ABSTRACT
The SARS-CoV-2 pandemic peak around March 2020 led to temporary closures of most fertility clinics. Many clinics reopened but required universal SARS-CoV-2 screening. However, the rate of positive results and the necessity for such testing is unknown. We report here on early results from asingle-center academic NewYork fertility practice utilizing universal SARS-CoV-2 screening. This mixed prospective retrospective cohort included 164 patients who underwent at least one SARS-CoV-2 screening test for fertility treatment between May and July2020. Patients completed 1 to 3 nasopharyngeal SARS-CoV-2 tests per cycle and remained symptom-free to continue fertility treatments. SARS-CoV-2 test results, past results, history of Covid-19 infection, and patient/cycle characteristics were recorded and tabulated through October2020. Outcomes included positive SARS-CoV-2 RNA tests, rate of prior Covid-19 infections, and clinical courses of patients testing positive. Patients underwent 263 cycles entailing 460 total SARS-CoV-2 screening tests. Fifteen patients reported astrong prior clinical history of Covid-19. Six patients experienced apositive SARS-CoV-2 test (2.3% of all cycles). Among 77 cycles (n = 58 patients) entailing one SARS-CoV-2 test, 2 cases (2.6%) were noted. Among 173 cycles (n = 121 patients) entailing two SARS-CoV-2 tests, 4 cycles (2.3%) were noted. Zero (0%) of 13 cycles (n = 13 patients) entailing 3 SARS-CoV-2 tests were positive. All patients were cleared to resume treatment within one month. Overall, anew asymptomatic infection was identified in 2 cycles (0.8%), while 4 of the 6 positive SARS-CoV-2 tests were among patients with aprior history of Covid-19. 3 of 4 also had adocumented prior positive RNA test. Our data suggest that universal SARS-CoV-2 screening among fertility patients is feasible, with an approximately 2% positive rate per cycle among the patients of this study. Most positive patients had aprior remote infection, but their infectiousness while being screened remains unclear.
Abbreviations: REI: reproductive endocrinology and infertility; IUI: intrauterine insemination; IVF: in vitro fertilization; sono: sonography; cryo: cryopreservation; FET: frozen embryo transfer.
Introduction
The SARS-CoV-2 virus responsible for the Covid-19 pandemic has led to massive worldwide health and economic disruption. Efficient human-to-human respiratory and droplet transmission, asymptomatic disease carriers, high disease morbidity, and lack of prior population immunity (Gandhi et al. Citation2020) have intersected to force large-scale changes in daily life and practice.
During the peak of Covid-19 infections in New York between March and early May of 2020, almost all fertility clinics were closed in compliance with temporary lockdown mandates by the state governor and following the American Society For Reproductive Medicine (ASRM) Covid-19 task force patient management and clinical recommendations, first published March 17, 2020 (ASRM; La Marca et al. Citation2020). Following a phased reopening beginning in May, most fertility clinics have been able to resume elective and non-emergent care. Limited guidance (De Santis et al. Citation2020; La Marca et al. Citation2020; Dellino et al. Citation2020) has been provided for how to resume operation while keeping patients and healthcare workers safe, forcing individual clinics to develop their own protocols. Some have suggested the use of individualized molecular or serologic testing for SARS-CoV-2 as a criterion for assisted reproductive treatment (Alviggi et al. Citation2020).
Even after a massive spike in Covid-19 cases and deaths around New York City (NYC), there is little evidence of herd immunity. A statewide seroprevalence study demonstrated SARS-CoV-2 antibody reactivity among only 22.7% of patients in NYC through late March 2020 (Rosenberg et al. Citation2020). Asymptomatic community transmission remains difficult to measure given biases among patients receiving testing and significant temporal effects.
In mid-May 2020, the Montefiore Institute for Reproductive Medicine and Health, located in Hartsdale, New York, reopened its fertility clinic while mandating universal testing for all patients undergoing procedures or repeated clinic visits. Patients would be tested for SARS-CoV-2 up to 3 times per cycle, based on the planned treatment.
We sought to measure the practicality of this testing regimen of universal screening and identify the rate of SARS-CoV-2 positivity among asymptomatic community members undergoing fertility treatment in order to better understand the implications of such a testing protocol in this population.
