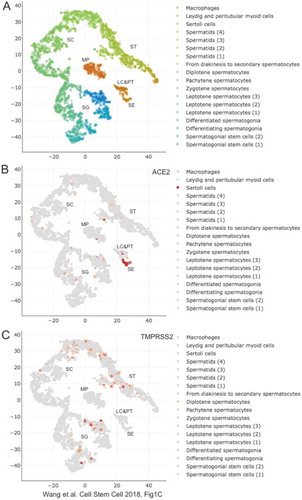
Using established single cell RNA sequencing datasets (Pan et al. Citation2020) there are reports that suggest limited expression of ACE2 and TMPRSS2 in testis tissue and that the expression of these genes does not overlap. This indirectly supports the notion that viral entry into testicular cells is unlikely to occur. Single-cell RNA-Sequencing data indeed confirm that ACE2 is predominantly expressed in Sertoli cells, while TMPRSS2 is transcribed only in a sub-population of germ cells at different stages of development () (Wang et al. Citation2018). Other studies have reported elevated levels of ACE2 and TMPRSS2 in the testis, with overlapping expression in Leydig cells, Sertoli cells, and spermatogonia (Pan et al. Citation2013; Verma et al. Citation2020; Wang and Xu Citation2020). These studies suggest that SARS-CoV-2 can enter testicular cells and potentially impact male fertility. Other members of the SARS-CoV family have been shown to cause testicular dysfunction including germ cell death, leukocyte infiltration, and orchitis (Xu et al. Citation2006) and therefore SARS-CoV-2 may potentially cause similar symptoms. Indeed, 19% of patients experienced testicular discomfort around the time of their SARS-CoV-2 diagnosis (Pan et al. Citation2020). Although the cause of this testicular discomfort is unknown, orchitis due to SARS-CoV-2 could be a contributing factor. A medRxiv unrefereed preprint study by Ma et al assessing 81 moderate-to-severe SARS-CoV-2 adult males compared to 100 age-matched controls indicated that LH levels in serum were elevated in infected patients (Ma et al. Citation2020). Additionally the authors reported that the ratios of T to LH and FSH to LH were significantly decreased in infected individuals compared to controls (Ma et al. Citation2020). Clinically, elevation of serum LH and decreased ratios (T:LH and FSH:LH) indicate testicular dysfunction that may impact sperm production.
Figure 3. scRNA-Seq expression data in testis for ACE2 and TMPRSS2. (A) A color-coded scatter plot identifies testicular cells types as shown in the legend. Regions showing clusters of macrophages (MP), Sertoli cells (SE), Leydig and peritubular cells (LE&PT), spermatogonia (SG), spermatocytes (SC) and spermatids (ST) are indicated. (B,C) Expression data are shown for genes as indicated at the top. The figure was created by M. Primig using the Reproductive Genomics Viewer’s scatter plot function (https://rgv.genouest.org; Darde et al., Bioinformatics Citation2019)
Future studies examining semen parameters in early and symptomatic stages of SARS-CoV-2 infection should be conducted to examine acute effects on spermatogenesis in infected men. Additionally, follow-up studies of at least 3+ months should be conducted to examine if there are any long-term impacts on male fertility following recovery from SARS-CoV-2 infection. Studies should also be conducted in relevant animal models and novel testicular organoid and in vitro spermatogenesis models to delineate mechanisms by which SARS-CoV-2 affect male fertility.
Impact of COVID-19 on humans according to gender
It has been made clear that disparities exist in the rate of spread and rates of cases and case-fatalities between countries, even when adjusted for population size. Some factors that may in part explain this include: gender-related differences, geographic location, genetic differences, population density and demographics, exposure to international travel, reporting criteria, pandemic preparedness and response timing, testing capabilities, and variations in the capacity for response of the different healthcare systems and political climate.
Gender-related differences have been detected in the susceptibility to SARS- CoV-2 infection (Zhu et al. Citation2020; Wu et al. Citation2020; Scully et al. Citation2020). These differences may originate from genetic or epigenetic differences between males and females resulting in differential gene expression and subsequently in different gender-related prognosis (Klein and Flanagan Citation2016; Takahashi et al. Citation2020). Although little is known about the origin of these differences, advances in this area should be very important to determining susceptibility and identifying prognostic biomarkers in step with designing better prevention and treatment strategies for male and female patients ().
Table 1. Main gender-genetic differences and possible effects caused by potential SARS-CoV-2 infection on male and female gonadal cell types that express the ACE2 receptor
One of the first genes potentially related to COVID-19 susceptibility to infection is ACE2 (Wan et al. Citation2020; Hoffmann et al. Citation2020). ACE2 is an X chromosome- encoded gene that is downregulated by estrogens (Liu J et al. Citation2010) and exhibits tissue- specific expression patterns in addition to polymorphisms which may affect functions and viral interaction (Li et al. Citation2020b). Different missense mutations have been detected in ACE2 with minor allele frequency differences between populations. Two of these variants, the K26R and the I468V, may affect binding characteristics between S protein of the virus and ACE2 receptor (Li et al. Citation2020c). In addition, the fact that the ACE2 gene is part of the X-chromosome, allows females to be potentially heterozygous compared to men who are hemizygous. Therefore, male cells always will express a single ACE2 allele because all cells contain an identical X chromosome. In contrast, females could be heterogenic because of the mosaicism of X chromosomes, which are stochastically inactivated between the cells. Thus, in the female, a potentially more efficient form of ACE2 receptor would be present in only half of the cells, therefore limiting the ability of SARS-CoV-2 viruses to infect their host. However, the magnitude of the difference is relatively small and the significance is not clear since functional studies are not available (Li et al. Citation2020b).
In males ACE2 expression is relatively high in testis and the corresponding protein is also present in the mature spermatozoa (Oliva et al. Citation2008; Castillo et al. Citation2018; Verma et al. Citation2020). This may also account for the gender differences and may result in related reproductive health concerns. In some COVID-19 patients’ inflammation of the testis has been reported although in most of the patients the lesions were mild (Yang et al. Citation2020). However, in most patients in which the semen has been analyzed, SARS-CoV-2 virus has not been detected in the ejaculate (Eisenberg Citation2020, Li et al. Citation2019). Although epidemiological data indicate that men are more severely affected than women, ACE2 is also highly expressed in females (Li et. Citation2020b). Therefore, it seems that other factors are present and account for the gender differences in disease prognosis.
The differences in severity could be attributed to the differential gene expression profiles between males and females and reflecting the X and Y chromosome related-genes, differential sex hormone profiles and the immune status (Dorus et al. Citation2012; Takahashi et al. Citation2020). The X chromosome contains many immune response related-genes, miRNAs and other non-coding regulatory RNAs (Kloc et al. Citation2020). Interestingly, the presence of the Y chromosome regulates many different immune responses and functions, although the mechanism by which the transcription of the other genes is largely unknown (Francisco and Lemos Citation2014). The Y chromosome among the males also differs according to the haplogroup (Parachinni et al. Citation2000; Krausz et al. Citation2009; Lo Giacco et al. Citation2014). Interestingly, males with one of the most common Y chromosome haplogroups present in European lineages have an up-regulated inflammatory response, downregulated adaptive immunity and a higher risk of coronary disease (Maan et al. Citation2017).
Both the innate and the adaptive immunological response are influenced by sex hormones, with the androgens being anti-inflammatory and the estrogens both pro-inflammatory and anti-inflammatory (Klein and Flanagan Citation2016). However, the mechanisms of these differences are not yet fully understood but seem to depend on very complex reciprocal interactions between sex chromosome-encoded and regulatory factors and hormones. These are also related to the microbiota inhabiting the human body. It is known that there are important differences between males and females in the microbiota gastrointestinal tract and other body organs. This differential microbiota (microgenderome) develops after puberty in parallel with the changes in sex hormones (Vemuri et al. Citation2019; Kloc et al. Citation2020). This aspect is gaining increasing importance as the microbiota regulates local and systemic inflammation responses and therefore may explain any substantial proportion of the gender associated susceptibility differences to infection. Differences in androgen sensitivity may also potentially explain some differences as well as the male to male variation (Wambier et al. Citation2020). Informatively, the androgen receptor regulates transcription of TMPRSS2 which is a prerequisite for infection. Foremost, genetic variants in the androgen receptor have been associated with androgenetic alopecia, prostatic hyperplasia, cancer and polycystic ovary syndrome. Preliminary studies have reported high rates of androgenetic alopecia in hospitalized COVID-19 patients due to severe symptoms (Gordon et al. Citation2020).
A radically different approach to identifying susceptibility genes has been through the application of genome wide association studies (Kachuri et al. Citation2020; Ellinghaus et al. Citation2020). Specifically, associations were detected at the 9q34.2 embedded in the ABO blood group locus and several other signals clustered at 3p21.31, which included genes for SLC6A20, LZTFL1, CCR9, FYCO1, CXCR6 and XCR1 (Ellinghaus et al. Citation2020). A significant higher risk and a protective effect in A and O blood groups, respectively, was detected in patients with severe COVID-19 as compared with other blood groups. Locus 3p21.31, contains several genes with functions that are potentially relevant to COVID-19 susceptibility or prognosis. One of the candidates detected is SLC6A20, which encodes a transporter and functionally interacts with ACE2 (Ellinghaus et al. Citation2020). In addition, the locus also contains genes that encode chemokine receptors (CCR9; CXCR6) related to the immune response to airway pathogens (Wein et al. Citation2019). Using similar approaches, the HLA region was also confirmed to be important in the host response to viral infection (Kachuri et al. Citation2020). Overall the results from these genomic approaches are consistent with the maladaptive cytokine release in response to infection and other stimuli, known as ‘cytokine storm’. At both the local and systemic levels this causes substantial immune damage that has been reported as part of the pathogenesis in severe COVID-19 patients (Debnath et al. Citation2020; Ye et al. Citation2020).
Although further studies are needed to determine the functional consequences of these associations, the aforementioned results represent a first step toward identifying genetic variation associated to COVID-19 susceptibility and prognosis. It will be also interesting to determine whether male and female mRNA and protein expression profile differences in these genes may also be related to the gender detected differences in disease response using an integrated systems biology approach.
