ABSTRACT
This study aimed to investigate the expression of Fos proto-oncogene, AP-1 transcription factor subunit (c-Fos) in the genital tubercle (GT) of rats with di(2-ethylhexyl) phthalate (DEHP)-induced hypospadias and in the prepuce of patients with hypospadias compared with unaffected controls. Pregnant rats were given 750 mg/kg/day DEHP orally from gestational days 12–19. Western blotting showed that c-Fos expression was increased in DEHP-induced hypospadiac male offspring. In addition, 30 prepuce tissue specimens obtained during hypospadias repair surgery were divided into 2 groups: the mild hypospadias group (n = 15) and the severe hypospadias group (n = 15). Fifteen normal prepuce tissue specimens were harvested during elective circumcision as normal controls. Real-time quantitative polymerase chain reaction, western blotting and immunohistochemistry analyses were used to assess c-Fos expression. c-Fos protein levels were higher in the GT of DEHP-induced rats than in that of control rats. c-Fos mRNA and protein levels were also higher in the hypospadias groups than in the control group (p < 0.05, p < 0.001), and c-Fos protein levels were significantly higher in the severe hypospadias group than in the mild hypospadias group (p < 0.01). The expression of c-Fos was increased in both the GT of DEHP-induced hypospadiac rats and the prepuce of hypospadias patients. Thus, c-Fos overexpression might contribute to hypospadias.
Abbreviations: DEHP: di(2-ethylhexyl) phthalate; c-Fos: Fos proto-oncogene, AP-1 transcription factor subunit; Mafb: the masculinization-regulatory gene v-maf musculoaponeurotic fibrosarcoma oncogene family, protein B; GT: genital tubercle; ED: embryonic day; AGD: anogenital distance; AGI: anogenital distance index; ED: embryonic day.
KEYWORDS:
Introduction
Hypospadias, defined as a defect in ventral foreskin development along with ectopic displacement of the urethral meatus, is a common congenital malformation in neonates (Cunha et al. Citation2015). It occurs in up to 1 per 125 live male births, and the incidence continues to rise (van der Horst and de Wall Citation2017). The severity of hypospadias can vary from mild to severe according to the location of the urethral opening. Patients with severe hypospadias are at risk of complications leading to lifelong difficulties with urination, sexual dysfunction, and psychological problems, and surgery is the main treatment option (Snodgrass et al. Citation2011). Although the etiology of hypospadias remains obscure, it is thought to be multifactorial, involving factors such as genetic predisposition, inadequate hormone levels, and environmental elements (Thorup et al. Citation2014; van der Horst and de Wall Citation2017).
Di(2-ethylhexyl) phthalate (DEHP), a common endocrine disruptor, is the dominant plasticizer used globally for polyvinyl chloride (PVC) production. The widespread use of plastic products leads to broad exposure in daily life. DEHP has been shown to induce a wide range of reproductive toxicities and developmental disorders, such as hypospadias, cryptorchidism and sperm and fertility issues (Foster Citation2006; Wang et al. Citation2015; Khasin et al. Citation2020). However, the potential mechanism of DEHP-induced hypospadias remains unknown.
Disrupted gene expression has been proposed to be related to the occurrence of hypospadias; however, few related genes have been reported. According to gene expression array analyses, Fos proto-oncogene, AP-1 transcription factor subunit (FOS) and FosB proto-oncogene, AP-1 transcription factor subunit (FOSB) are upregulated in the context of hypospadias, but further studies have not been conducted (Karabulut et al. Citation2013). In addition, the masculinization-regulatory gene v-maf musculoaponeurotic fibrosarcoma oncogene family, protein B (Mafb), which has been identified as an androgen-responsive target gene that regulates male-type external genitalia formation in mice, has attracted our attention (Suzuki et al. Citation2014). Our previous study found 13 proteins related to Mafb through coimmunoprecipitation (co-IP) combined with mass spectrometry; among these proteins, FOS was the most closely related according to protein score (Supplementry Figure1).