Results
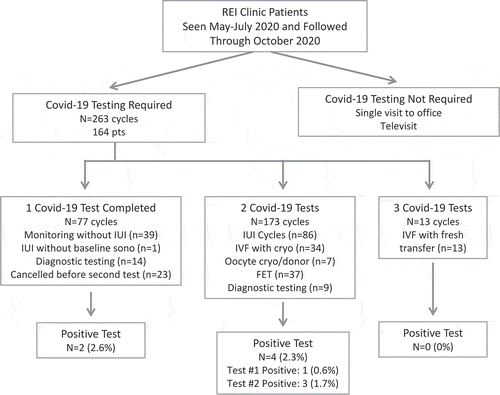
We identified 164 patients who underwent fertility-mandated SARS-CoV-2 screening through our clinic between mid-May 2020, when our clinic opened, and early July. These patients were followed through October, during which they underwent 263 treatment cycles requiring at least one set of SARS-CoV-2 screening tests (). A total of 460 SARS-CoV-2 screening tests were performed during the follow-up period. Patients had the opportunity to undergo multiple treatment cycles, potentially involving different numbers of SARS-CoV-2 tests per cycle based on treatment.
Figure 1. Workflow for SARS-CoV-2 screening in the fertility clinic. Cycles requiring universal SARS-CoV-2 screening are stratified based on the number of screening tests performed. Intended fertility treatment is reported for cycles completing the required number of SARS-CoV-2 screening test(s)
All patients consented in writing to universal testing and were able to coordinate testing in time for proceeding with planned fertility workup or treatment. Stratified by county of residence, 75 (45.7%) of treated patients were from the Bronx, 38 (23.2%) were from Westchester, 15 (9.1%) were from Rockland and the remainder were from other surrounding counties.
Fifteen (9.1%) of the patients reported a strong clinical or biochemical laboratory history of a Covid-19 infection prior to undergoing treatment, and 12 (7.3%) had a prior positive SARS-CoV-2 RNA test within our records or reported one. One of the patients with confirmed Covid-19 infection history (0.6%) had a history of hospitalization for Covid-19 pneumonia prior to her fertility treatment.
Six patients in total were identified to have a positive SARS-CoV-2 test during our universal screening while undergoing fertility diagnostic or treatment cycles (2.3% of all cycles) (). Among the 77 cycles (n = 58 patients) entailing one SARS-CoV-2 test, a positive SARS-CoV-2 test was identified in 2 cases (2.6%).
Among the 173 cycles (n = 121 patients) with patients undergoing two SARS-CoV-2 tests, a positive SARS-CoV-2 test was identified in 4 cycles (2.3%). Three of the 4 positive tests were noted during the second test, while one patient’s first SARS-CoV-2 test was positive but was negative on repeat as she continued estrogen while preparing for and completing a frozen-thawed embryo transfer (FET). Zero (0%) of the 13 cycles (n = 13 patients) entailing 3 SARS-CoV-2 tests were positive.
One patient planning intrauterine insemination (IUI) had her cycle converted to timed intercourse because the second SARS-CoV-2 test, though ultimately negative, did not result in time for an IUI.
We reviewed in detail the clinical history of patients who tested positive during their treatment during this period of universal testing (). One patient had a polymerase chain reaction (PCR)-confirmed symptomatic Covid-19 infection in March with complete recovery. She had been asymptomatic since April but continued to demonstrate positive tests, including one performed through the department of health in early May and again around the time of menses at our center in mid-May while preparing for a FET. She was started on estradiol and allowed to complete the FET approximately 2 weeks later after repeat testing was negative.
Table 1. Fertility patients testing positive for SARS-CoV-2 during universal screening
The second patient, with a prior history of Covid-19 infection in March, began an IUI cycle in June, which was converted to a timed intercourse cycle following a positive test collected on the day of ovulation trigger. She completed an IUI cycle the following month.
The third patient had an IUI cycle in June, converted to timed intercourse after a positive test on the day of ovulation trigger. She lacked evidence of prior Covid-19 infection and was able to resume her planned IUI cycle the next month.
The fourth patient also reported a history of Covid-19 symptoms in March but was not tested then. She demonstrated a positive SARS-CoV-2 test collected on the day of menses prior to beginning an IUI cycle in June, which was canceled and deferred until September.
The fifth patient, with no prior Covid-19 history, had planned to begin in vitro fertilization (IVF) in June. She was instead started on oral contraceptives and was able to begin gonadotropins following a negative test 3 weeks later.
