ABSTRACT
We investigated the feasibility of agarose-gel microcapsules to cryopreserve extremely small numbers of sperm for assisted reproductive technology. Semen samples were collected from 16 patients attending the center for reproductive medicine male infertility clinic at a university hospital. We used agarose microcapsules to cryopreserve extremely small numbers of sperm from 16 patients with male infertility (10 with sperm concentration ≥1 million/mL; 6 with sperm concentration <1 million/mL). Six spermatozoa were injected into agarose-gel microcapsules and cryopreserved in a liquid nitrogen tank for 7 days. The Crytop method was used for cryopreservation as a control. After thawing, spermatozoa were recovered. Sperm recovery rates, motility and viability, and recovery time were compared.
The post-thawing recovery rate, motility rate, and viability rate were higher whereas the recovery time was shorter in samples preserved using the agarose-gel microcapsule method compared to samples preserved using the Cryotop method in both the group with sperm concentrations of 1 million/mL or above and the group with sperm concentrations of less than 1 million/mL. This study demonstrated that using the agarose-gel microcapsule method increased post-thawing sperm recovery rate, sperm motility rate, and sperm viability rate, and reduced sperm recovery time compared with the conventional Cryotop method when cryopreserving samples with low sperm count. Although requiring further study, the agarose-gel microcapsule method shows much promise as a new option for freezing sperm.
Introduction
Oligozoospermia is a common condition in patients with male infertility. Some patients have such extremely low sperm counts that only a limited number of sperm can be collected after the centrifugation of the semen. In patients with severe conditions, sperm is commonly cryopreserved prior to the day intracytoplasmic sperm injection (ICSI) is performed to avoid the risk of canceling the procedure due to an insufficient amount of collected sperm on the day.
If the total sperm count is as low as a few sperm, a significant amount of time may be required to find clinically viable sperm among the many that become damaged through the post-thawing centrifugation process or among other contaminants. It is not rare to find no viable sperm despite the long time spent attempting to recover them (Said et al. Citation2010).
We hypothesized that the smaller the space where spermatozoa were cryopreserved, the lower the chance that recovery would fail after thawing.
In such cases, it is useful to have containers that can store individual spermatozoa in units of several cells and prevent them from being dispersed. To date, many different types of cryopreservation containers for sperm have been developed (AbdelHafez et al. Citation2009). Recently, Liu and Li (Citation2020) published a review of cryopreservation of single-sperm, and they described the agarose gel microcapsule method developed by Araki et al. (Citation2015) and the Cryotop method reported by Endo et al. (Citation2011) with illustrations. In Japan, many institutions that treat male factor infertile couples use the Cryotop method. However, in the daily clinical setting, we sometimes experience the loss of frozen sperm and cumbersome searching of cryopreserved sperm.
We used spermatozoa from two distinct patient populations. One consisted of six patients with extremely low sperm concentration whose live sperm recovery rate was considered low, and the other consisted of 10 patients with low to normal sperm concentration whose live sperm recovery rate was considered high.
In this study, we investigated whether sperm cryopreservation capsules made from agarose gels were effective for the cryopreservation of an extremely low number of sperm collected from the aforementioned two distinct patient populations in comparison to using the Cryotop method which was used as a standard procedure in Japan. Sperm recovery rate appeared higher and sperm recovery time was shorted using agarose gel microcapsules.
Results
Age and seminograms of patients
A total of 16 patients were enrolled in this study. The age and seminograms of patients are listed in . Of these 16 subjects, 10 had sperm concentrations of 1 million/mL and 6 had sperm concentrations of less than 1 million/mL.
Table 1. Age and Seminograms of patients
Five sets of six spermatozoa obtained from each patient’s semen sample were cryopreserved using the agarose gel capsule method and Cryotop method as summarized in . After thawing sperm recovery rate, sperm motility rate, sperm viability rate, and sperm recovery time were compared and the data are summarized in .