Impact of COVID-19 on Assisted Reproductive Technology (ART) and Pregnancy
Following the WHO announcement of the COVID-19 pandemic on March 11th 2020 (WHO Virtual Press Conference on COVID-19 Citation2020), the largest reproductive medicine societies ASRM and ESHRE responded acutely, decisively, and independently to recommend suspending specific aspects and adapting others when delivering reproductive medicine care (ASRM: Patient Management and Clinical Recommendations During the Coronavirus (COVID-19) Pandemic Citation2020; ESHRE News and Statements Citation2020). The key recommendations coming from initial society statements stressed minimizing patient-interaction by increasing the use of telemedicine and suspension of new fertility treatments including: ovulation induction, IUIs, IVF, gamete retrieval and cryopreservation in non-urgent cases and fresh, or frozen ET. Furthermore, recommendations extended to suspending elective surgeries and non-urgent diagnostic procedures (Alviggi et al. Citation2020). Some exceptions were incorporated into the initial recommendations and more were added subsequently to include patients that were currently undergoing treatment ‘in-cycle’ and those patients requiring urgent gamete cryopreservation (e.g., prior to initiating gonadotoxic therapy for cancer treatment) (Alviggi et al. Citation2020; ASRM: Patient Management and Clinical Recommendations During the Coronavirus (COVID-19) Pandemic Citation2020). These initial recommendations were also adopted/reaffirmed by many other reproductive medicine societies and organizations including the International Federation of fertility Societies (IFFS) (IFFS: COVID-19 Task Force Statements Citation2020). These guidelines were issued in part, out of an abundance of caution due to concerns regarding the unknown risks of COVID-19 in pregnant women and their fetuses. This specific concern was predicated by the prior experience with other coronavirus strains, including SARs and MERS, which were linked with adverse clinical outcomes, and higher rates of premature births and miscarriages (Monteleone et al. Citation2020; Schwartz and Graham Citation2020). Additionally, although unrelated to coronaviruses, the 2015 Zika virus epidemic was shown to affect the central nervous system that resulted in birth defects including microcephaly in some neonates of affected pregnant women (Hajra et al. Citation2017). Moreover, these recommendations facilitate the optimization of, and allocation of critical healthcare resources and the support of social distancing measures including limiting travel for care. These measures were intended to avoid overwhelming healthcare systems while maximizing the safety of patients, fetuses, newborns, and ART clinic staff by reducing the spread of the virus (Vaiarelli et al. Citation2020).
Following these recommendations, most ART clinics worldwide halted or were significantly limited, providing medically assisted reproduction services (MAR), infertility treatment exclusive of IVF and ART, including IVF (Ory et al. Citation2020). However, implementation of these recommendations has varied significantly among clinics and countries. In light of emerging evidence, and mitigation strategies that in some places have led to the relaxation of social distancing measures and important discussions on patient autonomy, ASRM, ESHRE, IFFS and others have regularly updated their recommendations. These updated recommendations are less stringent, endorsing a gradual resumption of ART services along with measured and thoughtful guidance on how best to get back to business while protecting patients, future offspring, and staff as much as possible (Albertini Citation2020; COVID-19 and Human Reproduction Joint Statement: ASRM, ESHRE, IFFS Citation2020). A joint statement from ASRM, ESHRE, IFFS issued on May 29, 2020 affirmed the importance of providing reproductive services during the COVID-19 pandemic (COVID-19 and Human Reproduction Joint Statement: ASRM, ESHRE, IFFS Citation2020). However, prior to resuming treatment, clinics were encouraged to monitor local conditions, including guidance from professional societies, disease prevalence, status of government or state regulations, and availability of resources. Clinics are also advised to develop and implement risk assessment strategies to reduce the risk of viral transmission. They face the challenge of continuing to provide essential patient care while ensuring the safety of both patients and staff. Patients should also be counseled about all available options, including delaying evaluation and treatment. Furthermore, the recommendations also suggest prioritization of care, for example subgroups of patients for whom time is a critical variable, such as advanced maternal age or reduced ovarian reserve. In these instances, postponing treatment for an indefinite period will increase the time to oocyte retrieval and could significantly reduce the patients’ chance to successfully conceive (Alviggi et al. Citation2020; Vaiarelli et al. Citation2020). Different countries and cultures have displayed different approaches to this challenge with some prioritizing the needs of the society in containing the virus and allocation of healthcare resources, and some patient autonomy in making informed choices. The majority of ART clinics worldwide are in a state of flux, clinics are currently at various stages between suspending ART treatments, preparing to or having to begun to resume ART services, alongside those clinics that have continued offering all patient services since the outset of the pandemic.
Perhaps one of the biggest challenges is the limited understanding of the current pathophysiology of COVID-19 and its impact on the reproductive system and pregnancy. ART clinics and the various reproductive medicine societies and organizations are well positioned to gather critical data to better understand the impacts that COVID-19 has on the reproductive system. The ASRM, ESHRE, IFFS joint statement includes a list of ongoing initiatives in this area (COVID-19 and Human Reproduction Joint Statement: ASRM, ESHRE, IFFS Citation2020). The COVID-19 pandemic has provoked a torrent of research and currently many studies are underway, including registries of COVID-19 infected pregnant women to address the maternal and neonatal outcome of exposed individuals.
During pregnancy, immunosuppression and other physiological changes can result in a higher susceptibility to respiratory pathogens and severe pneumonia in pregnant women (Jamieson et al. Citation2006). Insights regarding COVID-19 susceptibility in pregnant women and pregnancy complications may be gleamed from prior experiences with other respiratory virus epidemics (Monteleone et al. Citation2020). During the 2009 swine-origin influenza A (H1N1) epidemic, pregnant women were found to be at an increased risk of complications and were more likely to be hospitalized when compared to the general population (Jamieson et al. Citation2006). With respect to the other coronavirus epidemics (SARS and MERS) clinical outcomes during this epidemic were worse in pregnant women when compared to non-pregnant women, and higher rates of premature births and miscarriages were also reported (Monteleone et al. Citation2020; Schwartz and Graham Citation2020). Given the higher rates of complications in pregnant women for the aforementioned viruses (Schwartz and Graham Citation2020), it is not surprising that concerns have been raised for an increased risk of complications in pregnant women with COVID-19. At the present time, no unique risks of COVID-19 have emerged and the current maternal and fetal data published regarding COVID-19 infections in pregnancy women and maternal and fetal data in pregnancy offers limited reassurance. However, it is critical to note that the pandemic is ongoing and the current available data are limited. Thus, the findings reported are subject to change as more data is gathered and published. Initial studies suggested that the clinical course of patients with confirmed cases of COVID-19 is similar to non-pregnant women of similar age (Monteleone et al. Citation2020). However, recent data from the CDC suggests that COVID-19 positive pregnant women are more likely to be hospitalized and are at increased risk of being hospitalized or admitted to the ICU and receive mechanical ventilation compared with non-pregnant women of similar age, but the risk of death was similar between both groups (Ellington et al. Citation2020). The preterm delivery rate in pregnant women with COVID-19 infections appears to be similar to the general population (Monteleone et al. Citation2020). Informatively, no teratogenic effects or malformations have been reported in association with COVID-19 infections. However, the limited available data is primarily focused on third trimester pregnancies and to a lesser extent second trimester pregnancies, with a lack of data during the first trimester which is the critical period for fetal organ development. Currently, there is no reliable confirmation of vertical transmission of a COVID-19 infection from the mother to the fetus, with transmission proposed to be more likely following birth (Qiao Citation2020). Nevertheless, ACE2 has not been found to be expressed in the placenta providing a barrier for any transmission (Pique-Regi et al. Citation2020).
The available data is limited in the number of pregnant patients reported, the number of sites reporting, the adequacy of screening and testing and the available follow-up. As such, current guidance is incomplete. Undoubtedly, a clearer picture of specific risks (or reassurance) for maternal and fetal health is likely to emerge as this data becomes available over the next twelve to eighteen months.
Impact of COVID-19 on reproductive research
Large animal models have been used by physicians and scientists for millennia, dating back to second century Greek physician-philosopher, Galen (Zuidema and Sutovsky Citation2020). They are now gaining a foothold in modern biomedicine because of their suitability for genetic engineering, reasonable costs of maintenance and genetic similarity to humans that sets them apart from expensive non-human primate models and phylogenetically more distant laboratory rodent (Meyerholz et al. Citation2020). While sheep, goats and even donkeys have been used to mass produce antibodies, hormones and proteins for a long time, the domestic pig is increasingly becoming the model of choice. Transgenic pigs are being developed by reproductive biologists specializing in somatic cell nuclear transfer/cloning and genome modification with several porcine models created to study viral infections. Initially the main goal was to create virus resistant pigs for the swine industry but under the pressure of the ongoing pandemic, the focus is likely to shift toward developing porcine models for influenza research. At present, the domestic pig is indeed the only large animal model used for COVID-19 research, and it is restricted to examining transmission routes and responses to infection (Lakdawala and Menachery Citation2020). Although the pig does not seem permissive to SARS-CoV-2 infection (Shi et al. Citation2020), transgenic pigs expressing for example human ACE2 receptor could become a very important model, particularly if they display human like symptoms after forced infection. Such a COVID-19 prone pig could mimic already existing transgenic pigs sensitized to major porcine viruses such as PRRS (Prather et al. Citation2017) or swine influenza (Yan et al. Citation2014). Importantly, such transgenic pigs are now relatively easy and reasonably fast to generate, and given their anatomical and genetic similarity to humans, could be very important not only for virus transmission research, but for vaccine, drug and clinical procedure testing.
The adage that out of turmoil comes creativity will without a doubt hold true for the role of reproductive biologists in both COVID-19 and infertility research, both types of efforts being affected by ongoing pandemic. For understanding the genetic causes of infertility, pigs and cattle propagated by the means of artificial insemination (AI) are a valuable resource. With the advent of AI, one sire can be used to inseminate thousands of females and the pregnancy and birth rates are recorded. This resource has been made available to the genomics of fertility and biomarker communities as such research, simply impossible in humans.
COVID-19 and diagnostic tests
As the pandemic of COVID-19 continues to impact our health, it is crucial to understand the importance of the diagnostic tests and especially the implications raised by the interpretation of these tests for fertility patients. Since professional bodies have recommended reactivation of fertility services through the use of appropriate PPE and proper social distancing, the need for the appropriate interpretation of any diagnostic test used is highlighted. In other words, the knowledge of the diagnostic performance of each test used is essential for the fight against the pandemic.
