ABSTRACT
The present study was designed to evaluate whether tissue preparation by glutaraldehyde and glycol methacrylate (G/GMA) improves the diagnostic assessment of testicular biopsies from azoospermic men when compared to the standard tissue preparation using Bouin’s solution and paraffin. We prospectively included a total of 21 testicular biopsies of sexually mature men aged 29–50 years with infertility and azoospermia. One testicular biopsy fragment from each patient was processed by the G/GMA method, whereas another tissue fragment was contemporarily processed by the conventional Bouin/paraffin (B/P) method. The G/GMA method provided better resolution of cytological details of the seminiferous epithelium, changing the final diagnosis in four cases. The medians of Bergmann’s spermatogenesis scores were 0.25 (interquartile range 0.04–0.88) for B/P preparations and 0.79 (interquartile range 0.17–0.96) for G/GMA preparations. Both techniques allowed accurate prediction of sperm recovery from the biopsies (B/P, area under the receiver operating characteristics [ROC] curve 0.88, 95% confidence interval [CI] 0.75–1.00; G/GMA, area under the ROC curve 0.94, 95% CI 0.86–1.00). We conclude that human testicular biopsy preparation with G/GMA improved image resolution under light microscopy and produced more reliable results for the evaluation of spermatogenesis in comparison with B/P, allowing a more precise fertility-oriented diagnosis in azoospermic men.
Abbreviations: B/P: Bouin/paraffin; GMA: glycol methacrylate; G/GMA: glutaraldehyde and glycol methacrylate; ICSI: intracytoplasmic sperm injection; OA: obstructive azoospermia; NOA: nonobstructive azoospermia; TESE: testicular sperm extraction.
Introduction
Azoospermia is characterized by the absence of sperm in the ejaculate and can be classified as obstructive (OA) or nonobstructive (NOA) (Puhse et al. Citation2011). Men with OA have the spermatogenesis preserved, and therefore can benefit from sperm retrieval by epididymal or testicular extraction with high success rates (Corona et al. Citation2019). In men with NOA, an accurate etiological diagnosis is needed to increase the chances of subsequent sperm recovery for intracytoplasmic sperm injection (ICSI). A precise diagnosis is also essential to allow the isolation and in vitro culture of germ cells ranging from spermatogonia to early round spermatids (Sato et al. Citation2011; Sharma et al. Citation2019; Gul et al. Citation2020). The etiological diagnosis of azoospermia is fully based on histopathological findings and thus requires a testicular biopsy (Cito et al. Citation2018; Eken and Gulec Citation2018; Gnessi et al. Citation2018; Toksoz and Kizilkan Citation2019).
Traditionally, testicular biopsy specimens have been fixed in Bouin’s solution or formalin and embedded in paraffin, producing histological sections of low microscopic resolution (Howroyd et al. Citation2005). However, studies in non-human primates have shown that processing testicular specimens with low-resolution methods compromises tissue morphology, introducing major errors in qualitative and quantitative assessments and reducing the reliability of the histological diagnosis (Ehmcke and Schlatt Citation2006).
We have proposed the histological processing of testes using glutaraldehyde for fixation and plastic resins such as glycol methacrylate (GMA) (Chiarini-Garcia et al. Citation2011) or epoxy resin (Chiarini-Garcia and Meistrich Citation2008) for embedding, which improves both tissue preservation and microscopic resolution. In rodents, these methods allowed the description of cytological details of the testicular parenchyma at the level of light microscopy, particularly with regard to the spermatogonial biology (Chiarini-Garcia and Russell Citation2001; Chiarini-Garcia et al. Citation2003). It has been known for decades that GMA embedding has advantages, such as fast processing, hydrosolubility, easy handling, infiltration and polymerization at room temperature, with the possibility of obtaining semi-thin sections (0.5 μm), less distortion and artifacts, and better resolution under light microscopy (Cole and Sykes Citation1974; Bennett et al. Citation1976; Woodruff and Greenfield Citation1979; Chiarini-Garcia et al. Citation2011, Citation2017). Despite such advantages, it is still unknown whether this method is appropriate for the histopathological examination of testicular biopsies in the context of male infertility.