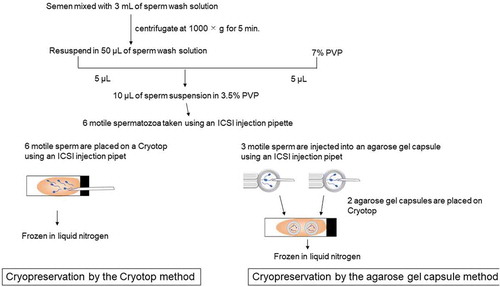
Figure 1. Freezing procedures using Cryotop method and agarose gel capsule method. Motile sperm suspension was prepared with washed sperm and 7% PVP. Six motile spermatozoa were aspirated and micro-injected into the droplet placed on Cryotop. It was frozen in liquid nitrogen (Cryotop method). Motile sperm suspension was prepared with washed sperm and 7% PVP. Six motile spermatozoa were aspirated and micro-injected into two agarose gel capsules (three sperm for each capsule), and capsules were placed on Cryotop. Then, it was frozen in liquid nitrogen (Agarose gel capsule method). PVP: polyvinylpyrrolidone
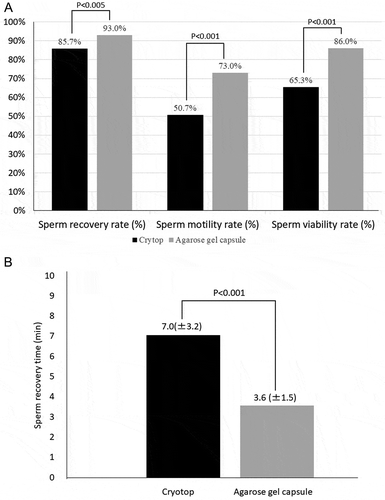
Investigation of samples with sperm concentration 1 million/mL or above
As illustrated in and shown in and illustrated in, the sperm recovery rate: 93% (279/300) for the agarose gel capsule method was significantly higher than the 86% (257/300) for the Cryotop method (P < 0.005). Sperm motility rate: 73% (219/300) for the agarose gel capsule method was significantly higher than the 51% (152/300) for the Cryotop method (P < 0.001). Sperm viability rate: 86% (258/300) for the agarose gel capsule method was significantly higher than the 65% (196/300) for the Cryotop method (P < 0.001). Sperm recovery time (mean ± SD): 3.56 ± 1.5 min for the agarose gel capsule method was significantly shorter than the 7.04 ± 3.2 min for the Cryotop method (P < 0.001).
Table 2. Comparison of agarose gel method and Cryotop method in samples with sperm concentration 1 million/mL or above
Figure 2. Comparison between the agarose gel capsule method and Cryotop method in samples with sperm concentration of 1 million/mL or above. A). Sperm recovery rate: 93% for the agarose gel capsule method was significantly higher than the 86%for the Cryotop method (P < 0.005). Sperm motility rate: 73% for the agarose gel capsule method was significantly higher than the 51%for the Cryotop method (P < 0.001). Sperm viability rate: 86% for the agarose gel capsule method was significantly higher than the 65% for the Cryotop method (P < 0.001). B). Sperm recovery time (mean ± SD): 3.56 ± 1.5 min for the agarose gel capsule method was significantly shorter than the 7.04 ± 3.2 min for the Cryotop method (P < 0.001)
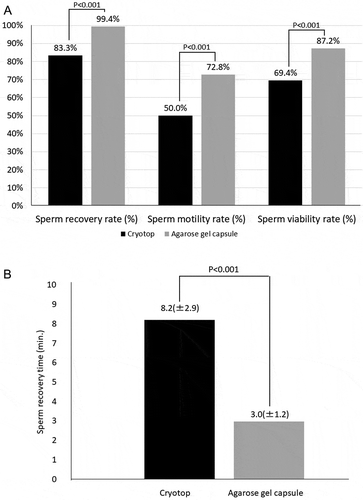
Investigation of samples with sperm concentration less than 1 million/mL
As summarized in and shown in , sperm recovery rate of 99% (179/180) for the agarose gel capsule method was significantly higher than the 83% (150/180) for the Cryotop method (P < 0.001). Sperm motility rate: 87% (157/180) for the agarose gel capsule method was significantly higher than the 69% (125/180) for the Cryotop method (P < 0.001). Sperm viability rate: 86% (258/300) for the agarose gel capsule method was significantly higher than the 65% (196/300) for the Cryotop method (P < 0.001). Sperm recovery time (mean ± SD): 2.97 ± 1.2 min for the agarose gel capsule method was significantly shorter than the 8.20 ± 2.9 min for the Cryotop method (P < 0.001).