There are basically two types of diagnostic tests for SARS-CoV-2, broadly classified as direct or indirect. The direct diagnostic test evolves the detection of the virus and viral load, while the indirect method relies on the early seroconversion or the seroconversion in an ongoing infection or in a previous infection, respectively. Both methods have advantages and disadvantages with the direct test displaying higher sensitivity and specificity values compared to the indirect method (Di et al. Citation2005). Apart from the technical implications including the variability of specimen collection, the sample itself (from the different parts of the respiratory track), the time of collection in regards to the time of disease, the direct method seems to be the most reliable test for the diagnosis of COVID-19. The CDC has recommended the collection of specimens from the upper respiratory track, and performing RT-PCR from specimens collected in nasopharyngeal swabs. The genes that have been targeted in RT-PCR tests encode the envelope, the nucleocapsid, the spike, the RNA-dependent RNA polymerase and the ORF1 genes. Nalla et al found that among seven different primer/probe sets the RNA-dependent RNA polymerase probe has the lowest sensitivity (Nalla et al. Citation2020). The viral RNA is measured by a Ct, where Ct values lower than 40 are defined as PCR positive and the severity of illness. Depending very much on the viral titer, early detection of the virus by RT-PCR is anticipated in a Ct less than 10 copies/reaction (Chu et al. Citation2020), showing a sensitivity of 95% (Corman et al. Citation2020). However, transportation for detection, even storage, may adversely impact a negative test result (He et al. Citation2020). A positive RT-PCR test does not always mean that the virus is infectious, but indicates that the virus’ genetic material is present. As the sensitivity of the test increases with the increasing severity of the infection, it is obvious that the decline in infectivity will be accompanied by an analogous increase in Ct values. Nevertheless, viral RNA has been detected in patients six weeks after the first positive RT-PCR test (Wölfel et al. Citation2020). This means that several PCR tests may produce false-positive results which may come from the cross-reactivity of the primers with DNA from other viruses that have co-infected the host during the course of the disease. Contamination of the laboratory by similar viruses is another issue that may generate false-positive results. On the contrary, false-negative results may come from the variability of the virus, or even by the mutations that may as the virus spreads worldwide.
Antibodies may be detected in nasopharyngeal swabs or even serum samples using the ELISA diagnostic assay, which identifies either IgM or IgG and IgA antibodies when the host has been previously exposed to SARS-CoV-2 (Okba et al. Citation2019). IgM antibodies are the first produced in response to the antigen as part of the early onset phase. One week later, IgG seems to be the most abundant antibody which lasts several days (perhaps up to a month) (To et al. Citation2020; Xiang et al. Citation2020), while IgA seems to play assistant role in the overall function of the immune system. Therefore, antibody testing does not inform about the infection status but of possible previous exposure.
The antibodies are generally produced in response to the viral nucleocapsid proteins. Concerns have been raised because the antibody tests may cross-react with similar proteins of other viruses. Therefore the sensitivity of the antibody tests is less than that of the RT-PCR tests. Positive detection rates for IgG and IgM of 85.4% and 93.1%, respectively (Guo et al. Citation2020) and serum detection rates for IgM and IgG of 48.1% and 88.9%, respectively have been recently reported (Jin et al. Citation2020). It seems that the predictive value of antibody tests is higher when it is measured in a population where there is widespread infection and is implemented as a tool to release people from the social isolation. Sensitivity and specificity increases when IgM and IgG ELISA antibody tests are both used. However, the detection of both antibodies does not always mean immunity against the SARS-CoV-2, while on the other hand negative antibody test does not rule out a COVID-19 infection (D’Cruz et al. Citation2020).
It is currently impossible to conclude which test is the most appropriate for use in the fertility service setting. The recommendations proposed by professional bodies for re-starting fertility services suggests that RT-PCR tests be performed at the onset of ovarian stimulation and prior to hCG with a patient management plan implemented based on the results. Even with the low possible false–negative result, in combination with the unknown incubation time constitutes a significant risk for the public and the healthcare workers. It has been suggested that the best choice is to presume that all fertility patients are infected or were previously infected (Bahadur et al. Citation2020). Laboratory prevention measures (Anifandis et al. Citation2020) in co-junction with PPE is the standard practice to overcome the unique challenges in the reproductive services during the pandemic.
COVID-19 and modern diagnostic tests: Application of NGS and SMS
The identification and monitoring of pathogens are being transformed by the incorporation of Next Generation Sequencing (NGS) and Single Molecule Sequencing (SMS) tools within the clinical setting (Lai et al. Citation1996; Cordeiro et al. Citation2017). They are now starting to be deployed directly in the field providing a host of technical solutions that do not require culture, an antibody or direct knowledge to detect, and/or identify the emerging microorganism (Buermans and den Dunnen Citation2014). The information garnered from their application encompasses both DNA and RNA. While DNA sequencing provides information on what is present in a sample and how it has evolved, RNA sequencing divulges information concerning which biological processes are active and affected in the sample (Wang et al. Citation2009).
NGS of pathogens was initially developed as a targeted approach similar to PCR based on what was known. The targeted approach excludes complexity by focusing on the pathogen of interest (Buermans and den Dunnen Citation2014). This permits the detection of sequence variants within the targeted region that can be used to monitor the evolution of the pathogen over-time. A 2019-Novel Coronavirus Real-Time RT-PCR assay is available for the diagnosis of COVID-19 (https://www.fda.gov/media/134922/download). This test is reliant on the amplification of the viral nucleocapsid gene and has been successfully used in combination with NGS genome sequencing of corresponding formalin fixed paraffin embedded (FFPE) tissue samples (Sekulic et al. Citation2020). NGS provides an ideal platform to assess synergistic and opportunistic mechanisms. For example, SARS-CoV2 in combination with herpes simplex virus type 1 and human herpesvirus type 6B virus in the tears and conjunctival secretions of a COVID-19 patient has been detected (CitationHu et al.).
NGS has also been employed in metagenomic and whole genome analysis, in which all DNA or RNA is sequenced from the same sample. The presence of pathogens is determined by directly comparing the sequences obtained from the subject samples to those from a ‘healthy’ control (Buermans and den Dunnen Citation2014). This approach is beneficial when the causative agent is unknown, or the genome is not fully sequenced. The metagenomic RNA sequencing approach was used to define the SARS-CoV2 genome (Chen et al. Citation2020c; Lu et al. Citation2020), and holds promise for the active surveillance of novel coronaviruses with epidemic potential in bats (Li et al. Citation2020a; Yadav et al. Citation2020). It has also been used for the evaluation of the host’s immune response upon infection (CitationWang et al.; Xiong et al. Citation2020).
SMS technologies quantitate each individual molecule independent of length through single-molecule sequencing. This provides a direct measurement of short or extremely long sequences of DNA and RNA (Ameur et al. Citation2019) without the need for amplification. In the absence of amplification, it is possible to limit the effects of GC-content bias which can obscure the results obtained using other technologies (Buermans and den Dunnen Citation2014). SMS is of sufficient sensitivity to identify viral co-infection, e.g. influenza virus, and coronavirus, in a single sample (Lewandowski et al. Citation2019) and has proven effective as a rapid viral diagnostic test (Xu et al. Citation2018; Burbelo et al. Citation2019). Sample multiplexing also enables a larger number of samples to be analyzed at a reduced cost, but care must be taken to minimize bar-code cross-over events so sequences are appropriately assigned to each original sample (Xu et al. Citation2018). Low-cost SMS has generally been associated with reduced sequence accuracy (< 85%) (Buermans and den Dunnen Citation2014), however, as algorithms improve, it is now approaching 99% (Eid et al. Citation2009; Eisenstein Citation2019).
SMS by nanopores has proven effective in sequencing the entire SARS-CoV2 genome (Viehweger et al. Citation2019) and has the capacity to identify gene variants and sites of methylation (Harel et al. Citation2019; Viehweger et al. Citation2019). Various bioinformatic approaches are now being developed to identify potential drug targets against SARS-CoV2 infection (Alakwaa Citation2020). Oxford Nanopore Loop-mediated isothermal amplification (LAMP), has also been developed (LamPORE) for the SMS platform. Simultaneously, viral RNA is linearly amplified, sequenced, aligned and analyzed. Once a sample reaches a specific SARS-CoV2 threshold, it is classified as COVID-19 positive. Depending on the analysis needs and platform 1 to 480 samples can be analyzed per hour (Burbelo et al. Citation2019). It is available in a single sample laptop or other small device formats that are suitable for both laboratory and clinical settings, e.g., the doctor’s office. This technology remains to be clinically certified as a COVID-19 diagnostic.
Assessing the impact of SARS-CoV2 on the reproductive tract and gamete, e.g., sperm, poses a few challenges. First, human sperm contains approximately 50 fg of long RNA (> 200 nts) and 0.3 fg of small RNAs (< 200 nts) per cell, some of which are imported from exosomes. This may limit the recovery of a foreign target (reviewed in Godia et al. Citation2018). Along with the above, mature RNA is fragmented (Sendler et al. Citation2013; Godia et al. Citation2018) appearing similar to that obtained from FFPE, tissue samples. To resolve these inherent issues, metagenomic amplification, and alignment of RNA using the RNA Element Discovery algorithm (REDa) was developed (Estill et al. Citation2019b). This approach was designed to consider exon sized sequences, i.e., RNA Elements (REs), by aligning all sequences to any genome. REDa has proven a powerful approach to assess the complexity of mature sperm (Estill et al. Citation2019a; Swanson et al. Citation2020a), and through the various stages of spermatogenesis (Estill et al. Citation2019b). Interestingly, microbiome sequences can be identified within human RNA-seq data (Strong et al. Citation2014; Uphoff et al. Citation2019; Swanson et al. Citation2020b). Thus it is possible to identify those individuals with a pathogen burden (Swanson et al. Citation2020b). In concert, this holistic approach of examining all of the transcripts within the sample (Swanson et al. Citation2020a; Swanson et al. Citation2020b) affords pathways to be constructed describing single exon-level changes in response to a stressor, e.g., SARS-CoV2 infection. This can provide a pathway to remediation. With the use of NGS and SMS approaches for pathogen evaluation, the identification of individuals infected with SARS-CoV2 is possible. By combining these techniques with algorithms like REDa, understanding how SARS-CoV2 interacts with the host’s system should facilitate personalized care.
COVID-19 and bioethical considerations
Very few milestone events have been documented to alter the course of history. These historical phenomena include wars, recessions, and pandemics (Huremović Citation2019). On one hand a pandemic outbreaks’ magnitude of effects have been reported to extend from decreasing the population to changing population dynamics, while causing breakdown of societal structures. On the other hand, they are also considered the driving force of innovations and advances in medicine in the midst of economic change (Scheidel Citation2018; Huremović Citation2019). A glance at the past could offer a glimpse of the future, therefore a comparison between the current COVID-19 pandemic and notorious pandemics throughout history could reveal ethical considerations and enhance physicians’ insight of the current context.