Thus, the present study was designed to investigate whether tissue preparation by glutaraldehyde and glycol methacrylate (G/GMA) improves the qualitative and quantitative assessment of testicular biopsies from azoospermic men when compared to the standard tissue preparation with Bouin’s solution and paraffin (B/P) and if such methodology could be able to improve the diagnosis and classification of spermatogenesis in azoospermic men.
Results
Morphological features
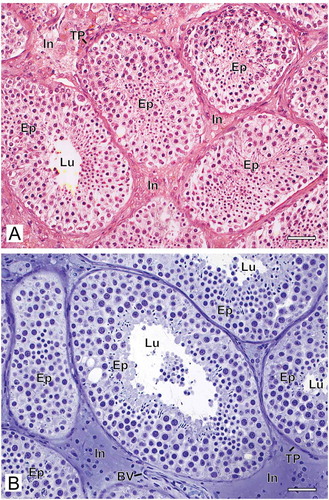
The method of histological preparation interfered directly with the morphological preservation of the testicular biopsies. When the samples were fixed with Bouin’s solution and embedded in paraffin, we observed tissue disorganization with retraction between the seminiferous tubules and poor distinction between the interstitial components. In general, the tissue was grainy with low definition of different structures and cell types ()). However, when the biopsy fragments were fixed with glutaraldehyde and embedded in GMA, the overall appearance of the tissue was quite different. The structures were better organized, with little tissue shrinkage, grain-free and well-defined cell types ()).
Figure 1. Photomicrographs at low magnification of human testicular biopsies. A) Bouin/paraffin (B/P) processing followed by hematoxylin & eosin staining. B) Glutaraldehyde/glycol methacrylate (G/GMA) processing followed by toluidine blue-sodium borate staining. Observe in the B/P processed sample (A) the reduced diameter of the seminiferous tubules as well as a poor morphological distinction between the different types of germ cells of the seminiferous epithelium (Ep) and the components of the interstitium (In) when compared to the processing with G/GMA (B). Lu: lumen; TP: tunica propria; BV: blood vessel. Bars: 50 μm
The nuclei and nucleoli of Sertoli cells were readily distinguished by their morphology after preparation in G/GMA ()), which was not easily observed in B/P sections ()). After B/P embedding, Sertoli cell nuclei were granular and irregularly stained without the typical folds of the nuclear envelope in addition to retraction of the cytoplasm and the nucleus.
Figure 2. Photomicrographs at higher magnification of human testicular biopsies. A,C,E,G) Glutaraldehyde/glycol methacrylate (G/GMA) processing followed by toluidine blue-sodium borate staining. B,D,F,H) Bouin/paraffin (B/P) processing followed by hematoxylin & eosin staining. After G/GMA (A), germ cells (Adark and Apale spermatogonia; P: pachytene spermatocyte; R: round spermatid; E: elongating spermatid) and somatic cells (S: Sertoli cell; My: myoid cell; Mc: mast cell) as well apoptosis (Ap) and tunica propria (TP) were promptly recognized. After B/P (B), it is difficult to confidently recognize different spermatogonial subtypes (Sg). Lu: lumen. The processes of mitosis (C,D) and apoptosis (E,F) were more easily recognized and the initial hyalinization of tunica propria (C,E, arrows) after G/GMA. After G/GMA (G), Leydig cells showed evident round nucleus and nucleolus and the cytoplasm was either less evident (L1) or full of lipid droplets (L2). These morphological details were not observed after processing with B/P (H). ST: seminiferous tubule; BV: blood vessel. Bars: A-B, 23 μm; C-D’, 10 μm; E-F, 15 μm