Table 3. Comparison of agarose gel method and Cryotop method in samples with sperm concentration less than 1 million/mL
Figure 3. Comparison between the agarose gel capsule method and Cryotop method in samples with sperm concentration less than 1 million/mL. A). Sperm recovery rate: 99% for the agarose gel capsule method was significantly higher than the 83% for the Cryotop method (P < 0.001). Sperm motility rate: 87% for the agarose gel capsule method was significantly higher than the 69% for the Cryotop method (P < 0.001). Sperm viability rate: 86% for the agarose gel capsule method was significantly higher than the 65% for the Cryotop method (P < 0.001). B). Sperm recovery time: 2.97 ± 1.2 min for the agarose gel capsule method was significantly shorter than the 8.20 ± 2.9 min for the Cryotop method (P < 0.001)
The results above showed that the post-thawing sperm recovery rate, sperm motility rate, and sperm viability rate were higher while the sperm recovery time was shorter in samples preserved using the agarose gel capsule method compared to samples preserved using the Cryotop method in both the group with sperm concentrations of 1 million/mL or above and the group with sperm concentrations of less than 1 million/mL.
Discussion
For the cryopreservation of small numbers of sperm, methods have been reported that involve placement of microdroplets of cryopreservation medium containing sperm on special equipment such as the CryoLoop (Hampton Research, Aliso Viejo, CA), Cell Sleeper (Nipro Corp., Osaka, Japan), or Cryotop (Kitazato Corp.) (Schuster et al. Citation2003; Desai et al. Citation2004; Endo et al. Citation2012a, Citation2012b). These methods are relatively easy to perform, but they have the disadvantage that sperm become dispersed within the cryopreservation medium microdroplet, making them difficult to recover.
To overcome this problem, several methods for capturing sperm in small vials have been reported, including the egg zona pellucida preservation method (Cohen et al. Citation1997), volvox globator algae method (Just et al. Citation2004), alginic acid capsules method (Herrler et al. Citation2006), hyaluronan microcapsules method (Tomita et al. Citation2016), and the agarose gel capsule method that was investigated in this study (Araki et al. Citation2015).
The agarose gel capsule is 80 to 120 μm in outer diameter, and 60 to 100 μm in inner diameter. It is a capsule with an empty core that mimics the structure of egg zona pellucida. Agarose, the main component of the capsule, is used in food ingredients such as agar, and many studies have reported a culture of mammalian cells in agarose (Sakai et al. Citation2005); therefore, it is assumed to be safe for humans.
Loss of sperm after thawing is a huge problem when cryopreserving a small number of sperm, for example, those collected by microdissection testicular sperm extraction from nonobstructive azoospermia patients or ejaculated sperm from cryptozoospermia patients. Furthermore, it is important to reduce the amount of time needed to search for sperm during recovery to prevent the aging of collected ovum (Nagy et al. Citation1993; Van Steirteghem et al. Citation1993; Miao et al. Citation2009; Lord and Aitken Citation2013; Prasad et al. Citation2015).
The difficulty in searching for sperm that have been cryopreserved and thawed from low-count samples may be due to contamination with components other than sperm, or because the volume of vials or freezing media is too large in comparison with the sperm. In this investigation, thawed sperm was searched for within as little as 5 µL of the medium, and the results obtained were better using the agarose gel capsule method than the conventional Cryotop method.
The advantage of the agarose capsule method is that sperm are captured within the capsule, which prevents their dispersal and leads to an improved recovery time and recovery rate. In cases where capturing thawed sperm is difficult due to low sperm count, the agarose gel method may offset the issue of aging ovum by reducing the time required before ICSI can be performed, which may lead to improved fertilization rates.