The sixth patient had a documented history of Covid-19 infection in April. She began ovulation induction medications in September following a negative test but demonstrated a positive test on the day of ovulation trigger, prompting a cancellation. She is planning to return to the clinic.
Overall, 4 of the 6 positive SARS-CoV-2 tests were in patients with a presumed prior history of Covid-19, and 3 of the 4 had a prior recorded positive test.
Discussion
Our work is the first formal report on the early clinical experiences following the implementation of a universal SARS-CoV-2 screening protocol, though several other groups have reported preliminary data in abstracts presented at the ASRM 2020 Annual Meeting (Armstrong et al. Citation2017; Romanski et al. Citation2020; Foulk et al. Citation2020; Katz-Jaffe et al. Citation2020; Prados et al. Citation2020; Robles et al. Citation2020; Seidman et al. Citation2020). Our findings demonstrating a relatively low prevalence among patients screened in fertility centers even in regions with high historical Covid-19 infection rates essentially match the results of other groups (). However, with an ongoing, dramatic rise in infection rates across the United States and no vaccine widely available to reproductive-age patients at the writing of this study (January 2021), the prevalence of Covid-19 among patients in fertility centers has the potential to rise significantly.
Table 2. Summary of studies of universal SARS-CoV-2 RNA screening in fertility clinics
To our knowledge, none of the previously presented studies reported on the prevalence of a prior Covid-19 infection history among all patients, though one study identified up to 2.2% seroprevalence of SARS-CoV-2 IgG antibodies among patients and clinic staff screened in a clinic in Massachusetts (Foulk et al. Citation2020), another reported 0.7% IgM seroprevalence among patients tested across multiple clinics in Spain (Prados et al. Citation2020) and another reported one patient with a prior history of Covid-19 among the 5 who tested positive for viral RNA in a NYC clinic (Bortoletto et al. Citation2020) ().
Because fertility patients are typically young and healthy and are now being tested repeatedly with 100% participation, this population may serve as an excellent barometer of SARS-CoV-2 infections and transmissions in the community, potentially aiding public health surveillance.
While all patients with a positive test experienced treatment delays as a consequence of our protocol to defer treatment until a subsequent negative result, these patients generally were able to resume treatment within a month if desired (). Our clinic protocol also led to 1 patient converting her treatment from IUI to timed intercourse due to pending a SARS-CoV-2 test on the planned day of insemination, highlighting the potential for unintended consequences from a mandatory universal SARS-CoV-2 screening protocol.
Recent evidence suggesting that an IVF treatment delay of up to 180 days among patients with diminished ovarian reserve does not impair reproductive outcomes (Romanski et al. Citation2020) supports the overall lack of harm to patient reproductive potential from policies mandating a deferral until a negative SARS-CoV-2 test.
This study has several limitations. Notably, the majority of patients (4 of 6) whose care was delayed due to a positive test from universal screening already had a prior Covid-19 infection documented several months before. Patients who ultimately clear a Covid-19 infection typically demonstrate detectable viral RNA for 20 days after illness onset (Alviggi et al. Citation2020), but RNA has been demonstrated up to 63 days (Widders et al. Citation2020) or even longer in some instances (Choi et al. Citation2020; Turner et al. Citation2021) after symptom onset. This study could not determine whether patients screening positive after a prior Covid-19 infection experienced viral RNA persistence or were reinfected.
This study qualitatively measured SARS-CoV-2 RNA at discrete snapshots but could not determine whether patients were shedding virus in the weeks before or after treatment or quantify viral levels once positive. Antibody IgG and IgM titers were not performed universally either, precluding identification of patients who had recovered from an asymptomatic infection in the immediate (IgM) or more remote (IgG) past. Lack of standardization on a single RT-PCR assay for all patients also complicates estimating the false positive/negative test rate. This study’s patient population may not reflect the population of patients undergoing fertility treatments nationally and may even differ from patients undergoing treatment pre-pandemic.
The cost-effectiveness and clinical utility of our strict universal testing approach remains unclear. A total of 460 tests were performed in order to identify 2 cases of new asymptomatic Covid-19 infection and 4 patients with detectable SARS-CoV-2 RNA months after a previously cleared infection.