During the bubonic plague, the ‘Plague Doctor’ was a profession severely affected. A shortage of medical professionals was described, forcing local authorities to request the assistance of young doctors without expertise (Sehdev Citation2002). Along the same lines, health care workers are now in the frontline of the COVID-19 pandemic, with media reporting hundreds of lives lost in the line of duty. Young doctors are called to arms in war on COVID-19 to revive the health care system which experiences a tremendous crisis. Moving further down history lane, the Spanish flu pandemic constituted the first global crisis modern medicine encountered. Similarly to today, medical specialists such as epidemiologists and infectious disease experts examined aspects of the disease and attempted to decipher its identity (Huremović Citation2019). It is believed that the silencing of the press censored the true extent and impact of Spanish flu. Nations involved in war covered up the pandemic to ensure that the combatants’ morale would not be compromised (Martini et al. Citation2019). International politics have been influenced since emergence of COVID-19. This is inevitable as global affairs were subject to the influence of the pandemic with Governments being expected to respond and issue formal theses regarding COVID-19 and its’ management (Davies and Wenham Citation2020). Hence, politics still are and will continue to be at the heart of this issue (The Politics of the Coronavirus Outbreak | Think Global Health), as we have yet to experience the full impact of COVID-19. However, the pandemic of 2020 in contrast to the Spanish Flu and the Plague has an ally in the face of the World Health Organization. Further to that, a distinguishing line between COVID-19 and previous pandemics such as the Spanish flu are the massive diffusion of information that has been ensued through the internet. However, there is another side to the coin of the abundance of shared information. The perils of this global epidemic of misinformation threaten public health and as aptly described this ‘infodemic’ adds another level of complexity in the management of the COVID-19 crisis (Zarocostas Citation2020; Cinelli et al. Citation2020). If we look into what we could learn from our acquaintance with a most recent threat presented in the form of a virus over the past decades, we’ll find that our experience with HIV being an insidious disease that has eventually grown into a slowly progressing pandemic has some insight to offer (Huremović Citation2019; Challenges and Similarities in HIV, COVID-19 Crises). Initially, HIV was perceived as a disease that did not affect everybody, similarly to COVID-19 being described as a virus principally affecting older age people (Mueller et al. Citation2020). Hence initial responses from the medical and public authorities did not focus tothe adoption of a common strategy to face this emergency as the danger was considered initially to target principally a specific population. Public awareness was raised in regards to HIV to highlight that the homosexual population was at risk, stigmatizing these people and lifting a barrier to prevention, care and treatment (Chesney and Smith Citation1999). Similarly, it has been reported that patients diagnosed with SARS-CoV-2 may experience social ostracism and discrimination (Bagcchi Citation2020) that isolate them. Despite the plethora of information and evidence that we have accumulated over the years on the pathogenesis of HIV, its treatment still remains the holy grail of unaccomplished goals in the medical field. Could that also be the case for COVID-19? Researchers are focusing their strengths on how to eradicate the current pandemic nonetheless the scenario of the virus establishing itself forever may not be inconceivable at this point in time. What is more, a percentage of population still actively and worrisomely ignores the reality of COVID-19 consciously adopting the belief that “this is not a real threat, ‘it does not exist’, ‘it is a product of a conspiracy theory’. There are documented attitudes on conspiracy theories (Rieger and He-Ulbricht Citation2020) that negatively affect successful adoption of preventative measures and vaccination intentions (Bertin et al. Citation2020), while most importantly they may play a detrimental role in the future.
History showcases that the detrimental effect of pandemics on humanity can be terminated either with developing an efficient treatment or when the virus naturally drives itself to extinction. Based on the availability of resources, it appears that we may be stronger than in the past in fighting a global threat of this magnitude. Hence, we are hopeful that treating and eradicating COVID-19 will not present as challenging as previous pandemic crises have. Nonetheless, the unprecedented challenges and consequences instill fear and threaten to paralyze societal infrastructures as we are emphatically missing the full picture of this ongoing pandemic and the full extent of its impact, therefore it may be too soon to even draw conclusions. Yet, to offer some relief, this is not the first nor will it be the last global pandemic that the human kind will face. History has taught us that we will prevail. We should be wise and to make use of these history lessons and count them as our ally in this battle.
Countries issued directives for partial or total lockdown, while the distinction between essential and non-essential health care fueled a heated debate. One of the most impacted divisions in health care systems was reproductive medicine, raising bioethical concerns. These pertained to patients’ access to treatment, and the responsibility of the reproductive medicine practitioners toward encouraging pregnancy at a time of turmoil and uncertainty, while concurrently concerns were voiced with regard to the measures undertaken ascertaining safety.
Highly-esteemed Reproductive Societies namely the ESRHE and the ASRM recommended that all treatments be postponed cryopreserving embryos for future use, emphasizing the unknown impact this new virus may exert in the reproductive system and pregnancy. Discrepant recommendations followed and were anticipated as some countries issued limited treatments while others, namely the United Kingdom and Italy, prohibited all IVF treatments until the infection curve flattened and the risk of infection was mitigated (La Marca et al. Citation2020). Diverse views were expressed concerning the optimal strategy, taking into consideration the concerns regarding the financial consequences for ART centers (Alviggi et al. Citation2020). On another note, valid concerns surfaced that postponing treatments may interfere with the infertile patients’ right to access to treatment. Two points of view have been expressed and published on the matter of whether delays in treatment could lead to detrimental outcomes. On the one hand it was suggested that time-sensitive patients such as those with diminished ovarian reserve should be given treatment priority (Vaiarelli et al. Citation2020). On the contrary, retrospective data have indicated that a potential 6-month delay does not affect clinical outcome for such patients (Romanski et al. Citation2020).
With reduced infection risk and flattening of the infection curve, the IVF world had been invited to restart treatments. New recommendations were published by ESHRE and ASRM, and protocol modification to ensure the safety of clinical and laboratory personnel and patient, have been proposed (Anifandis et al. Citation2020; Arav Citation2020). The ART world awaits with bated breath on how safe these measures will prove to be, along with the safety of pregnancies using embryos originating in an IVF lab during COVID-19. From a bioethical perspective contradicting data are worrisome, as it makes clinical application more difficult. On one hand there is evidence to suggest that SARS-CoV-2 may be harmful for pregnant women (Breslin et al. Citation2020; Chen et al. Citation2020b; Mehan et al. Citation2020), and that IVF treatments should be limited and performed under strict protocols. On the other hand, there is now published data on the first and second trimester pregnancies indicating that no unusual issues are present (Liang and Acharya Citation2020).
During our time of crisis, the field of ART faces a great challenge as a non-essential healthcare provider. The consequences and numerous bioethical concerns should be weighted prior to concurring on how we move forward (Agarwal et al. Citation2020). In the meantime, whether resuming ART treatment was a hasty decision remains to be seen. IVF specialists are called to respond with commitment to the infertile couples requesting treatment, while at the same time securing optimized safe and efficient services for all involved. The task of providing a platform of protection the extends from staff, to patients, to embryos, to fetuses, to children born in the time of COVID-19, is a challenging one-to say the least-and cannot be circumvented as ART has a moral obligation to future children and future parents. The importance of confirming that patients understand their choices and options available, along with the risks involved is key, and the concept of shared-decision-making should be emphasized here (Michel Citation2003) uniting both the patient and physician while ensuring that risks are underlined and the practitioners’ ethical obligation to serve patients’ welfare is fulfilled. Abiding by the shared-decision-making process during COVID-19 is a necessity.
Evidence accumulates and the influx of proposals on how to best navigate safety issues regarding health care interventions is considerable (Anifandis et al. Citation2020; Stephens et al. Citation2020; Chen et al. Citation2020a), nonetheless, we still do not know whether it is sufficient, or when it will be sufficient and foolproof to the extent one would require. Proposals must be scrutinized and hypotheses tested. When evidence-based medicine should dictate practice, it may be challenging to provide medical services at the time of COVID-19 on the grounds that ART treatment along with socioeconomic aspects of life should ‘go on’. Nonetheless, as we navigate into what is still considered as ‘the unknown’ the driver of ART practice should be shaped by several components and these should all be considered from a bioethical perspective. Are data on safe practice robust and adequate? Does patient demand reflect the patients’ autonomy in claiming access to treatment to address infertility? Is the concern for the viability of the future of the IVF sector and the concern for the business aftermath filtered through bioethical principles such as beneficence and autonomy? We have yet to determine the way forward, and new influx of data on COVID-19 are expected to be crucial, until then we learn to adapt abiding by the basic principles of evolution and the aphorism ‘primum non nocere’, do no harm.
Conclusions
The novel virus SARS-CoV-2 causes COVID-19, which was declared by the World Health Organization (WHO) on 11th March 2020 to be a pandemic. Every country has adopted specific risk-reduction measures to flatten the curve of infected cases and to reduce the spread of the virus. At present the WHO assessment shows the viral curve is not flattening but continues to increase, placing the COVID-19 pandemic among the greatest in modern history. Apart from the organs known to be affected by this virus, the reproductive organs may also be susceptible to infection. Given that the ACE2 receptor is important for viral spread and is also found in the human testicle, attention has been given to the study of COVID-19, spermatogenesis and male fertility. Similarly, the study of the disparity in severe COVID-19 infections in males vs. females has already produced interesting research in the field of genetics, epigenetics and hormone metabolism. It has been also shown that males suffered a much higher mortality rate in comparison to women in part reflecting the genetic and epigenetic differences, originated likely in part by the respective sex chromosome constitution and other male-specific and female specifically expressed genes. Furthermore, work at fertility clinics was affected by the pandemic as services were temporarily halted. The impact of COVID-19 extended to research, as laboratories were mustered to understand the mechanisms and the pathways that this virus follows to exert its action throughout the human body. Diagnostic tests have been developed for examining present or post-infectivity but molecular and antibody tests, especially at fertility services, need to be improved. New NGS and SMS methodologies seem to improve the diagnostic reliability of COVID-19 infection. The COVID-19 pandemic has also brought to light several bioethical issues regarding the safe and effective delivery of reproductive healthcare (). And of course, as we are in the middle of the pandemic, we need to remain clinically and scientifically agile and adaptable, to continue the effectively fight the COVID-19 pandemic together as even with a vaccine on the horizon we are now in the second wave.
Figure 4. The impact of SARS-CoV-2. An overview of the current impact of COVID-19. As the viral curve is not flattening but continues to increase, COVID-19 raised a number of issues including the impact on reproductive research. Various diagnostic tests have been developed with NGS and SMS having the more diagnostic reliability in comparison to other molecular and antibody tests. Reproductive organs have been also affected by COVID-19, especially testis and ovaries raising the possibility that this virus may exert negative effects on reproduction and assisted reproduction. Nonetheless, COVID-19 brought to us bioethical issues as well, placing this pandemic, caused by this virus, as one of the biggest in the modern history
Authors’ contributions
Conceptualization: GA, SAK; writing—original draft preparation: GA, HGT, RO, GMS, MS, CAE, MP, CIM, PJT, PS, SJO, SAK; editing: GA, SAK. All authors have read and agreed to the version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Abobaker A, Raba AA. 2020. Does COVID-19 affect male fertility? World J Urol. doi:10.1007/s00345-020-03208-w.