However, cryopreservation using the agarose gel capsule method is more complicated than the widely used conventional method (Endo et al. Citation2011), and additional time will be required to train staff.
For the agarose gel capsule method to become widely adopted in the future, selection criteria for applicable cases must be investigated, and the establishment of manuals and training programs will also be required. We are planning to further investigate whether cryopreservation using the agarose gel method is effective for testicular sperm collected by the testicular sperm extraction method.
There are several limitations in this study.
We had checked the viability of sperm by HOS test. But DNA integrity of immotile sperm was not evaluated.
Intracellular and extracellular ice formation and osmotic stress during freezing and thawing processes may cause cryoinjury of sperm DNA. A recent report indicated application of ultrasonic vibration can modify the water molecules as the main compositions of the freezing medium used for human sperm cryopreservation and consequently decreased apoptosis rate (Dariush et al. Citation2019). Future research should be conducted to compare the results of the agarose gel capsule method and the application of the ultrasonic vibration method.
In conclusion, this study demonstrated that using the agarose gel capsule method increased post-thawing sperm recovery rate, sperm motility rate, and sperm viability rate, and reduced sperm recovery time compared with the conventional Cryotop method when cryopreserving samples with very low sperm count from male infertility patients.
The agarose gel capsule method may be a promising treatment option for male infertility patients with extremely small numbers of sperm. But more research work is mandatory to establish a better cryopreservation method for extremely small numbers of human spermatozoa.
Materials and methods
Subjects
Between September 1st and September 30th 2019, a total of 57 patients consulted the male infertility clinic at the International Center for reproductive Medicine (Dokkyo Medical University Saitama Medical Center). They were screened for the possible cause of infertility (e.g., varicocele, endocrine disorders, erectile dysfunction, or chronic disease). All but azoospermic patients were asked to participate in this study. Finally, a total of 16 patients (age = 19–24 years) agreed to produce semen samples for this study. Araki et al. (Citation2015) described the effectiveness of agarose gel microcapsule for cryopreservation of single sperm from a fertile donor. We evaluated the live sperm recovery rate and sperm recovery time using agarose gel microcapsules in the real clinical setting.
Written informed consent was obtained from all 16 subjects who participated in this study. A semen screening test was performed for each patient in accordance with the World Health Organization guidelines (World Health Organization Citation2010). Patients were divided into two groups by the sperm concentration (sperm concentration less than 1 million/ml vs. 1 million/ml or above).
Of the 16 subjects, 10 had sperm concentrations of 1 million/mL or above; this is considered a high recovery rate of live sperm following cryopreservation and thawing of semen using the Crytop method, which is the standard method used for samples with low sperm counts (Endo et al. Citation2011). The median age of subjects was 34 years (range: 24–45 years), and median sperm concentration was 28.5 million/mL (range: 1 million/mL to 63 million/mL).
Six subjects had sperm concentrations of less than 1 million/mL; this is considered to be a lower recovery rate of live sperm. The median age was 36 years (range: 19–41 years), and median sperm concentration was 0.6 million/mL (range: 0.9 million/mL to 0.9 million/mL).
Sperm preparation
As illustrated in , semen that were planned to be discarded after testing were mixed with 3 mL of sperm wash solution (SepaSperm Wash Solution; Kitazato Corp., Shizuoka, Japan) and were centrifuged at 1000 × g for 5 min using a centrifuge (5702 RH; Eppendorf, Hamburg, Germany). After removal of supernatant, sperm pellet was resuspended with 50 µL of sperm wash solution. On the bottom of a petri dish (FALCON1006; Corning Incorporated, Corning, NY), sperm suspension droplets were created by mixing 5 µL of 7% polyvinylpyrrolidone (PVP; Nakamedical Inc., Tokyo, Japan) with 5 µL of sperm suspension. The droplet was covered with mineral oil (WO500, Nakamedical Inc.) so that motility of spermatozoa within the drop could be observed.