Given the universal protections implemented for protection of patients and providers in the clinic, including universal mask-wearing, use of N95 masks and face shields for all providers during procedures, limiting guests, and distancing of patients in the waiting room, the requirement for mandatory universal SARS-CoV-2 testing may perhaps be relaxed as long as the population prevalence remains low.
Despite these generally reassuring findings, IVF treatment during a pandemic continues to pose important bioethical challenges. Suspensions in treatment impose significant psychosocial costs (Turner et al. Citation2021). The ASRM task force emphasized as early as March 30th, 2020, that infertility care, even during a pandemic, is not elective (ASRM). Nonetheless, continued treatment may divert essential medical providers or resources, expose patients and vulnerable staff to infections and expose additional pregnancies to risks that may not be manifest until many years in the future.
Given the potential risks of SARS-CoV-2 during pregnancy, including higher rates of invasive ventilation and intensive care unit admission (Zambrano et al. Citation2020), the testing regimen employed in our clinic may have the added benefit of allowing patients to defer attempts at conception until after recovery. In addition, having a universal testing protocol may help to reassure clinic patients and staff of their safety while continuing treatment.
Partners were not tested at our institution for SARS-CoV-2 during treatment. While the possibility of SARS-CoV-2 isolation from semen remains controversial (Turner et al. Citation2021), some clinics may also consider testing partners to minimize staff and patient exposure risks and limit virus transmission potential duringl fertilization and the possibility of embryonic effects.
In summary, implementing universal SARS-CoV-2 screening among fertility patients is feasible. Approximately 2% of patients undergoing diagnostic or treatment fertility cycles between May and July 2020 with follow-up through October in our center in the metropolitan New York area demonstrated a positive SARS-CoV-2 RNA test. All were able to resume treatment shortly thereafter if desired.
Over half had tested positive after a prior documented infection more than 2 weeks in the past. It remains unclear what fraction of positive patients identified from screening are truly infectious. However, given the limited time-frame of reported shedding of live viral particles following infection, many are likely not. Therefore, the medical necessity of universal SARS-CoV-2 testing in fertility clinics remains dubious but a conservative screening approach may still be warranted.
Materials and methods
We performed a mixed prospective-retrospective longitudinal cohort study for all patients treated at MIRMH in-person between May 19th, 2020 (the first day of reopening) and July 7th, 2020. This cohort of patients was followed through October 31st, 2020. Chart review was performed on electronic medical records.
All patients seen at MIRMH who underwent SARS-CoV-2 testing as a condition of their fertility evaluation and treatment were included. Patients and their partners were required to sign consents detailing testing requirements, responsibility of both partners to reduce risk of exposure, reasons for cycle cancellation and potential risks to patients or pregnancies from infections during treatment. Only patients underwent testing. Partners were expected to reduce exposure risks but were not required to undergo testing as they were not permitted in the facility during treatment and were required to produce semen outside of the clinic.
SARS-COV-2 testing was performed by either real-time polymerase chain reaction (RT-PCR) assay or antigen fluorescent immunoassay (FIA) on nasopharyngeal (NP) or oropharyngeal swab (OP) specimens for qualitative detection of nucleic acid or nucleocapsid protein antigen from SARS-CoV-2, respectively. Specimens were typically collected (>90%) at affiliate Montefiore testing sites willing to provide testing with an approximately 24-hour turnaround, though patients were given the option to obtain testing at outside sites (public health testing stations, outside clinics, laboratory testing sites) if they could provide documentation of test results in time for their visits/procedures. Testing for patients screened at Montefiore sites was performed using the Panther Fusion RT-PCR SARS-COV-2 assay (Hologic, San Diego, CA). Outside testing was performed using various systems, including Sofia SARS Antigen FIA (Quidel Corporation,San Diego, CA), cobas RT-PCR SARS-COV-2 assay (Roche, Pleasanton, CA) and Quest RT-PCR SARS-COV-2 assay (Quest Diagnostics Infectious Disease, San Juan Capistrano, CA).
Patients unable to complete SARS-CoV-2 testing or with unavailable testing records were excluded. Patients undergoing pre-operative testing for reproductive surgeries were excluded. Male patients were not tested and were excluded from this cohort.
Testing for SARS-CoV-2 was performed between 1 and 3 times per treatment cycle, based on planned treatment (). Patients were instructed to self-quarantine during their treatment cycle and between test days.