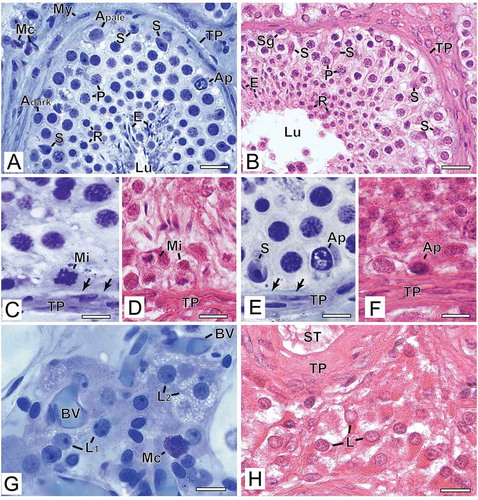
The germ cells identified with G/GMA showed a good definition of nuclear chromatin allowing safe characterization of spermatogonial subtypes, different steps of spermatocytes along the meiotic process and spermatid steps ()). Conversely, after B/P preparation, morphological details of the nucleus were barely visible; therefore, the distinction between the different types of germ cells was less precise. One feature that caught our attention was the retraction of the cytoplasm of germ cells that had empty space in the perinuclear region, suggesting cytoplasmic emptying ()). The artifact observed more frequently in biopsies embedded in B/P was the retraction of the seminiferous epithelium, leaving an empty space between Sertoli cells/germ cells and the basal lamina ()). This artifact was less frequent in samples prepared with G/GMA ()). The figures of spermatogonial mitosis ()) and germ cell apoptosis ()) were best visualized in the tissue embedded in G/GMA due to a better definition of the chromatin/nuclear morphology.
The tunica propria had no apparent morphological distinction between its components when visualized in B/P sections, but revealed its cytological details when observed in G/GMA specimens ()). In most patients, there was a hyalinized layer of variable thickness between myoid cells and the basal lamina of the seminiferous epithelium. When this layer was thin, it could only be observed in biopsies embedded in G/GMA ().
Leydig cells were well preserved with the cytological distinction between cytoplasm and nucleus after G/GMA embedding, and it was even possible to distinguish lipid droplets in their cytoplasm suggesting greater cell synthesis activity ()). Conversely, after B/P embedding, Leydig cell nucleus and cytoplasm were poorly visualized presenting a homogeneous appearance ()). Regarding blood vessels, they were well visualized with both methodologies.
Comparative analysis of spermatogenesis scores between the two methods
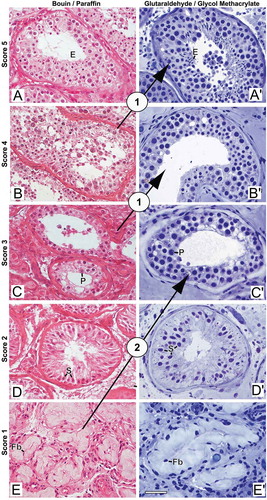
The G/GMA method provided better resolution of cytological details of the seminiferous epithelium, which were sufficient to cause changes in the diagnosis of azoospermia as compared to the B/P method, according to the score proposed by Levin (Levin Citation1979) and revised by McLachlan (McLachlan et al. Citation2007) (). After G/GMA processing, the diagnosis changed in four cases and all of them were upgraded. These diagnostic changes occurred in those patients in whom more advanced germ cells were rare and thus difficult to be recognized during histopathological evaluation in B/P. In two cases, after the diagnosis had been given as testicular fibrosis when the tissue was processed with B/P, it changed to maturation arrest, once rare early pachytene spermatocytes were found with G/GMA. In the other two cases, there was a change from maturation arrest to hypospermatogenesis and from hypospermatogenesis to full spermatogenesis. The patterns that did not change were preserved spermatogenesis and germ cell aplasia (Sertoli cell only). Quantitatively, these four cases of diagnostic change did not represent a statistically significant discordance between the two histological methods (McNemar test, p = 0.219).