- Agarwal S, Booth R, Madeira JL, Trolice M, Petrozza J, Lindheim SR. 2020. Core bioethical principles and the ethics of continued care during the COVID-19 war. Fertil Steril Dialog [Internet]. [accessed 2020 Jun 6]. http://www.fertstertdialog.com/users/16110-fertility-and-sterility/posts/66118-core-bioethical-principles-and-the-ethics-of-continued-care-during-the-covid-19-war.
- Alakwaa FM. 2020. Repurposing didanosine as a potential treatment for COVID-19 using single-cell RNA sequencing data. mSystems. 5(2):e00297–00220. eng. doi:10.1128/mSystems.00297-20.
- Albertini DF. 2020. Buffering reproductive science in the era of COVID-19. J Assist Reprod Gen. 37:1017. doi:10.1007/s10815-020-01824-4.
- Alchon SA. 2003. A pest in the land: new world epidemics in a global perspective. ALBUQUERQUE: University of New Mexico Press.
- Alviggi C, Esteves SC, Orvieto R, Conforti A, La Marca A, Fischer R, Andersen CY, Bühler K, Sunkara SK, Polyzos NP, et al. 2020. COVID-19 and assisted reproductive technology services: repercussions for patients and proposal for individualized clinical management. Reprod Biol Endocrinol. 18:45. doi:10.1186/s12958-020-00605-z.
- Ameur A, Kloosterman WP, Hestand MS. 2019. Single-molecule sequencing: towards clinical applications. Trends Biotechnol. 37(1):72–85. doi:10.1016/j.tibtech.2018.07.013.
- Anifandis G, Messini CI, Daponte A, Messinis IE 2020. COVID-19 and fertility: a virtual reality. Reprod Biomed Online [Internet]. [accessed 2020 Jun 25]. https://www.rbmojournal.com/article/S1472-6483(20)30263-7/abstract.
- Arav A. 2020. A recommendation for IVF lab practice in light of the current COVID-19 pandemic. J Assist Reprod Genet. 37(7):1543. doi:10.1007/s10815-020-01841-3.
- ASRM: Patient Management and Clinical Recommendations During the Coronavirus (COVID-19) Pandemic. 2020. [accessed 2020 Jun 14]. https://www.asrm.org/globalassets/asrm/asrm-content/news-and-publications/covid-19/covidtaskforce.pdf.
- Bagcchi S. 2020. Stigma during the COVID-19 pandemic. Lancet Infect Dis. 20(7):782. doi:10.1016/S1473-3099(20)30498-9.
- Bahadur G, Acharya S, Muneer A, Huirne J, Łukaszuk M, Doreski PA, Homburg R. 2020. SARS-CoV-2: diagnostic and design conundrums in the context of male factor infertility. Reprod Biomed Online. 41:S1472-6483(20)30313–8. doi:10.1016/j.rbmo.2020.05.014.
- Bertin P, Nera K, Delouvée S. 2020. Conspiracy beliefs, rejection of vaccination, and support for hydroxychloroquine: a conceptual replication-extension in the COVID-19 pandemic context. Front Psychol. 11. [accessed 2020 Oct 23]. doi:10.3389/fpsyg.2020.565128.
- Breslin N, Baptiste C, Miller R, Fuchs K, Goffman D, Gyamfi-Bannerman C, D’Alton M. 2020. Coronavirus disease 2019 in pregnancy: early lessons. Am J Obstet Gynecol MFM. 2(Supplement):100111. doi:10.1016/j.ajogmf.2020.100111.
- Buermans HPJ, den Dunnen JT. 2014. Next generation sequencing technology: advances and applications. Biochim Biophys Acta Mol Basis Dis. 1842(10):1932–1941. doi:10.1016/j.bbadis.2014.06.015.
- Burbelo PD, Iadarola MJ, Chaturvedi A. 2019. Emerging technologies for the detection of viral infections. Future Virol. 14(1):39–49. eng. doi:10.2217/fvl-2018-0145.
- Burton KA, McKnight GS. 2007. PKA, germ cells, and fertility. Physiology (Bethesda). 22:40–46. doi:10.1152/physiol.00034.2006.
- Castillo J, Jodar M, Oliva R. 2018. The contribution of human sperm proteins to the development and epigenome of the preimplantation embryo. Hum Reprod Update. 24(5):535–555. doi:10.1093/humupd/dmy017.
- Challenges and Similarities in HIV, COVID-19 Crises. AJMC [Internet]. [accessed 2020 Oct 22]. https://www.ajmc.com/view/challenges-and-similarities-in-hiv-covid19-crises-a-qa-with-anthony-fauci-md.
- Chalmel F, Lardenois A, Evrard B, Mathieu R, Feig C, Demougin P, Gattiker A, Schulze W, Jegou B, Kirchhoff C, et al. 2012. Global human tissue profiling and protein network analysis reveals distinct levels of transcriptional germline-specificity and identifies target genes for male infertility. Human Reprod. 27(11):3233–3248. doi:10.1093/humrep/des301.
- Chen D, Yang H, Cao Y, Cheng W, Duan T, Fan C, Fan S, Feng L, Gao Y, He F, et al. 2020a. Expert consensus for managing pregnant women and neonates born to mothers with suspected or confirmed novel coronavirus (COVID-19) infection. Int J Gynaecol Obstet Off Organ Int Fed Gynaecol Obstet. 149(2):130–136. doi:10.1002/ijgo.13146.
- Chen H, Guo J, Wang C, Luo F, Yu X, Zhang W, Li J, Zhao D, Xu D, Gong Q, et al. 2020b. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 395(10226):809–815. doi:10.1016/S0140-6736(20)30360-3.
- Chen L, Liu W, Zhang Q, Xu K, Ye G, Wu W, Sun Z, Liu F, Wu K, Zhong B, et al. 2020c. RNA based mNGS approach identifies a novel human coronavirus from two individual pneumonia cases in 2019 Wuhan outbreak. Emerg Microbes Infect. 9(1):313–319. eng. doi:10.1080/22221751.2020.1725399.
- Chesney MA, Smith AW. 1999. Critical delays in HIV testing and care: the potential role of stigma. Am Behav Sci. 42(7):1162–1174. doi:10.1177/00027649921954822.
- Chu DKW, Pan Y, Cheng SMS, Hui KPY, Krishnan P, Liu Y, Ng DYM, Wan CKC, Yang P, Wang Q, et al. 2020. Molecular diagnosis of a Novel Coronavirus (2019-nCoV) causing an outbreak of pneumonia. Clin Chem. 66(4):549–555. doi:10.1093/clinchem/hvaa029.
- Cinelli M, Quattrociocchi W, Galeazzi A, Valensise CM, Brugnoli E, Schmidt AL, Zola P, Zollo F, Scala A. 2020. The COVID-19 social media infodemic. Sci Rep. 10(1):16598. doi:10.1038/s41598-020-73510-5.
- Cordeiro CN, Bano R, Washington Cross CI, Segars JH. 2017. Zika virus and assisted reproduction. Curr Opin Obstet Gynecol. 29(3):175–179. doi:10.1097/GCO.0000000000000366.
- Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DK, Bleicker T, Brünink S, Schneider J, Schmidt ML, et al. 2020. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 25(3):2000045. doi:10.2807/1560-7917.ES.2020.25.3.2000045.
- COVID-19 and Human Reproduction Joint Statement: ASRM, ESHRE, IFFS. 2020. [accessed 2020 Jun 14]. https://www.iffsreproduction.org/news/510203/COVID-19-and-Human-Reproduction-Joint-Statement-ASRM-ESHRE-IFFS.htm.
- Crosby AW. 2003. America’s forgotten pandemic: the Influenza of 1918. 2nd ed. 32 Avenue of the Americas New York, NY 10013-2473, USA: Cambridge University Press.
- D’Cruz RJ, Currier AW, Sampson VB. 2020. Laboratory testing methods for novel severe acute respiratory syndrome-Coronavirus-2 (SARS-CoV-2). Front Cell Dev Biol. 8:468. doi:10.3389/fcell.2020.00468.
- Darde TA, Lecluze E, Lardenois A, Stévant I, Alary N, Tüttelmann F, Collin O, Nef S, Jégou B, Rolland AD, et al. 2019. The ReproGenomics Viewer: a multi-omics and cross-species resource compatible with single-cell studies for the reproductive science community. Bioinformatics. 35(17):3133–3139. doi:10.1093/bioinformatics/btz047.
- Davies SE, Wenham C. 2020. Why the COVID-19 response needs international relations. Int Aff. 96(5):1227–1251. doi:10.1093/ia/iiaa135.
- Debnath M, Banerjee M, Berk M. 2020. Genetic gateways to COVID-19 infection: implications for risk, severity, and outcomes. Faseb J. 34(7):8787–8795. Jun 11. doi:10.1096/fj.202001115R.
- Devlin DJ, Agrawal Zaneveld S, Nozawa K, Han X, Moye AR, Liang Q, Harnish JM, Matzuk MM, Chen R. 2020. Knockout of mouse receptor accessory protein 6 leads to sperm function and morphology defectsdagger. Biol Reprod. 102(6):1234–1247. doi:10.1093/biolre/ioaa024.
- Di B, Hao W, Gao Y, Wang M, Wang YD, Qiu LW, Wen K, Zhou DH, Wu XW, Lu EJ, et al. 2005. Monoclonal antibody-based antigen capture enzyme-linked immunosorbent assay reveals high sensitivity of the nucleocapsid protein in acute-phase sera of severe acute respiratory syndrome patients. Clin Diagn Lab Immunol. 12(1):135–140. doi:10.1128/CDLI.12.1.135-140.2005.
- Djureinovic D, Fagerberg L, Hallstrom B, Danielsson A, Lindskog C, Uhlen M, Ponten F. 2014. The human testis-specific proteome defined by transcriptomics and antibody-based profiling. Mol Hum Reprod. 20(6):476–488. doi:10.1093/molehr/gau018.
- Dorus S, Skerget S, Karr TL. 2012. Proteomic discovery of diverse immunity molecules in mammalian spermatozoa. Syst Biol Reprod Med. 58:218–228. doi:10.3109/19396368.2012.700442.
- Eid J, Fehr A, Gray J, Luong K, Lyle J, Otto G, Peluso P, Rank D, Baybayan P, Bettman B, et al. 2009. Real-time DNA sequencing from single polymerase molecules. Science. 323(5910):133–138. doi:10.1126/science.1162986.