The proto-oncogene c-Fos (Piechaczyk and Blanchard Citation1994) is involved in the regulation of numerous genes that control growth and differentiation in many tissues (Barrett et al. Citation2017). c-Fos functions largely to promote growth, cell survival, oncogenesis, tumor invasion, and metastasis (Zhang et al. Citation2007). The c-Fos protein regulates gene expression as part of a dimeric AP-1 complex, typically with a member of the c-Jun family of transcription factors. Of note, c-jun N-terminal kinase (JNK) 1/2 has been reported to be upregulated in patients with hypospadias and thus might play a role in the development of external male genitalia defects (Li et al. Citation2013). The evidence described above suggests that c-Fos might play a role in the etiology of hypospadias. Thus, we sought to verify the changes in c-Fos in hypospadiac patients.
Results and discussion
Role of c-fos in DEHP-treated rats
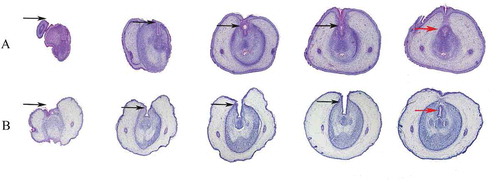
DEHP, an endocrine disruptor, has attracted attention regarding the onset of hypospadias. In this study, DEHP was used to generate the hypospadiac rodent model. The statistical information of fetal pups, of which the body weight and anogenital distance were measured completely, is shown in the . The incidence of hypospadias was significantly higher among DEHP-treated male fetuses (42%) than among control fetuses (). In addition, the anogenital distance (AGD) and anogenital index (AGI) were significantly lower in the DEHP group than in the control group (), which were consistent with prior work that showed reduced AGD in male infants were associated with increasing urinary concentrations of DEHP metabolites in prenatal maternity (Swan et al. Citation2015). Other workers have shown that AGD could be a stable anatomical landmark, which reflected the action of androgen in utero(Kita et al. Citation2016; Dorman et al. Citation2018). Genital tubercles (GTs) can be observed under scanning electron microscopy (SEM) to monitor the development of external genitalia. In the control group, the penile shaft and glans penis were divided by the coronary sulcus and clearly differentiated. The midline urethral groove was obvious, and the lateral preputial fold completely covered the corpus cavernosum ( & C). However, in the DEHP group, the skin folds did not merge completely in the middle, indicating disordered penile foreskin development ( & D). Examination of serial histological sections of GTs revealed DEHP-induced hypospadias, characterized by delayed preputial development and abnormalities in urethral seam closure (). In the DEHP group, the urethral plate remained from proximal to distal (). However, in the control group, the tubular urethra formed in the proximal region (). These morphological observations verified that DEHP can induce hypospadias in rats with disordered preputial development and delayed urethral plate tubularization.
Table 1. The effect of DEHP on hypospadias induction in male rat fetuses at ED19
Figure 1. Gross SEM images of genital tubercles (GTs) at GD19. In the control group (A & C), a midline groove was clearly observed in external genitalia on ED19. In addition, the penile shaft and glans penis were divided by the coronary sulcus and clearly differentiated. However, in the DEHP group (B&D), the skin folds did not completely cover the corpus cavernosum, which indicates disordered penile foreskin development. PF: preputial fold; UG: urethral groove; ED: embryonic day
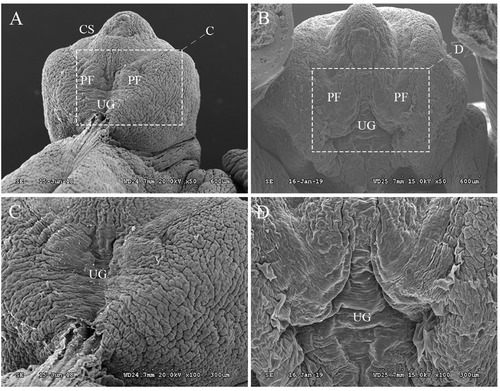
Figure 2. Serial histological sections of rat embryonic external genitalia at ED19. Histological analysis of embryonic external genitalia at ED19 by H&E staining. The orientation is from the distal end (left) to the proximal aspect of the GT (right). Black arrow, urethral plate; red arrow, tubular urethra. Notably, the GT of a male rat treated with 750 mg/kg/d DEHP (hypospadias group) showed delays in preputial development and abnormal closure of the urethral seam (B), which differed significantly from the findings in a control male rat (A). (original magnification, ×40)
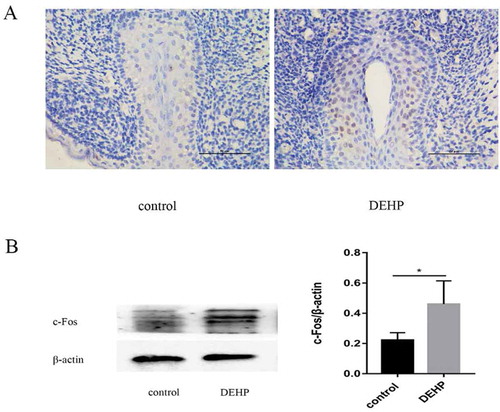