Cryopreservation medium
A cryopreservation medium was prepared by mixing equal volumes of sperm wash solution and cryoprotectant (Sperm Freeze; Kitazato Corp.).
We compared the following two cryopreservation methods as summarized in .
2. Cryopreservation by the Cryotop method.
The procedure was conducted as reported previously (Endo et al. Citation2011).
Briefly, approximately 0.5 µL to 1.0 µL cryopreservation medium drop was prepared on the Cryotop, and 6 motile spermatozoa aspirated from the droplet covered with mineral oil. Then, they were injected into the cryopreservation medium drop on the Cryotop using an ICSI injection pipette under inverted microscope (IX73; Olympus Corporation, Tokyo, Japan). The cryopreservation medium drop containing six spermatozoa was placed on a polystyrene foam board that was placed in a container of liquid nitrogen 4 cm above the liquid nitrogen surface. Samples were frozen for 2 minutes after which Cryotops were immersed in liquid nitrogen, capped, placed in cryocanes (C10 straw type; My Sciences Co., Ltd., Tokyo, Japan), and stored in a cryopreservation tank (MVE XC47/11; Chart Industries, Inc., Luxembourg City, Luxembourg).
(2) Cryopreservation by the agarose gel capsule method
Six motile spermatozoa aspirated from the droplet covered with mineral oil. They were then injected into two agarose gel capsules (three sperm per capsule) filled with cryopreservation medium using an ICSI injection pipette under inverted microscope. The agarose gel capsules and approximately 0.5 µL of cryopreservation medium were placed on top of the Cryotop. A polystyrene foam boat made especially for the agarose capsule method was floated on the freezing equipment (SP Cryostyrole A-101,002; The Institute for Advanced Reproductive Medical Technology, Maebashi, Japan). The polystyrene foam boat was equipped with a digital thermometer (SK-1110; Sato Keiryoki Mfg. Co., Ltd., Tokyo, Japan) and a thermo probe (TSU-25; Tokai Hit Co., Ltd., Shizuoka, Japan). Cryopreservation was performed for 2 min in accordance with the manufacturer’s instructions. After freezing, Cryotops were immersed in liquid nitrogen, capped, placed in cryocanes, and stored in a cryopreservation tank.
Thawing and sperm collection
Samples were thawed and sperm were collected following storage in liquid nitrogen for 1 week using the abovementioned Cryotop and agarose gel capsule methods.
For samples preserved using the Cryotop method, a 5-µL sperm wash solution droplet was placed on a petri dish (FALCON1006; Corning) covered with mineral oil for thawing and collecting sperm. The dish was heated to 37°C, and the end of the Cryotop was removed from the liquid nitrogen and thawed within the oil on the dish. The portion of the sheet containing the sperm was immersed in the sperm wash solution droplet. Sperm were collected using an ICSI injection pipette under inverted microscope.
For samples preserved using the agarose gel capsule method, a petri dish for thawing and collecting sperm was prepared and heated as described above. The end of the Cryotop was removed from the liquid nitrogen and thawed within the oil on the dish. The portion of the sheet part containing the capsules was then immersed in the droplet to wash off the cryopreservation medium. Five µL of sperm wash solution droplet was placed on a plastic petri dish covered with mineral oil for sperm collection. The washed agarose gel capsules were immersed in the droplet, and sperm were collected from the capsules using an ICSI injection pipette under inverted microscope.
Sperm were observed under the inverted microscope at 400 × magnification. Sperm whose movement was confirmed were defined as motile sperm. The viability of immotile sperm was determined by the hypo-osmotic swelling (HOS) test, which was performed as described previously (Jeyendran et al. Citation1984; Smikle and Turek Citation1997). Viable sperm were defined as those that had swollen tails within the HOS medium made up of equal volumes of sperm wash solution and distilled water.
Time taken for sperm recovery
The time taken for sperm to recover was measured from the point when the dish containing thawed sperm was placed on the inverted microscope. The time of completion was set as the time when all sperm had been collected with the ICSI injection pipette.