Patients undergoing monitored cycles without procedures but possibly with medications were tested only once, typically on cycle day 1 or 2, while those undergoing diagnostic-only or treatment procedures without baseline monitoring were screened during the 3 days prior to their procedure. Patients would also be tested only once if they underwent monitoring at our clinic for a procedure performed at an outside site.
Patients undergoing IUI, oocyte retrieval with freeze-all disposition (including IVF with freeze-all, oocyte cryopreservation, and oocyte donor) and FET cycles would be tested on cycle day 1–2 as well as within the 3 days prior to their procedure. To ensure that the second test could be reliably completed, patients were not permitted to await for spontaneous luteinizing hormone (LH) surges and would undergo testing on the day of administration of ovulation trigger medication.
Finally, patients undergoing IVF with fresh embryo transfer would undergo 3 tests, with testing comparable to IVF freeze-all cycles plus a third test performed during the 3 days preceding embryo transfer.
Patients whose treatment cycle was canceled for any reason would not be required to complete second or third SARS-CoV-2 tests. Patients who tested positive for SARS-CoV-2 at any point during their treatment cycle would be canceled or converted to an alternative treatment plan requiring no further clinic visits (e.g., timed intercourse) following counseling about potential risks of Covid-19 in pregnancy until a subsequent negative test was demonstrated.
The primary outcome was the rate of SARS-CoV-2 positive tests by NP/OP swab among universally screened patients undergoing fertility treatment and duration of delay among patients with positive tests before fertility treatment could resume. Secondary outcomes included rates of prior SARS-CoV-2 RNA test positivity, history- or laboratory-indicated Covid-19 infection and prior hospitalization for Covid-19 among screened patients.
Patient data were extracted from electronic medical records and entered into a secure, HIPAA-compliant database accessible only to investigators.
Data analysis and summary statistics were computed in R (version 4.0.3, R Foundation for Statistical Computing, Vienna, Austria) (R Development Core Team Citation2013).
Ethics approval
The study was performed following Montefiore Institutional Review Board (IRB) approval under IRB 2020–12,029, dated August 19, 2020. Patient informed consent for participation was not required as this study was considered exempt.
Authors' contributions
Conceived of and designed the project: JAG, SP, MS, HL, SJ; performed data collection and analysis: JAG, SG, SF, MK; authored and edited the manuscript: JAG, SG, SF, HL, SJ.
Acknowledgments
We would like to thank the Montefiore IRB for granting expedited approval of this research project.
Disclosure statement
The authors declare no competing interests.
References
- Alviggi C, Esteves SC, Orvieto R, Conforti A, La Marca A, Fischer R, Andersen CY, Bühler K, Sunkara SK, Polyzos NP, et al. 2020. COVID-19 and assisted reproductive technology services: repercussions for patients and proposal for individualized clinical management. Reprod Biol Endocrinol. 18:1. doi:10.1186/s12958-020-00605-z.
- Armstrong SC, Showell M, Stewart EA, Rebar RW, Vanderpoel S, Farquhar CM. 2017. Baseline anatomical assessment of the uterus and ovaries in infertile women: A systematic review of the evidence on which assessment methods are the safest and most effective in terms of improving fertility outcomes. Hum Reprod Update. 23(5):534–547. doi:10.1093/humupd/dmx019.
- ASRM Covid-19 Task Force. Patient management and clinical recommendations during the coronavirus (COVID-19) pandemic | ASRM [Internet]. [accessed 2021 Jan 14]. https://www.asrm.org/news-and-publications/covid-19/statements/patient-management-and-clinical-recommendations-during-the-coronavirus-covid-19-pandemic/
- Bortoletto P, Romanski PA, Stewart J, Schattman G, Rosenwaks Z. 2020. Universal severe acute respiratory syndrome coronavirus 2 (sars-cov-2) testing in a New York city reproductive medicine practice. Fertil Steril. 114(3):e17. doi:10.1016/j.fertnstert.2020.08.073.
- Choi B, Choudhary MC, Regan J, Sparks JA, Padera RF, Qiu X, Solomon IH, Kuo -H-H, Boucau J, Bowman K, et al. 2020. Persistence and evolution of SARS-CoV-2 in an immunocompromised host. N Engl J Med. 383(23):2291–2293. doi:10.1056/NEJMc2031364.