- Eisenberg ML. 2020. Coronavirus disease 2019 and men’s reproductive health. Fertil Steril. 113(6):1154. Jun. doi:10.1016/j.fertnstert.2020.04.039.
- Eisenstein M. 2019. Playing a long game. Nat Methods. 16(8):683–686. doi:10.1038/s41592-019-0507-7.
- Ellinghaus D, Degenhardt F, Bujanda L, Buti M, Albillos A, Invernizzi P, Fernández J, Prati D, Baselli G, Asselta R, et al. 2020. Genomewide association study of severe Covid-19 with respiratory failure. N Engl J Med. NEJMoa2020283. Jun 17. doi:10.1056/NEJMoa2020283.
- Ellington S, Strid P, Tong VT, Woodworth K, Galang RR, Zambrano LD, Nahabedian J, Anderson K, Gilboa SM. 2020. Characteristics of women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status - United States, January 22-June 7, 2020. MMWR Morb Mortal Wkly Rep. 69:769–775. doi:10.15585/mmwr.mm6925a1.
- ESHRE News and Statements. 2020. https://www.eshre.eu/Press-Room/ESHRE-News
- Estill M, Hauser R, Nassan FL, Moss A, Krawetz SA. 2019a. The effects of di-butyl phthalate exposure from medications on human sperm RNA among men. Sci Rep. 9(1):12397. doi:10.1038/s41598-019-48441-5.
- Estill MS, Hauser R, Krawetz SA. 2019b. RNA element discovery from germ cell to blastocyst. Nucleic Acids Res. 47(5):2263–2275. doi:10.1093/nar/gky1223.
- Fei C, Jing Y, Run-Qian L, Ya-Bin L, Hao-Ran W. 2020. Reply: COVID-19 and human reproduction: hypothesis needs to be investigated. Mol Hum Reprod. 26(7):551–552. doi:10.1093/molehr/gaaa042.
- Francisco FO, Lemos B. 2014. How do Y-chromosomes modulate genome-wide epigenetic states: genome folding, chromatin sinks, and gene expression. J Genom. 2:94–103.20. doi:10.7150/jgen.8043.
- Fu J, Zhou B, Zhang L, Balaji KS, Wei C, Liu X, Chen H, Peng J, Fu J. 2020. Expressions and significances of the angiotensin-converting enzyme 2 gene, the receptor of SARS-CoV-2 for COVID-19. Mol Biol Rep. 47(6):4383–4392. doi:10.1007/s11033-020-05478-4.
- Gagnon A, Miller MS, Hallman SA, Bourbeau R, Herring DA, Earn DJ, Madrenas J, Digard P. 2013. Age-specific mortality during the 1918 influenza pandemic: unravelling the mystery of high young adult mortality. Plos One. 8(8). doi:10.1371/journal.pone.0069586.
- Godia M, Swanson G, Krawetz SA. 2018. A history of why fathers’ RNA matters. Biol Reprod. 99(1):147–159. doi:10.1093/biolre/ioy007.
- Gordon DE, Jang GM, Bouhaddou M, Xu J, Obernier K, White KM, O’Meara MJ, Rezelj VV, Guo JZ, Swaney DL, et al. 2020. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 583(7816):459–468. doi:10.1038/s41586-020-2286-9.
- Guo L, Ren L, Yang S, Xiao M, Chang D, Yang F, Dela Cruz CS, Wang Y, Wu C, Xiao Y, et al. 2020. Profiling early humoral response to diagnose Novel Coronavirus disease (COVID-19). Clin Infect Dis. 71(15):778–785. doi:10.1093/cid/ciaa310.
- Hadziselimovic F, Hadziselimovic NO, Demougin P, Krey G, Hoecht B, Oakeley EJ. 2009. EGR4 is a master gene responsible for fertility in cryptorchidism. Sex Dev. 3(5):253–263. eng. doi:10.1159/000249147.
- Hajra A, Bandyopadhyay D, Heise LR, Bhadra R, Ball S, Hajra SK. 2017. Zika and pregnancy: A comprehensive review. Am J Reprod Immunol. 77:e12607. doi:10.1111/aji.12607.
- Hannigan MM, Zagore LL, Licatalosi DD. 2017. Ptbp2 controls an alternative splicing network required for cell communication during spermatogenesis. Cell Rep. 19(12):2598–2612. doi:10.1016/j.celrep.2017.05.089.
- Harel N, Meir M, Gophna U, Stern A. 2019. Direct sequencing of RNA with MinION Nanopore: detecting mutations based on associations. Nucleic Acids Res. 47(22):e148–e148. eng. doi:10.1093/nar/gkz907.
- He X, Lau EHY, Wu P, Wang J, Hao X, Lau YC, Wong JY, Guan Y, Tan X, Mo X, et al. 2020. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 26(5):672–675. doi:10.1038/s41591-020-0869-5.
- Hikmet F, Méar L, Edvinsson Å, Micke P, Uhlén M, Lindskog C 2020. The protein expression profile of ACE2 in human tissues. Mol. Syst. Biol. 16: e9610. doi:10.15252/msb.20209610.
- Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, et al. 2020. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 181(2):271–280 e278. doi:10.1016/j.cell.2020.02.052.
- Hu Y, Chen T, Liu M, Zhang L, Wang F, Zhao S, Liu H, Xia H, Wang Y, Li L. 2020. Positive detection of SARS-CoV-2 combined HSV1 and HHV6B virus nucleic acid in tear and conjunctival secretions of a non-conjunctivitis COVID-19 patient with obstruction of common lacrimal duct. Acta Ophthalmol. 98(8):859–863
- Huremović D. 2019. Brief History of Pandemics (Pandemics Throughout History). Psychiatry Pandemics A Mental Health Response to Infection Outbreak. Springer. 7–35.
- IFFS: COVID-19 Task Force Statements. 2020. [accessed 2020 Jun 14]. https://www.iffsreproduction.org/page/COVIDStatements.
- Illiano E, Trama F, Costantini E. 2020. Could COVID-19 have an impact on male fertility? Andrologia. 52(6):e13654. doi:10.1111/and.13654.
- Jamieson DJ, Theiler RN, Rasmussen SA. 2006. Emerging infections and pregnancy. Emerg Infect Dis. 12:1638–1643. doi:10.3201/eid1211.060152.
- Jin Y, Wang M, Zuo Z, Fan C, Ye F, Cai Z, Wang Y, Cui H, Pan K, Xu A. 2020. Diagnostic value and dynamic variance of serum antibody in coronavirus disease 2019. Int J Infect Dis. 94:49–52. doi:10.1016/j.ijid.2020.03.065.
- Johns Hopkins COVID-19 Dashboard. 2020. [accessed 2020 Jun 14]. https://coronavirus.jhu.edu/map.html.
- Kachuri L, Francis SS, Morrison M, Bossé Y, Cavazos TB, Rashkin SR, Ziv E, Witte JS 2020. The landscape of host genetic factors involved in infection to common viruses and SARS-CoV-2. medRxiv. May. doi: 10.1101/2020.05.01.20088054.
- Klein SL, Flanagan KL. 2016. Sex differences in immune responses. Nat Rev Immunol. 16(10):626–638. doi:10.1038/nri.2016.90.
- Kloc M, Ghobrial RM, Kubiak JZ. 2020. The role of genetic sex and mitochondria in response to COVID-19 infection. Int Arch Allergy Immunol. 19:1–6. doi:10.1159/000508560.
- Krausz C, Giachini C, Xue Y, O’Bryan MK, Gromoll J, Rajpert-de Meyts E, Oliva R, Aknin-Seifer I, Erdei E, Jorgensen N, et al. 2009. Phenotypic variation within European carriers of the Y-chromosomal gr/gr deletion is independent of Y-chromosomal background. J Med Genet. 46(1):21–31. Jan. doi:10.1136/jmg.2008.059915.
- La Marca A, Niederberger C, Pellicer A, Nelson SM. 2020. COVID-19: lessons from the Italian reproductive medical experience. Fertil Steril. 113(5):920–922. doi:10.1016/j.fertnstert.2020.03.021.
- Lai YM, Yang F-P, Pao CC. 1996. Human papillomavirus deoxyribonucleic acid and ribonucleic acid in seminal plasma and sperm cells**Supported by research grant NSC83-0412-B182-001 from National Science Council of Republic of China and by medical research grant CMRP-343 from Chang Gung College of Medicine and Technology and Memorial Hospital, Taipei, Taiwan, Republic of China, both awarded to C.C.P. Fertil Steril. 65(5):1026–1030.
- Lakdawala SS, Menachery VD. 2020. The search for a COVID-19 animal model. Science. 368(6494):942–943. doi:10.1126/science.abc6141.
- Lardenois A, Gattiker A, Collin O, Chalmel F, Primig M. 2010. GermOnline 4.0 is a genomics gateway for germline development, meiosis and the mitotic cell cycle. Database (Oxford). 2010:baq030. eng. doi:10.1093/database/baq030.
- Lewandowski K, Xu Y, Pullan ST, Lumley SF, Foster D, Sanderson N, Vaughan A, Morgan M, Bright N, Kavanagh J, et al. 2019. Metagenomic nanopore sequencing of influenza virus direct from clinical respiratory samples. J Clin Microbiol. 58(1):e00963–00919. eng. doi:10.1128/JCM.00963-19.
- Li B, Si H-R, Zhu Y, Yang X-L, Anderson DE, Shi Z-L, Wang L-F, Zhou P. 2020a. Discovery of Bat Coronaviruses through Surveillance and Probe Capture-Based Next-Generation Sequencing. mSphere. 5(1):e00807–00819. eng. doi:10.1128/mSphere.00807-19.
- Li D, Jin M, Bao P, Zhao W, Zhang S. 2019. Clinical Characteristics and Results of Semen Tests Among Men With Coronavirus Disease. JAMA Netw Open. 3(5):e208292. doi:10.1001/jamanetworkopen.2020.8292.
- Li Q, Cao Z, Rahman P. 2020b Jun. Genetic variability of human angiotensin-converting enzyme 2 (hACE2) among various ethnic populations. Mol Genet Genomic Med. 18:e1344. doi:10.1002/mgg3.1344.
- Li Y, Xu Q, Ma L, Wu D, Gao J, Chen G, Li H. 2020c. Systematic profiling of ACE2 expression in diverse physiological and pathological conditions for COVID-19/SARS-CoV-2. J Cell Mol Med. doi:10.1111/jcmm.15607.
- Liang H, Acharya G. 2020. Novel corona virus disease (COVID-19) in pregnancy: what clinical recommendations to follow? Acta Obstet Gynecol Scand. 99(4):439–442. doi:10.1111/aogs.13836.