Statistical analysis
From each patient, five sets of six motile sperm (total = 30) each were cryopreserved using the Cryotop method and the agarose gel capsule method. Sperm recovery rate after thawing (defined as the number of collected sperm divided by the number of cryopreserved sperm), motile sperm rate, sperm viability, and sperm recovery time were compared between samples preserved using the Cryotop and the agarose gel capsule methods for both the group with sperm concentrations of 1 million/mL or more (10 subjects), and the group with sperm concentrations of less than 1 million/mL (6 subjects).
SPSS statistical analysis software (SPSS Inc., Chicago, IL) was used to perform a chi-square test comparing sperm recovery rate after thawing, motile sperm rate, and sperm viability. The t-test was used to compare sperm recovery time. P < 0.05 was considered to be statistically significant.
Ethical approval
All patients involved in this study provided informed consent. The study protocol was approved by the IRB of Dokkyo Medical University Saitama Medical Center (approval no. 1576).
Authors’ contribution
Design of the study was conceived and spermatozoa cryopreservation using the new agarose gel capsule method was performed by MK. Semen analysis and cryopreservation using Cryotop method were done by MF. Acquisition and analysis of the data was done by AM. Semen analysis and statistical analysis of the data was done by TT. Data analysis and interpretation was performed by KS. Design of the study was prepared, data analysis and interpretation were done, and the revised manuscript was drafted by HO. All authors approved the final version of the manuscript.
Acknowledgments
The authors would like to thank Yasuhisa Araki, PhD and Yasuyuki Araki, PhD at the Institute for Advanced Reproductive Medical Technology for their technical assistance.
Disclosure statement
The authors report no conflict of interest.
Additional information
Funding
References
- AbdelHafez F, Bedaiwy M, El-Nashar SA, Sabanegh E, Desai N. 2009. Techniques for cryopreservation of individual or small numbers of human spermatozoa: a systematic review. Hum Reprod Update. 15(2):153–164. doi:10.1093/humupd/dmn061.
- Araki Y, Yao T, Asayama Y, Matsuhisa A, Araki Y. 2015. Single human sperm cryopreservation method using hollow-core agarose capsules. Fertil Steril. 104(4):1004–1009. doi:10.1016/j.fertnstert.2015.06.043.
- Cohen J, Garrisi GJ, Congedo-Ferrara TA, Kieck KA, Schimmel TW, Scott RT. 1997. Cryopreservation of single human spermatozoa. Hum Reprod. 12(5):994–1001. doi:10.1093/humrep/12.5.994.
- Dariush G, Gholamhossein R, Rouhollah F, Mahmood GS, Abdolhossein S, Mohsen S, Loghman A. 2019. The application of ultrasonic vibration in human sperm cryopreservation as a novel method for the modification of physicochemical characteristics of freezing media. Sci Rep. 9(1):10066. doi:10.1038/s41598-019-46424-0.
- Desai NN, Blackmon H, Goldfarb J. 2004. Single sperm cryopreservation on cryoloops: an alternative to hamster zona for freezing individual spermatozoa. Reprod Biomed Online. 9(1):47–53. doi:10.1016/S1472-6483(10)62109-8.
- Endo Y, Fujii Y, Kurotsuchi S, Motoyama H, Funahashi H. 2012a. Successful delivery derived from vitrified-warmed spermatozoa from a patient with nonobstructive azoospermia. Fertil Steril. 98(6):1423–1427. doi:10.1016/j.fertnstert.2012.07.1128.
- Endo Y, Fujii Y, Shintani K, Seo M, Motoyama H, Funahashi H. 2011. Single spermatozoon freezing using cryotop [Original article]. J Mamm Ova Res. 28(1):47–52. doi:10.1274/jmor.28.47.
- Endo Y, Fujii Y, Shintani K, Seo M, Motoyama H, Funahashi H. 2012b. Simple vitrification for small numbers of human spermatozoa. Reprod Biomed Online. 24(3):301–307. doi:10.1016/j.rbmo.2011.11.016.