Figure 3. Photomicrographs illustrating changes in spermatogenesis scoring. (A-E) Bouin/paraffin (B/P) processing followed by hematoxylin & eosin staining. (A’-E’) Glutaraldehyde/glycol methacrylate (G/GMA) processing followed by toluidine blue-sodium borate staining. The arrows show diagnostic changes that occurred when samples initially scored with B/P were reevaluated with G/GMA. Numbers inside the circles represent the number of cases with diagnostic change after rescoring. P: pachytene spermatocyte; E: elongating spermatid; S: Sertoli cell; Fb: fibroblast. Bar: 50 μm
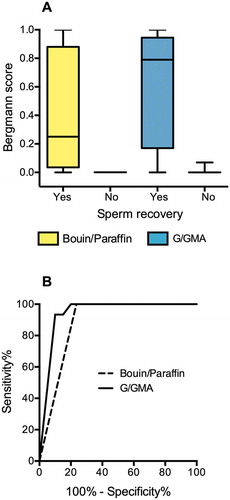
As shown in , the method of tissue preparation also made a difference in Bergmann’s score of spermatogenesis of patients who ultimately had sperm recovered from the testicular biopsy. The median scores were 0.25 (interquartile range 0.04–0.88) for B/P preparations and 0.79 (interquartile range 0.17–0.96) for G/GMA preparations ()). The Bergmann’s scores assigned to samples prepared with B/P correlated positively with their counterparts prepared with G/GMA, but the correlation was not perfect (Spearman's r = 0.84), as in four samples, there was an increase of the score when the material was evaluated by G/GMA. However, the accuracy to predict sperm recovery did not differ significantly between B/P (area under the curve 0.88, 95% CI 0.75–1.00) and G/GMA (area under the curve 0.94, 95% CI 0.86–1.00, )).
Figure 4. Quantitative Results. (A) Spermatogenesis scores assigned to paired fragments of testicular biopsies (n = 21) processed with Bouin/paraffin (B/P, yellow bars) or glutaraldehyde/glycol methacrylate (G/GMA, blue bars). The cases are divided according to the recovery of sperm for ICSI. (B) Receiver operating characteristics (ROC) curves of spermatogenesis score to predict sperm recovery for ICSI. The curves represent the scores assigned to biopsies processed by B/P or by G/GMA
Discussion
Testicular biopsy remains the standard test to characterize the severity of parenchymal damage and detect residual spermatogenesis in men with NOA, as well as to clarify the etiology of NOA and to predict sperm recovery for ICSI (Cito et al. Citation2018; Eken and Gulec Citation2018; Gnessi et al. Citation2018; Toksoz and Kizilkan Citation2019). However, the procedure is not free from risks (Janosek-Albright et al. Citation2015; Eliveld et al. Citation2018) and all efforts to maximize its accuracy through the best tissue handling are justified to extract accurate information from this invasive test. Here we show that tissue processing by G/GMA yields better images with higher cytological resolution and more precise diagnoses compared to B/P. Quantitatively, the Bergmann’s score of spermatogenesis of patients who ultimately had sperm recovered from the testicular biopsy was higher for G/GMA preparations than for B/P preparations. These results support the preferential use of G/GMA to process human testicular biopsies to optimize the histological diagnosis and to obtain a more faithful assessment of residual spermatogenesis in men with NOA.