- Liu J, Ji H, Zheng W, Wu X, Zhu JJ, Arnold AP, Sandberg K. 2010. Sex differences in renal angiotensin converting enzyme 2 (ACE2) activity are 17β-oestradiol-dependent and sex chromosome-independent. Biol Sex Differ. 1(1):6. doi:10.1186/2042-6410-1-6.
- Lo Giacco D, Chianese C, Sánchez-Curbelo J, Bassas L, Ruiz P, Rajmil O, Sarquella J, Vives A, Ruiz-Castañé E, Oliva R, et al. 2014. Clinical relevance of Y-linked CNV screening in male infertility: new insights based on the 8-year experience of a diagnostic genetic laboratory. Eur J Hum Genet. 22(6):754–761. doi:10.1038/ejhg.2013.253.
- Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu N, et al. 2020. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 395(10224):565–574. eng. doi:10.1016/S0140-6736(20)30251-8.
- Ma L, Xie W, Li D, Shi L, Mao Y, Xiong Y, Zhang Y, Zhang M 2020. Effect of SARS-CoV-2 infection upon male gonadal function: A single center-based study. medRxiv.2020.2003.2021.20037267.
- Maan AA, Eales J, Akbarov A, Rowland J, Xu X, Jobling MA, Charchar FJ, Tomaszewski M. 2017. The Y chromosome: a blueprint for men’s health? Eur J Hum Genet. 25(11):1181–1188. doi:10.1038/ejhg.2017.128.
- Martini M, Gazzaniga V, Bragazzi NL, Barberis I. 2019. The Spanish Influenza Pandemic: a lesson from history 100 years after 1918. J Prev Med Hyg. 60(1):E64–E67. doi:10.15167/2421-4248/jpmh2019.60.1.1205.
- Mehan A, Venkatesh A, Girish M. 2020. COVID-19 in pregnancy: risk of adverse neonatal outcomes. J Med Virol. 92(11):2295–2297. doi:10.1002/jmv.25959.
- Meyerholz DK, Beck AP, Singh B. 2020. Innovative use of animal models to advance scientific research. Cell Tissue Res. 380(2):205–206. doi:10.1007/s00441-020-03210-z.
- Michel L. 2003. Ethical and philosophical foundations of the informed consent. Acta Chir Belg. 103(1):1–3. doi:10.1080/00015458.2003.11679356.
- Mizuno Y, Ninomiya Y, Nakachi Y, Iseki M, Iwasa H, Akita M, Tsukui T, Shimozawa N, Ito C, Toshimori K, et al. 2013. Tysnd1 deficiency in mice interferes with the peroxisomal localization of PTS2 enzymes, causing lipid metabolic abnormalities and male infertility. PLoS Genet. 9(2):e1003286. doi:10.1371/journal.pgen.1003286.
- Monteleone PA, Nakano M, Lazar V, Gomes AP, Martin H, Bonetti TC. 2020. A review of initial data on pregnancy during the COVID-19 outbreak: implications for assisted reproductive treatments. JBRA Assist Reprod. 24:219–225. doi:10.5935/1518-0557.20200030.
- Mueller AL, McNamara MS, Sinclair DA. 2020. Why does COVID-19 disproportionately affect older people? Aging. 12(10):9959–9981. doi:10.18632/aging.103344.
- Nalla AK, Casto AM, Huang MW, Perchetti GA, Sampoleo R, Shrestha L, Wei Y, Zhu H, Jerome KR, Greninger AL. 2020. Comparative performance of SARS-CoV-2 detection assays using seven different primer-probe sets and one assay kit. J Clin Microbiol. 58(6):e00557–20. doi:10.1128/JCM.00557-20.
- Ni FD, Hao SL, Yang WX. 2020. Molecular insights into hormone regulation via signaling pathways in Sertoli cells: with discussion on infertility and testicular tumor. Gene. 753:144812. doi:10.1016/j.gene.2020.144812.
- Okba NMA, Raj VS, Widjaja I, GeurtsvanKessel CH, de Bruin E, Chandler FD, Park WB, Kim NJ, Farag EABA, Al-Hajri M, et al. 2019. Sensitive and specific detection of low-level antibody responses in mild middle east respiratory syndrome coronavirus infections. Emerg Infect Dis. 25(10):1868–1877. doi:10.3201/eid2510.190051.
- Oliva R, Martínez-Heredia J, Estanyol JM. 2008. Proteomics in the study of the sperm cell composition, differentiation and function. Syst Biol Reprod Med. 54(1):23–36. doi:10.1080/19396360701879595.
- Ory SJ, Miller KA, Horton M, Giudice L. 2020. The global impact of COVID-19 on infertility services. Global Reprod Health. 5(2):e43-e43. In Press. doi:10.1097/GRH.0000000000000043.
- Pan F, Xiao X, Guo J, Song Y, Li H, Patel DP, Spivak AM, Alukal JP, Zhang X, Xiong C, et al. 2020. No evidence of severe acute respiratory syndrome-coronavirus 2 in semen of males recovering from coronavirus disease 2019. Fertil Steril. 113(6):1135–1139. doi:10.1016/j.fertnstert.2020.04.024.
- Pan PP, Zhan QT, Le F, Zheng YM, Jin F. 2013. Angiotensin-converting enzymes play a dominant role in fertility. Int J Mol Sci. 14(10):21071–21086. doi:10.3390/ijms141021071.
- Paracchini S, Stuppia L, Gatta V, Palka G, Moro E, Foresta C, Mengual L, Ballesca JL, Oliva R, Kremer JAM, et al. 2000. Y-chromosomal DNA haplotypes in infertile European males carriyng Y-microdeletions. J Endocrinol Invest. 23:671–676. doi:10.1007/BF03343792.
- Peng Q, Peng R, Yuan B, Zhao J, Wang M, Wang X, Wang Q, Sun Y, Fan Z, Qi J, et al. 2020. Structural and biochemical characterization of the nsp12-nsp7-nsp8 core polymerase complex from SARS-CoV-2. Cell Rep. 31(11):107774. doi:10.1016/j.celrep.2020.107774.
- Pique-Regi R, Romero R, Tarca AL, Luca F, Xu Y, Alazizi A, Leng Y, Hsu CD, Gomez-Lopez N. 2020. Does the human placenta express the canonical cell entry mediators for SARS-Co V–2? Pique-Regi R, Romero R, Tarca AL, Luca F, Xu Y, Alazizi A, Leng Y, Hsu CD, Gomez-Lopez N. 2020. Does the human placenta express the canonical cell entry mediators for SARS-CoV–2? Elife. 9:e58716. Published 2020 Jul 14. doi:10.7554/eLife.58716
- Prather RS, Wells KD, Whitworth KM, Kerrigan MA, Samuel MS, Mileham A, Popescu LN, Rowland RRR. 2017. Knockout of maternal CD163 protects fetuses from infection with porcine reproductive and respiratory syndrome virus (PRRSV). Sci Rep. 7(1):13371. Published 2017 Oct 17. doi:10.1038/s41598-017-13794-2.
- Qiao J. 2020. What are the risks of COVID-19 infection in pregnant women? Lancet. 395(10226):760–762. doi:10.1016/S0140-6736(20)30365-2.
- Rieger MO, He-Ulbricht Y. 2020. German and Chinese dataset on attitudes regarding COVID-19 policies, perception of the crisis, and belief in conspiracy theories. Data Brief. 33:106384. doi:10.1016/j.dib.2020.106384.
- Romanski PA, Bortoletto P, Rosenwaks Z, Schattman GL. Delay in IVF treatment up to 180 days does not affect pregnancy outcomes in women with diminished ovarian reserve. Hum Reprod [Internet]. [accessed 2020 Jun 23]. https://academic.oup.com/humrep/advancearticle/doi/10.1093/humrep/deaa137/5858161.
- Satarker S, Nampoothiri M. 2020. Structural proteins in severe acute respiratory syndrome Coronavirus-2. Arch Med Res. 51(6):482–491. doi:10.1016/j.arcmed.2020.05.012.
- Scheidel W. 2018. The great leveler: violence and the history of inequality from the stone age to the twenty-first century. Princeton and Oxford: Princeton University Press.
- Schwartz DA, Graham AL. 2020. Potential maternal and infant outcomes from (Wuhan) Coronavirus 2019-nCoV infecting pregnant women: lessons from SARS, MERS, and other human Coronavirus infections. Viruses. 12:194. doi:10.3390/v12020194.
- Scully EP, Haverfield J, Ursin RL, Tannenbaum C, Klein SL. 2020. Considering how biological sex impacts immune responses and COVID-19 outcomes. Nat Rev Immunol. 11:1–6. doi:10.1038/s41577-020-0348-8.
- Sehdev PS. 2002. The origin of quarantine. Clin Infect Dis Off Publ Infect Dis Soc Am. 35(9):1071–1072. doi:10.1086/344062.
- Sekulic M, Harper H, Nezami BG, Shen DL, Sekulic SP, Koeth AT, Harding CV, Gilmore H, Sadri N. 2020. Molecular detection of SARS-CoV-2 infection in FFPE samples and histopathologic findings in fatal SARS-CoV-2 cases. Am J Clin Pathol. 154(2):190–200. doi:10.1093/ajcp/aqaa091.
- Sendler E, Johnson GD, Mao S, Goodrich RJ, Diamond MP, Hauser R, Krawetz SA. 2013. Stability, delivery and functions of human sperm RNAs at fertilization. Nucleic Acids Res. 41(7):4104–4117. eng. doi:10.1093/nar/gkt132.
- Shen C, Yu J, Zhang X, Liu CC, Guo YS, Zhu JW, Zhang K, Yu Y, Gao TT, Yang SM, et al. 2019. Strawberry Notch 1 (SBNO1) promotes proliferation of spermatogonial stem cells via the noncanonical Wnt pathway in mice. Asian J Androl. 21(4):345–350. doi:10.4103/aja.aja_65_18.
- Shi CS, Qi HY, Boularan C, Huang NN, Abu-Asab M, Shelhamer JH, Kehrl JH. 2014. SARS-coronavirus open reading frame-9b suppresses innate immunity by targeting mitochondria and the MAVS/TRAF3/TRAF6 signalosome. J Immunol. 193(6):3080–3089. doi:10.4049/jimmunol.1303196.
- Shi J, Wen Z, Zhong G, Yang H, Wang C, Huang B, Liu R, He X, Shuai L, Sun Z, et al. 2020. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS-coronavirus 2. Science. 368(6494):1016–1020. doi:10.1126/science.abb7015.
- Shu T, Huang M, Wu D, Ren Y, Zhang X, Han Y, Mu J, Wang R, Qiu Y, Zhang DY, et al. 2020. SARS-Coronavirus-2 Nsp13 Possesses NTPase and RNA Helicase Activities That Can Be Inhibited by Bismuth Salts. Virol Sin. 35(3):321–329. doi:10.1007/s12250-020-00242-1.