The urethral plate, an early developmental epithelium fused at the midline of the GT from the proximal region, is essential for urethral formation. In our experiments, c-Fos expression was significantly higher in the DEHP-treated GTs than in the control GTs. In the DEHP-treated group, c-Fos exhibited more widespread and stronger expression in the urethral epithelium, especially in the urethra plate region (). Western blot analysis showed a significant increase in c-fos expression in the DEHP group compared to the control group ().
Figure 3. Immunoreactivity (x400) and western blotting analysis of male GTs in the control and DEHP groups. Image of anti-c-Fos antibody staining (brown) indicating c-Fos expression in the urethral epithelium of the male GT, especially in the urethra plate region at ED19 (A). c-Fos staining was stronger in the DEHP group than in the control group. Western blot analysis showed a significant difference in c-fos expression in the GT between the control and DEHP groups (p < 0.05)
It is generally accepted that male external genital development is a complex process involving hormone signaling, cell proliferation and differentiation and tissue remodeling that follows an orderly progression in a time- and concentration-dependent manner (Utsch et al. Citation2004). The balance between androgens and estrogens seems to be essential for genital development (Matsushita et al. Citation2018). Both estrogen receptors (ERs) and androgen receptors (ARs) have been detected in differentiating male external genitalia, and colocalization of these receptors in the GT has been identified as a requirement for urethral closure (Crescioli et al. Citation2003). In addition, a recent study reported that either too much estrogen during early urethra formation or too little estrogen during delamination events results in hypospadias (Govers et al. Citation2019). Synthetic estrogen exposure during pregnancy can induce hypospadias in model mice (Kim et al. Citation2004). This evidence suggests that estrogen signaling plays a critical role in penile development.
Interestingly, c-Fos, a member of the AP-1 family, is induced by 17β-estradiol through nongenomic and genomic pathways, and the nongenomic pathway may contribute to the activation of ER/AP-1 (Safe and Kim Citation2008). The AP-1 superfamily comprises dimeric transcription factors including activating transcription factors (ATFs), Jun family members, Fos family members, and MAF transcription factors. Some AP-1 members, including ATF3 and Mafb, have been reported to be associated with hypospadias (Gurbuz et al. Citation2010; Suzuki et al. Citation2014). However, the role of c-Fos in the onset of hypospadias has not been verified. This study is the first to show that c-Fos was increased in the penile tubercles of DEHP-induced hypospadiac offspring. DEHP is a widespread plasticizer with estrogen-like activity. Although the effect of estrogen on c-fos in relation to hypospadias risk is not yet known, some hypotheses can be suggested.
Previous research has revealed that c-Fos can be stimulated by 17β-estradiol in estrogen-responsive human and mouse cells (Weisz and Rosales Citation1990; Hyder et al. Citation1991). In the male mouse reproductive tract, c-Fos was reported to be increased in the prostatic urethra by genistein, an estrogen agonist, via ERs (Strauss et al. Citation1998). These findings suggest a complex interrelation between estrogen signaling and c-fos transcription that could be investigated in the context of urethral development in future studies. In addition, epithelial cell proliferation and apoptosis play roles in urethral seam fusion (Zhou et al. Citation2017). Excessive proliferation and reduced apoptosis of urethral plate fibroblasts (UPFs) have been found in finasteride-treated hypospadiac rats(An et al. Citation2018) . Notably, c-Fos was reported to be critical for certain inducers of apoptosis (Zhang et al. Citation2007). Increased apoptosis in GTs after high-dose estrogen treatment has been identified in vitro(Ma et al. Citation2009). FOS is also involved in controlling of apoptosis–survival balance during limb morphogenesis(Suda et al. Citation2014) . Hence, the effects of c-Fos upregulation on cell proliferation and apoptosis during genital development may be worth exploring.