Although the technique of tissue preparation with G/GMA is not new, historical reasons explain its underuse and the persistence of the B/P method in routine practice. Before the first report of a pregnancy obtained by ICSI with testicular sperm extraction (TESE) in 1993 (Schoysman et al. Citation1993), NOA was an intractable condition and the value of testicular biopsy to guide prognosis was less evident. Subsequently, the lack of clinical studies comparing G/GMA with the usual paraffin-based methods discouraged the use of G/GMA because it was considered more laborious and not clearly superior (Dajani Citation1998). Following the advancement of surgical and laboratory techniques, men with NOA now have a realistic perspective of biological parenthood since the live birth rates with TESE and ICSI approach 25% (Corona et al. Citation2019). Additionally, experimental studies have shown the possibility of obtaining sperm in vitro from spermatogonial culture (Sato et al. Citation2011; Sharma et al. Citation2019). Hence, when this method becomes standardized in human, the identification and isolation of few spermatogonial stem cells in NOA testicular biopsies will open the possibility of spermatogonial transplantation or its in vitro culture and further sperm production for use in ICSI (Gul et al. Citation2020). Considering this scenario, a high-quality histological preparation is needed for the precise identification of germ cells, improving diagnostic accuracy and avoiding ambiguity in the histopathological evaluation of testicular biopsy. Indeed, the present results show that approximately 20% of the cases were histologically reassigned by the pathologists using G/GMA sections, which had a better microscopic definition of the seminiferous epithelium compared to the ordinary B/P sections from the same biopsies.
Paraffin-embedding has the advantage of using xylene for section clearing, which permits the penetration of paraffin into the tissues. However, this organic solvent is toxic to cells. Moreover, all processing requires the use of high temperatures, which can damage the tissue cytoarchitecture (Amaral et al. Citation2004). In contrast, the hydrophilic GMA is easy to use, inexpensive, does not use xylene and the staining is done directly on the histological section (Chiarini-Garcia et al. Citation2011), being a viable method to be employed in histological evaluation of delicate structures, such as the seminiferous epithelium. Other studies corroborate our findings of the GMA employment for histopathological analysis (Cole and Sykes Citation1974; Bennett et al. Citation1976; Fox et al. Citation1985).
The type of fixative used affects the structural preservation of biopsies (Bennett et al. Citation1976; Chiarini-Garcia et al. Citation2017). Bouin is a coagulant fixative, consisting of a mixture of different chemical compounds, including picric acid that induces protein precipitation, resulting in tissue retraction, while being explosive (Latendresse et al. Citation2002; Singh et al. Citation2019; Ellenburg et al. Citation2020). In comparison, glutaraldehyde, a non-coagulant fixative, forms molecular bridges between its aldehyde groups and the amino groups of the proteins making them insoluble in the form of gel, besides reacting less intensely with lipids, carbohydrates and nucleic acids, thus being able to fix the cell as a whole (Chiarini-Garcia et al. Citation2011; Singh et al. Citation2019).
Despite all advantages in relation to morphological preservation, it is known that G/GMA has a restricted application to immunohistochemistry (Chiarini-Garcia et al. Citation2011), which is used in some cases to identify germ cell neoplasia in situ, to characterize immune infiltrations or to assess the maturation level of somatic cells (Xiao et al. Citation2017). Nevertheless, the major goal of assessing testicular biopsies for fertility evaluation is the identification of germ cells, followed by procedures to isolate them for in vitro manipulation.
In the present study, we analyzed the testicular biopsies using histopathological scores of spermatogenesis to compare the diagnostic classification yielded by the morphological features of the same samples processed by different techniques. In all analyses, testicular samples fixed with glutaraldehyde and embedded in GMA revealed more cytological details due to better preservation of the testicular structures compared to the material prepared with B/P, allowing more assertiveness in the histological diagnosis, especially to distinguish the limit between scores.
A strength of this study is the representation of NOA patients, with clinical characteristics typically found in this population, such as the common etiologies, low testicular volume and high serum FSH levels (Agarwal et al. Citation2020). However, the study has some limitations. We included participants with various causes of azoospermia and the sample size was not sufficient to allow the stratification of patients according to the etiologic agent of testicular damage. While we found consistent qualitative differences between the histological methods evaluated, further quantitative evidence from a larger number of patients is needed to assess more precisely the diagnostic performance of each method.