- Stephens AJ, Barton JR, Bentum N-A-A, Blackwell SC, Sibai BM. 2020. General Guidelines in the Management of an Obstetrical Patient on the Labor and Delivery Unit during the COVID-19 Pandemic. Am J Perinatol. 37(8): 829–836
- Strong MJ, Xu G, Morici L, Splinter Bon-Durant S, Baddoo M, Lin Z, Fewell C, Taylor CM, Flemington EK. 2014. Microbial contamination in next generation sequencing: implications for sequence-based analysis of clinical samples. PLoS Pathog. 10(11):e1004437. doi:10.1371/journal.ppat.1004437.
- Swanson GM, Estill M, Diamond MP, Legro RS, Coutifaris C, Barnhart KT, Huang H, Hansen KR, Trussell JC, Coward RM, et al. 2020a. Human chromatin remodeler cofactor, RNA interactor, eraser and writer sperm RNAs responding to obesity. Epigenetics. 15(1–2):32–46. doi:10.1080/15592294.2019.1644880.
- Swanson GM, Moskovtsev S, Librach C, Pilsner JR, Goodrich R, Krawetz SA. 2020b. What human sperm RNA-Seq tells us about the microbiome. J Assist Reprod Genet. 37(2):359–368. doi:10.1007/s10815-019-01672-x.
- Takahashi T, Wong P, Ellingson M, Lucas C, Klein J, Israelow B, Silva J, Oh J, Mao T, Tokuyama M, et al. 2020. Sex differences in immune responses to SARS-CoV-2 that underlie disease outcomes. medRxiv. Jun 9. doi:10.1101/2020.06.06.20123414.
- The Politics of the Coronavirus Outbreak | Think Global Health. Counc Foreign Relat. [accessed 2020 Oct 23]. https://www.thinkglobalhealth.org/article/politics-coronavirus-outbreak.
- To KK, Tsang OT, Leung WS, Tam AR, Wu TC, Lung DC, Yip CC, Cai JP, Chan JM, Chik TS, et al. 2020. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 20(5):565–574. doi:10.1016/S1473-3099(20)30196-1.
- Uhlen M, Fagerberg L, Hallstrom BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson A, Kampf C, Sjostedt E, Asplund A, et al. 2015. Proteomics. Tissue-based map of the human proteome. Science. 347(6220):1260419. doi:10.1126/science.1260419.
- Uphoff CC, Pommerenke C, Denkmann SA, Drexler HG. 2019. Screening human cell lines for viral infections applying RNA-Seq data analysis. PloS One. 14(1):e0210404–e0210404. eng. doi:10.1371/journal.pone.0210404.
- Vaiarelli A, Bulletti C, Cimadomo D, Borini A, Alviggi C, Ajossa S, Anserini P, Gennarelli G, Guido M, Levi-Setti PE, et al. 2020. COVID-19 and ART: the view of the italian society of fertility and sterility and reproductive medicine. Reprod Biomed Online. 40:755–759. doi:10.1016/j.rbmo.2020.04.003.
- Vemuri R, Sylvia KE, Klein SL, Forster SC, Plebanski M, Eri R, Flanagan KL. 2019. The microgenderome revealed: sex differences in bidirectional interactions between the microbiota, hormones, immunity and disease susceptibility. Semin Immunopathol. 41(2):265–275. doi:10.1007/s00281-018-0716-7.
- Verma S, Saksena S, Sadri-Ardekani H. 2020. ACE2 receptor expression in testes: implications in COVID-19 pathogenesis. Biol Reprod. 103(3):ioaa080. May 19. doi:10.1093/biolre/ioaa080.
- Viehweger A, Krautwurst S, Lamkiewicz K, Madhugiri R, Ziebuhr J, Hölzer M, Marz M. 2019. Direct RNA nanopore sequencing of full-length coronavirus genomes provides novel insights into structural variants and enables modification analysis. Genome Res. 29(9):1545–1554. eng. doi:10.1101/gr.247064.118.
- Walker L, Levine H, Jucker M. 2006. Koch’s postulates and infectious proteins. Acta Neuropathol. 112(1):1–4. PMID 16703338. doi:10.1007/s00401-006-0072-x.
- Wambier CG, Goren A, Vaño-Galván S, Ramos PM, Ossimetha A, Nau G, Herrera S, McCoy J. 2020. Androgen sensitivity gateway to COVID −19 disease severity. Drug Dev Res. 81(7):771–776. May 15. doi:10.1002/ddr.21688.
- Wan Y, Shang J, Graham R, Baric RS, Li F. 2020. Receptor Recognition by the Novel Coronavirus from Wuhan: an Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. J Virol. 94(7):e00127–20. Mar 17. doi:10.1128/JVI.00127-20.
- Wang M, Liu X, Chang G, Chen Y, An G, Yan L, Gao S, Xu Y, Cui Y, Dong J, et al. 2018. Single-cell RNA sequencing analysis reveals sequential cell fate transition during human spermatogenesis. Cell Stem Cell. 23(4):599–614.e4. doi:10.1016/j.stem.2018.08.007.
- Wang W, Zhang W, Zhang J, He J, Zhu F Distribution of HLA allele frequencies in 82 Chinese individuals with coronavirus disease-2019 (COVID-19). HLA
- Wang Z, Gerstein M, Snyder M. 2009. RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet. 10(1):57–63. doi:10.1038/nrg2484.
- Wang Z, Xu X. 2020. scRNA-seq profiling of human testes reveals the presence of the ACE2 receptor, a target for SARS-CoV-2 infection in spermatogonia, leydig and sertoli cells. Cells. 9(4):920. doi:10.3390/cells9040920.
- Wein AN, McMaster SR, Takamura S, Dunbar PR, Cartwright EK, Hayward SL, McManus DT, Shimaoka T, Ueha S, Tsukui T, et al. 2019. CXCR6 regulates localization of tissue-resident memory CD8 T cells to the airways. J Exp Med. 216(12):2748–2762. doi:10.1084/jem.20181308.
- WHO Virtual Press Conference on COVID-19. 2020. [accessed 2020 Jun 14]. https://www.who.int/docs/default-source/coronaviruse/transcripts/who-audio-emergencies-coronavirus-press-conference-full-and-final-11mar2020.pdf?sfvrsn=cb432bb3_2.
- Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, Niemeyer D, Jones TC, Vollmar P, Rothe C, et al. 2020. Virological assessment of hospitalized patients with COVID-2019. Nature. 581(7809):465–469. doi:10.1038/s41586-020-2196-x.
- Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, Graham BS, McLellan JS. 2020. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 367(6483):1260–1263. doi:10.1126/science.abb2507.
- Wu Y, Guo W, Liu H, Qi B, Liang K, Xu H, Peng Z, Xiao SY. 2020. Clinical outcomes of 402 patients with COVID-2019 from a single center in Wuhan, China. J Med Virol. 92(11):2751–2757. Jun 12. doi:10.1002/jmv.26168.
- Xiang F, Wang X, He X, Peng Z, Yang B, Zhang J, Zhou Q, Ye H, Ma Y, Li H, et al. 2020. Antibody detection and dynamic characteristics in patients with COVID-19. Clin Infect Dis. 71(8):ciaa461. doi:10.1093/cid/ciaa461.
- Xiong Y, Liu Y, Cao L, Wang D, Guo M, Jiang A, Guo D, Hu W, Yang J, Tang Z, et al. 2020. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerg Microbes Infect. 9(1):761–770. eng. doi:10.1080/22221751.2020.1747363.
- Xu J, Qi L, Chi X, Yang J, Wei X, Gong E, Peh S, Gu J. 2006. Orchitis: a complication of severe acute respiratory syndrome (SARS). Biol Reprod. 74(2):410–416. doi:10.1095/biolreprod.105.044776.
- Xu Y, Lewandowski K, Lumley S, Pullan S, Vipond R, Carroll M, Foster D, Matthews PC, Peto T, Crook D. 2018. Detection of viral pathogens with multiplex nanopore MinION sequencing: be careful with cross-talk. Front Microbiol. 9:2225–2225.eng. doi:10.3389/fmicb.2018.02225.
- Yadav P, Shete-Aich A, Nyayanit D, Pardeshi P, Majumdar T, Balasubramanian R, Ullas P, Mohandas S, Dighe H, Sawant P, et al. 2020. Detection of coronaviruses in species of bats from different States of India [Original Article]. Indian J Med Res. 151(2):226–235.
- Yan Q, Yang H, Yang D, Zhao B, Ouyang Z, Liu Z, Fan N, Ouyang H, Gu W, Lai L. 2014. Production of transgenic pigs over-expressing the antiviral gene Mx1. Cell Regen (Lond). 3(1):11. Published 2014 Sep 26. doi:10.1186/2045-9769-3-11.
- Yang M, Chen S, Huang B, Zhong JM, Su H, Chen YJ, Cao Q, Ma L, He J, Li XF, et al. 2020. Pathological Findings in the Testes of COVID-19 Patients: clinical Implications. Eur Urol Focus. S2405-4569(20)30144–9. May 31. doi:10.1016/j.euf.2020.05.009.
- Ye Q, Wang B, Mao J. 2020. The pathogenesis and treatment of the `Cytokine Storm’ in COVID-19. J Infect. 80(6):607–613. doi:10.1016/j.jinf.2020.03.037.
- Yoshida S. 2018. Open niche regulation of mouse spermatogenic stem cells. Dev Growth Differ. 60(9):542–552. doi:10.1111/dgd.12574.
- Zagore LL, Grabinski SE, Sweet TJ, Hannigan MM, Sramkoski RM, Li Q, Licatalosi DD. 2015. RNA binding protein Ptbp2 is essential for male germ cell development. Mol Cell Biol. 35(23):4030–4042. doi:10.1128/MCB.00676-15.
- Zarocostas J. 2020. How to fight an infodemic. Lancet. 395(10225):676. doi:10.1016/S0140-6736(20)30461-X.
- Zhou P, Yang XL, Wang X-G, Hu B, Zhang L, Zhang W, Si H-R, Zhu Y, Li B, Huang C-L, et al. 2020. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 579(7798):270–273. doi:10.1038/s41586-020-2012-7.
- Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, et al. 2020. China Novel Coronavirus investigating and research team. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 382(8):727–733. doi:10.1056/NEJMoa2001017.
- Zuidema D, Sutovsky P. 2020. The domestic pig as a model for the study of mitochondrial inheritance. Cell Tissue Res. 380(2):263–271. doi:10.1007/s00441-019-03100-z.