Role of c-fos in the human prepuce
In this study, human prepuces were collected to verify c-Fos expression. Of the 15 prepuces with mild hypospadias, 4 had a glandular meatus (26.7%), 5 a coronal or subcoronal meatus (33.3%), and 6 a midshaft meatus (40%). Of the 15 subjects with severe hypospadias, 7 had a penoscrotal meatus (46.7%), and 8 had a scrotal meatus (53.3%).
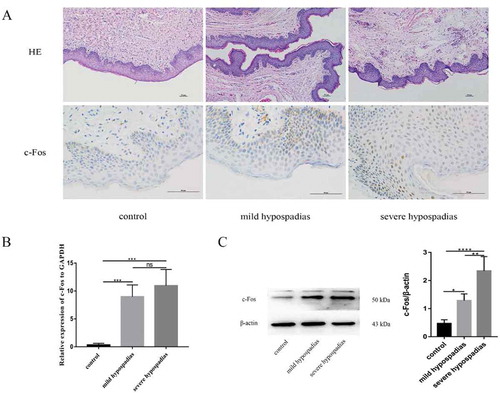
The expression of c-Fos mRNA was significantly different between the control and hypospadias groups (p < 0.05) (). Immunohistochemical analysis revealed that c-Fos was expressed in preputial tissue; c-Fos expression was observed in both dermal and epidermal cells in human preputial genital skin, especially in the basal layer. In certain patients with hypospadias, the staining was mostly nuclear, and the staining intensity appeared to be stronger in patients with more severe hypospadias than in those with milder hypospadias (). Western blotting analysis revealed that c-Fos protein levels differed significantly between the hypospadias and control groups (p < 0.05, p < 0.0001). Additionally, c-Fos expression was significantly different between the mild and severe hypospadias groups (p < 0.01) (), suggesting that c-Fos expression levels are related to the occurrence of hypospadias.
Figure 4. H&E staining (x100), immunoreactivity (x400), real-time PCR (RT-PCR) and western blot analysis of c-Fos in hypospadiac and control tissues. c-Fos expression was observed in both dermal and epidermal cells in human preputial genital skin. In patients with mild or severe hypospadias, the staining was mostly nuclear (A). c-Fos mRNA expression was significantly higher in subjects with hypospadias than in controls (p < 0.01, p < 0.01). However, no such difference was found between subjects with mild and severe hypospadias (B). c-Fos protein levels were significantly different between the control and hypospadias groups (p < 0.05, p < 0.001) and between the mild hypospadias group and the severe group (p < 0.01), suggesting a severity-dependent increase (C)
These results of our study verified that c-Fos is upregulated in patients with hypospadias, which is consistent with the previous finding by microarray analysis that among twin pairs, Fos was upregulated more than 2-fold in boys with hypospadias compared with controls (Wang et al. Citation2007) . Higher c-fos expression and lower ERβ expression have been found in the testes of men with obstructive azoospermia compared with men with nonobstructive azoospermia (Araujo et al. Citation2009). In the context of breast cancer, c-Fos is a fundamental factor for ERα-mediated transcription and enhances cell proliferation (Dahlman-Wright et al. Citation2012). In addition, ERs have been determined to be associated with hypospadias in humans (Ban et al. Citation2008). Although the effect of estrogen on c-fos in relation to hypospadias risk is not yet known, this potential relationship should be explored.