- De Santis L, Anastasi A, Cimadomo D, Klinger FG, Licata E, Pisaturo V, Sosa Fernandez L, Scarica C. 2020. COVID-19: the perspective of Italian embryologists managing the IVF laboratory in pandemic emergency. Hum Reprod. 35(4):1004–1005. doi:10.1093/humrep/deaa074.
- Dellino M, Minoia C, Paradiso AV, De Palo R, Silvestris E. 2020. Fertility preservation in cancer patients during the coronavirus (COVID-19) pandemic. Front Oncol. 10:1009. doi:10.3389/fonc.2020.01009.
- Foulk RA, Sakkas D, Kayali R, Valbuena D, Simon C, Cuzzi J. 2020. SARS-COV-2 herd immunity, infective and naïve incidence in fertility clinics after pandemic lockdown. A multicenter study. Fertil Steril. 114(3):e539. doi:10.1016/j.fertnstert.2020.09.069.
- Gandhi RT, Lynch JB, Del Rio C. 2020. Mild or moderate covid-19. Solomon CG, editor. N Engl J Med. 383:1757-1766 doi: 10.1056/NEJMcp2009249 2009249.
- Katz-Jaffe MG, Henry L, McCubbin N, Tucci R, Mann RS, McReynolds S, Schoolcraft WB. 2020. A comprehensive covid-19 risk mitigation strategy for safe patient care and staff wellness during a global pandemic. Fertil Steril. 114(3):e534–e535. doi:10.1016/j.fertnstert.2020.09.058.
- La Marca A, Niederberger C, Pellicer A, Nelson SM. 2020. COVID-19: lessons from the Italian reproductive medical experience. Fertil Steril. 113(5):920–922. doi:10.1016/j.fertnstert.2020.03.021.
- Prados N, González-Ravina C, Vergara V, Herrero J, Cruz M, Requena A. 2020. risk factor analysis for SARS-COV-2 seropositivity within assisted reproductive. Fertil Steril. 114(3):e542. doi:10.1016/j.fertnstert.2020.09.077.
- R Development Core Team. 2013. R: A language and environment for statistical computing. R Found Stat Comput Vienna Austria. 0:{ISBN} 3-900051-07–0.
- Robles A, Robles BN, Gemmell LC, Brady PC, Forman EJ, Williams Z. 2020. Universal screening oF COVID-19 in asymptomatic patients starting fertility treatment in New York city. Fertil Steril. 114(3):e179. doi:10.1016/j.fertnstert.2020.08.509.
- Romanski PA, Bortoletto P, Rosenwaks Z, Schattman GL. 2020. Delay in IVF treatment up to 180 days does not affect pregnancy outcomes in women with diminished ovarian reserve. Hum Reprod. 35(7):1630–1636. doi:10.1093/humrep/deaa137.
- Rosenberg ES, Tesoriero JM, Rosenthal EM, Chung R, Barranco MA, Styer LM, Parker MM, John Leung SY, Morne JE, Greene D, et al. 2020. Cumulative incidence and diagnosis of SARS-CoV-2 infection in New York. Ann Epidemiol. 48:23–29.e4. doi:10.1016/j.annepidem.2020.06.004.
- Seidman DS, Kahane A, Shulman A, Schiff E, Shavit T. 2020. Is universal screening of IVF patients for SARS-COV-2 justified? Fertil Steril. 114(3):e534. doi:10.1016/j.fertnstert.2020.09.056.
- Turner JS, Day A, Alsoussi WB, Liu Z, O’Halloran JA, Presti RM, Patterson BK, Whelan SPJ, Ellebedy AH, Mudd PA. 2021. SARS-CoV-2 Viral RNA shedding for more than 87 days in an individual with an impaired CD8+ T cell response. Front Immunol. 11:3597. doi:10.3389/fimmu.2020.618402.
- Widders A, Broom A, Broom J. 2020. SARS-CoV-2: the viral shedding vs infectivity dilemma. Infect Dis Heal. 25(3):210–215. doi:10.1016/j.idh.2020.05.002.
- Zambrano LD, Ellington S, Strid P, Galang RR, Oduyebo T, Tong VT, Woodworth KR, Nahabedian JF, Azziz-Baumgartner E, Gilboa SM, et al. 2020. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status — United STATES, January 22–October 3, 2020. MMWR Morb Mortal Wkly Rep. 69(44):1641–1647. doi:10.15585/mmwr.mm6944e3.