- Herrler A, Eisner S, Bach V, Weissenborn U, Beier HM. 2006. Cryopreservation of spermatozoa in alginic acid capsules. Fertil Steril. 85(1):208–213. doi:10.1016/j.fertnstert.2005.06.049.
- Jeyendran RS, Van Der Ven HH, Perez-Pelaez M, Crabo BG, Zaneveld LJ. 1984. Development of an assay to assess the functional integrity of the human sperm membrane and its relationship to other semen characteristics. J Reprod Fertil. 70(1):219–228. doi:10.1530/jrf.0.0700219.
- Just A, Gruber I, Wöber M, Lahodny J, Obruca A, Strohmer H. 2004. Novel method for the cryopreservation of testicular sperm and ejaculated spermatozoa from patients with severe oligospermia: a pilot study. Fertil Steril. 82(2):445–447. doi:10.1016/j.fertnstert.2003.12.050.
- Liu S, Li F. 2020. Cryopreservation of single-sperm: where are we today? Reprod Biol Endocrinol. 18(1):41. doi:10.1186/s12958-020-00607-x.
- Lord T, Aitken RJ. 2013. Oxidative stress and ageing of the post-ovulatory oocyte. Reproduction. 146(6):R217–227. doi:10.1530/REP-13-0111.
- Miao YL, Kikuchi K, Sun QY, Schatten H. 2009. Oocyte aging: cellular and molecular changes, developmental potential and reversal possibility. Hum Reprod Update. 15(5):573–585. doi:10.1093/humupd/dmp014.
- Nagy ZP, Joris H, Liu J, Staessen C, Devroey P, Van Steirteghem AC. 1993. Intracytoplasmic single sperm injection of 1-day-old unfertilized human oocytes. Hum Reprod. 8(12):2180–2184. doi:10.1093/oxfordjournals.humrep.a138000.
- Prasad S, Tiwari M, Koch B, Chaube SK. 2015. Morphological, cellular and molecular changes during postovulatory egg aging in mammals. J Biomed Sci. 22(1):36. doi:10.1186/s12929-015-0143-1.
- Said TM, Gaglani A, Agarwal A. 2010. Implication of apoptosis in sperm cryoinjury. Reprod Biomed Online. 21(4):456–462. doi:10.1016/j.rbmo.2010.05.011.
- Sakai S, Kawabata K, Tanaka S, Harimoto N, Hashimoto I, Mu C, Salmons B, Ijima H, Kawakami K. 2005. Subsieve-size agarose capsules enclosing ifosfamide-activating cells: a strategy toward chemotherapeutic targeting to tumors. Mol Cancer Ther. 4(11):1786–1790. doi:10.1158/1535-7163.MCT-05-0227.
- Schuster TG, Keller LM, Dunn RL, Ohl DA, Smith GD. 2003. Ultra-rapid freezing of very low numbers of sperm using cryoloops. Hum Reprod. 18(4):788–795. doi:10.1093/humrep/deg162.
- Smikle CB, Turek PJ. 1997. Hypo-osmotic swelling can accurately assess the viability of nonmotile sperm. Mol Reprod Dev. 47(2):200–203. doi:10.1002/(SICI)1098-2795(199706)47:2<200::AID-MRD11>3.0.CO;2-3.
- Tomita K, Sakai S, Khanmohammadi M, Yamochi T, Hashimoto S, Anzai M, Morimoto Y, Taya M, Hosoi Y. 2016. Cryopreservation of a small number of human sperm using enzymatically fabricated, hollow hyaluronan microcapsules handled by conventional ICSI procedures. J Assist Reprod Genet. 33(4):501–511. doi:10.1007/s10815-016-0656-x.
- Van Steirteghem AC, Nagy Z, Joris H, Liu J, Staessen C, Smitz J, Wisanto A, Devroey P. 1993. High fertilization and implantation rates after intracytoplasmic sperm injection. Hum Reprod. 8(7):1061–1066. doi:10.1093/oxfordjournals.humrep.a138192.
- World Health Organization. 2010. WHO laboratory manual for the examination and processing of human semen. 5th ed. World Health Organization.