Overall, our present study provides new insight into the association between c-Fos and hypospadias. The upregulation of c-Fos was identified in both the prepuce of patients with hypospadias and the GT of DEHP-treated rats. However, the small study population could somewhat limit our conclusions. Additional investigations of in vitro c-Fos expression and in vivo estrogen or ER regulation in the fetal GT and further examinations of cell proliferation and apoptosis are required to supplement the data presented herein.
Material and methods
Animals
All experiments in this study were approved by the Ethics Committee of Chongqing Medical University. In this study, twenty pregnant Sprague-Dawley rats were randomly divided into 2 groups, the oil control group (n = 10) and the DEHP exposure group (n = 10), which were administered oil or 750 mg/kg DEHP, respectively, by gavage from gestational day (GD) 12 to 19. The day on which the vaginal plug was found in the morning was considered embryonic day (ED) 0. All the animals were obtained from the Experimental Animal Center of Chongqing Medical University and housed at the Experimental Animal Center of the Children’s Hospital of Chongqing Medical University in an air-conditioned facility at an ambient temperature of 17–25°C with 50%-80% relative humidity. All the animals received standard rodent feed and water ad libitum and were maintained on a 12 h light–dark cycle. After the male fetuses were harvested, a dissection microscope (Nikon SMZ1500, Japan) furnished with an ocular micrometer was used to measure the AGD.
Clinical data
Preputial tissue specimens were collected from 30 male patients with hypospadias aged 1–11 years (mean, 4 years) during surgical repair and 15 boys aged 5–7 years (mean, 6 years) during elective circumcision at the Children’s Hospital of Chongqing Medical University within the same period. All the excess preputial tissue specimens were obtained with informed consent from the parents. Children who exhibited known endocrine abnormalities or undescended testes or who had undergone primary hypospadias surgery were excluded from the study. The position of the ectopic urethral meatus determines the degree of hypospadias, which is classified as mild (meatus at or distal to the midshaft) and severe (meatus proximal to the midshaft and scrotum or perineum).
Scanning electron microscopy
The GT samples were observed under a scanning electron microscope (Hitachi, S-3000 N, Japan) after being washed with normal saline, fixed with glutaraldehyde, dehydrated, and gold-coated.
Real-time polymerase chain reaction
Total RNA was isolated using the TRIzol method (Invitrogen) according to the manufacturer’s instructions. The 260/280 nm ratio was 1.92 (range, 1.80 to 2.0). Subsequently, total mRNA was transcribed into cDNA using reverse transcription kits (Takara Biotechnology). Reverse transcriptase polymerase chain reaction (PCR) was performed using a QuantiNovaTM SYBR Green PCR Kit (Qiagen) according to the standard protocol. The PCR conditions included predenaturation at 95°C for 2 min, 30 cycles of 95°C for 5 s and 59°C for 10 s, and final extension at 72°C for 5 min. Primers were designed with Primer 5.0 software according to the sequence obtained on PubMed or downloaded from PrimerBank. The PCR primers used in this study were as follows: c-Fos, ccggggatagcctctcttact (forward), ccaggtccgtgcagaagtc (reverse); and GAPDH, gtcaaggctgagaacgggaa (forward), aaatgagccccagccttctc (reverse). PCR product specificity was confirmed by the identification of a single peak in each dissociation curve.
Histology and immunohistochemistry
The foreskin tissue and male GT samples were fixed in 4% paraformaldehyde for 48 h, dehydrated, embedded in paraffin wax, and sectioned (4 µm) before being baked on slides in an oven at 37°C for 24 h. For histological examinations, GTs were subjected to hematoxylin and eosin (H&E) staining. For immunohistochemistry, a citrate solution was used for antigen retrieval after deparaffinization and rehydration of the sections. Subsequently, the slides were incubated sequentially with 0.2% Triton X-100, 3% hydrogen peroxide, and horse serum. Then, the slides were incubated with c-Fos antibody (1:200 dilution, ab208942) at 4°C overnight. On the following day, the slides were incubated with goat anti-mouse secondary antibody for 1 h at 25°C. The signals on tissue slides were visualized with a DAB color reagent kit (Zhongshan, China) under a microscope, followed by hematoxylin counterstaining.
Western blot analysis
Total protein was extracted from GT and foreskin tissue samples and quantified. The GT and foreskin tissue samples were homogenized in RIPA lysis buffer containing PMSF and centrifuged at 12,000 × g for 25 min at 4°C. After collecting the protein lysate (supernatant), a BCA assay reagent (Bioteke, China) was used to measure the protein concentration. For western blotting, 20 µg of whole lysate was resolved by SDS-PAGE (BioRad), and the separated proteins were subsequently transferred onto PVDF membranes. The membranes were blocked with 5% nonfat milk in Tris-buffered saline with 0.05% Tween-20 (TBST) containing 5% bovine serum albumin for 1 h and then incubated overnight at 4°C with primary antibodies against c-Fos (1:1000 dilution, ab208942) and β-actin (1:500 dilution, Zhongshan) in TBST. On the following day, the blots were washed and incubated with peroxidase-conjugated goat anti-mouse IgG secondary antibodies (1:5000 dilution, Zhongshan) for 1 h. Then, the blots were washed 4 times with TBST. Immunolabeling was detected using an electrochemiluminescence detection kit (Bioteke). Relative c-Fos expression levels were qualified as the ratio between c-Fos levels and those of the housekeeping gene β-actin using ImageJ software.
Statistical analysis
All data are expressed as the mean ± standard deviation. ANOVA followed by Dunnett’s multiple comparison test was used to compare significant differences among multiple groups using GraphPad Prism. The criterion for statistical significance was set at p < 0.05.
Compliance with ethical standards
The studies involving both humans and animals were conducted in accordance with the ethical standards of the Declaration of Helsinki and were approved by the Ethics Committee of Chongqing Medical University (File No. 2016–124). Informed consent was obtained from all individual participants or their parents.
Authors’ contributions
Contributed to study conception and design: XL, HX; performed the experiments: HX, SW, XYK, YHY; analyzed the data: HX; contributed materials and facilities: LJS, LCL, GHW; drafted the manuscript: HX, XL. All authors approved any revisions and the final paper.
Supplemental Material
Download Zip (521.6 KB)Disclosure statement
There is no potential conflict of interest noted on the cover page, and there are no financial conflicts of interest related to products in this study for any author.
Supplemental data
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- An N, Peng J, He G, Fan X, Li F, Chen H. 2018. Involvement of activation of Mitogen-Activated Protein Kinase (MAPK)/Extracellular Signal-Regulated Kinase (ERK) signaling pathway in proliferation of urethral plate fibroblasts in finasteride-induced rat hypospadias. Med Sci Monit. 24:8984–8992. eng. doi:10.12659/MSM.911271.
- Araujo FC, Oliveira CA, Reis AB, Del Puerto HL, Martins AS, Reis FM. 2009. Expression of the proto-oncogene c-fos and the immunolocalization of c-fos, phosphorylated c-fos and estrogen receptor beta in the human testis. Histol Histopathol. 24(12):1515–1522. doi:10.14670/HH-24.1515.
- Ban S, Sata F, Kurahashi N, Kasai S, Moriya K, Kakizaki H, Nonomura K, Kishi R. 2008. Genetic polymorphisms of ESR1 and ESR2 that may influence estrogen activity and the risk of hypospadias. Hum Reprod (Oxford, England). 23(6):1466–1471. eng. doi:10.1093/humrep/den098.
- Barrett CS, Millena AC, Khan SA. 2017. TGF-beta effects on prostate cancer cell migration and invasion require FosB. Prostate. 77(1):72–81. eng. doi:10.1002/pros.23250.
- Crescioli C, Maggi M, Vannelli GB, Ferruzzi P, Granchi S, Mancina R, Muratori M, Forti G, Serio M, Luconi M. 2003. Expression of functional estrogen receptors in human fetal male external genitalia. J Clin Endocrinol Metab. 88(4):1815–1824. eng. doi:10.1210/jc.2002-021085.
- Cunha GR, Sinclair A, Risbridger G, Hutson J, Baskin LS. 2015. Current understanding of hypospadias: relevance of animal models. Nat Rev Urol. 12(5):271–280.
- Dahlman-Wright K, Qiao Y, Jonsson P, Gustafsson JA, Williams C, Zhao C. 2012. Interplay between AP-1 and estrogen receptor alpha in regulating gene expression and proliferation networks in breast cancer cells. Carcinogenesis. 33(9):1684–1691. eng. doi:10.1093/carcin/bgs223.
- Dorman DC, Chiu W, Hales BF, Hauser R, Johnson KJ, Mantus E, Martel S, Robinson KA, Rooney AA, Rudel R. et al. 2018. Systematic reviews and meta-analyses of human and animal evidence of prenatal diethylhexyl phthalate exposure and changes in male anogenital distance. J Toxicol Environ Health B Crit Rev. 214:207–226. eng. doi:10.1080/10937404.2018.1505354.
- Foster PM. 2006. Disruption of reproductive development in male rat offspring following in utero exposure to phthalate esters. Int J Androl. 29(1):140–147; discussion 181–145. doi:10.1111/j.1365-2605.2005.00563.x.
- Govers LC, Phillips TR, Mattiske DM, Rashoo N, Black JR, Sinclair A, Baskin LS, Risbridger GP, Pask AJ. 2019. A critical role for estrogen signaling in penis development. Faseb J. 33:fj201802586RR. doi:10.1096/fj.201802586RR.
- Gurbuz C, Demir S, Zemheri E, Canat L, Kilic M, Caskurlu T. 2010. Is activating transcription factor 3 up-regulated in patients with hypospadias? Korean J Urol. 51(8):561–564. doi:10.4111/kju.2010.51.8.561.
- Hyder SM, Stancel GM, Loose-Mitchell DS. 1991. Presence of an estradiol response region in the mouse c-fos oncogene. Steroids. 56(10):498–504. eng. doi:10.1016/0039-128X(91)90114-B.
- Karabulut R, Turkyilmaz Z, Sonmez K, Kumas G, Ergun S, Ergun M, Basaklar A. 2013. Twenty-four genes are upregulated in patients with hypospadias. Balkan J Med Genet. 16(2):39–44. eng. doi:10.2478/bjmg-2013-0030.
- Khasin LG, Della Rosa J, Petersen N, Moeller J, Kriegsfeld LJ, Lishko PV. 2020. The impact of Di-2-Ethylhexyl phthalate on sperm fertility. Front Cell Dev Biol. 8:426. eng. doi:10.3389/fcell.2020.00426.
- Kim KS, Torres CR Jr., Yucel S, Raimondo K, Cunha GR, Baskin LS. 2004. Induction of hypospadias in a murine model by maternal exposure to synthetic estrogens. Environ Res. 94(3):267–275. eng. doi:10.1016/S0013-9351(03)00085-9.
- Kita DH, Meyer KB, Venturelli AC, Adams R, Machado DL, Morais RN, Swan SH, Gennings C, Martino-Andrade AJ. 2016. Manipulation of pre and postnatal androgen environments and anogenital distance in rats. Toxicology. 368–369:152–161. eng. doi:10.1016/j.tox.2016.08.021.
- Li M, Qiu L, Lin T, He D, Hua Y, Yuan X, Liu X, Wei G. 2013. c-Jun N-terminal kinase is upregulated in patients with hypospadias. Urology. 81(1):178–183. eng. doi:10.1016/j.urology.2012.09.010.
- Ma LM, Wang Z, Wang H, Li RS, Zhou J, Liu BC, Baskin LS. 2009. Estrogen effects on fetal penile and urethral development in organotypic mouse genital tubercle culture. J Urol. 182(5):2511–2517. eng. doi:10.1016/j.juro.2009.07.008.
- Matsushita S, Suzuki K, Murashima A, Kajioka D, Acebedo AR, Miyagawa S, Haraguchi R, Ogino Y, Yamada G. 2018. Regulation of masculinization: androgen signalling for external genitalia development. Nat Rev Urol. 15(6):358–368. doi:10.1038/s41585-018-0008-y.
- Piechaczyk M, Blanchard JM. 1994. c-fos proto-oncogene regulation and function. Crit Rev Oncol Hematol. 17(2):93–131. eng. doi:10.1016/1040-8428(94)90021-3.
- Safe S, Kim K. 2008. Non-classical genomic estrogen receptor (ER)/specificity protein and ER/activating protein-1 signaling pathways. J Mol Endocrinol. 41(5):263–275. eng. doi:10.1677/JME-08-0103.
- Snodgrass W, Macedo A, Hoebeke P, Mouriquand PD. 2011. Hypospadias dilemmas: a round table. J Pediatr Urol. 7(2):145–157. eng. doi:10.1016/j.jpurol.2010.11.009.
- Strauss L, Mäkelä S, Joshi S, Huhtaniemi I, Santti R. 1998. Genistein exerts estrogen-like effects in male mouse reproductive tract. Mol Cell Endocrinol. 144(1-2):83–93. eng. doi:10.1016/s0303-7207(98)00152-x.
- Suda N, Itoh T, Nakato R, Shirakawa D, Bando M, Katou Y, Kataoka K, Shirahige K, Tickle C, Tanaka M. 2014. Dimeric combinations of MafB, cFos and cJun control the apoptosis-survival balance in limb morphogenesis. Development. 141(14):2885–2894. eng. doi:10.1242/dev.099150.
- Suzuki K, Numata T, Suzuki H, Raga DD, Ipulan LA, Yokoyama C, Matsushita S, Hamada M, Nakagata N, Nishinakamura R, et al. 2014. Sexually dimorphic expression of Mafb regulates masculinization of the embryonic urethral formation. Proc Natl Acad Sci USA. 111(46):16407–16412. doi:10.1073/pnas.1413273111.
- Swan SH, Sathyanarayana S, Barrett ES, Janssen S, Liu F, Nguyen RH, Redmon JB. 2015. First trimester phthalate exposure and anogenital distance in newborns. Hum Reprod (Oxford, England). 30(4):963–972. eng. doi:10.1093/humrep/deu363.
- Thorup J, Nordenskjold A, Hutson JM. 2014. Genetic and environmental origins of hypospadias. Curr Opin Endocrinol Diabetes Obes. 21(3):227–232. doi:10.1097/MED.0000000000000063.
- Utsch B, Albers N, Ludwig M. 2004. Genetic and molecular aspects of hypospadias. Eur J Pediatr Surg [Et Al] = Zeitschrift Fur Kinderchirurgie. 14(5):297–302. eng. doi:10.1055/s-2004-821275.
- van der Horst HJ, de Wall LL. 2017. Hypospadias, all there is to know. Eur J Pediatr. 176(4):435–441. doi:10.1007/s00431-017-2864-5.
- Wang Y, Liu W, Yang Q, Yu M, Zhang Z. 2015. Di (2-ethylhexyl) phthalate exposure during pregnancy disturbs temporal sex determination regulation in mice offspring. Toxicology. 336:10–16. doi:10.1016/j.tox.2015.07.009.
- Wang Z, Liu BC, Lin GT, Lin CS, Lue TF, Willingham E, Baskin LS. 2007. Up-regulation of estrogen responsive genes in hypospadias: microarray analysis. J Urol. 177(5):1939–1946. eng. doi:10.1016/j.juro.2007.01.014.
- Weisz A, Rosales R. 1990. Identification of an estrogen response element upstream of the human c-fos gene that binds the estrogen receptor and the AP-1 transcription factor. Nucleic Acids Res. 18(17):5097–5106. eng. doi:10.1093/nar/18.17.5097.
- Zhang X, Zhang L, Yang H, Huang X, Otu H, Libermann TA, DeWolf WC, Khosravi-Far R, Olumi AF. 2007. c-Fos as a proapoptotic agent in TRAIL-induced apoptosis in prostate cancer cells. Cancer Res. 67(19):9425–9434. eng. doi:10.1158/0008-5472.CAN-07-1310.
- Zhou Y, Liu X, Huang F, Liu Y, Cao X, Shen L, Long C, He D, Lin T, Wei G. 2017. Epithelial-mesenchymal transformation and apoptosis in rat urethra development. Pediatr Res. 82(6):1073–1079. eng. doi:10.1038/pr.2017.185.