We, therefore, conclude that processing testicular biopsy specimens with G/GMA improves image resolution under light microscopy yielding accurate results in the evaluation of spermatogenesis. This method might be preferable for a fertility-oriented diagnosis in azoospermic men.
Materials and methods
We prospectively included a total of 21 testicular biopsies of sexually mature men aged 29–50 years (mean 36 ± 6 years). The patients were selected from different etiologies and their clinical characteristics are described in . The inclusion criteria were infertility, defined as failing to achieve a successful pregnancy after ≥12 months of regular unprotected intercourse, and azoospermia in at least two seminal analyses, defined as the absence of spermatozoa in ejaculated semen samples after specimen centrifugation and pellet examination (Practice Committee of the American Society for Reproductive, Citation2015). The causes of azoospermia were bilateral cryptorchidism (n = 3), mumps orchitis (n = 3), chemotherapy (n = 2), severe bilateral varicocele (n = 2), vasectomy (n = 2), testicular trauma (n = 1) or unknown etiology (n = 8). As expected, the testicular volume was ≤20 cm3 in 17 cases, and serum FSH levels were higher than 7.6 mIU/mL in 14 cases ().
Table 1. Clinical characteristics of the study participants
The biopsies were performed under spermatic cord block anesthesia as part of the routine care of infertile men with azoospermia, and the tissue samples, measuring approximately 10 × 6 mm in size, were cut longitudinally into two fragments.
One testicular biopsy fragment from each patient was processed by the conventional B/P method. This tissue fragment was routinely fixed in Bouin’s solution for 24 hours and embedded longitudinally in paraffin. Five sections of 5 µm thickness obtained semi-serially (depth of 125 µm) were stained with hematoxylin-eosin. The other tissue fragment was processed by the G/GMA method as described elsewhere (Chiarini-Garcia et al. Citation2011). Succinctly, the tissue was fixed with 5% glutaraldehyde (EMS, biological grade) in 0.05 M phosphate buffer pH 7.3 for 24 hours at 4◦C, and embedded in plastic GMA resin (Historesin, Leica). This fragment was positioned facing exactly in the same cutting position as the one embedded in B/P. Five sections of 3 µm thickness, obtained semi-serially (depth of 75 µm) were stained with toluidine blue-sodium borate. The staining techniques used were the most appropriate for each type of embedding medium (Chiarini-Garcia et al. Citation2011).
The tissue sections prepared by both methods were evaluated by two examiners in an independent and blind way. All sections were evaluated initially with 200x magnification for a general description of the testicular parenchyma and subsequently with 400x magnification for a more detailed study of the seminiferous epithelium cells. Spermatogenesis was classified into five patterns according to the score proposed by Levin (Levin Citation1979) and revised by McLachlan (McLachlan et al. Citation2007). The five patterns represent: normal testicular biopsy (score 5, full spermatogenesis and normal inter-tubular tissue), hypospermatogenesis (score 4, reduced spermatogenesis to a varying degree, but indicating the capacity to generate sperm), germ cell arrest (score 3, total arrest at a particular stage, mainly at the spermatogonial or primary spermatocyte stage), Sertoli cell-only (score 2, absence of germ cells) and fibrosis (score 1, absence of germ and Sertoli cells and seminiferous tubule hyalinization). Additionally, we applied Bergmann’s score which assesses changes in spermatogenesis in relation to the presence of elongated spermatids in the testicular parenchyma (Bergmann et al. Citation1994). The latter method is valuable in clinical practice when the goal is to find sperm in the testes for use in ICSI (Zitzmann et al. Citation2006).
Statistics
The spermatogenesis scores departed from a normal distribution (D’Agostino-Pearson test) and therefore are summarized as medians and interquartile intervals. Linear correlations were tested with Spearman's rank correlation coefficient. The accuracy of spermatogenesis scores to predict subsequent sperm recovery for ICSI was evaluated by receiver operating characteristics (ROC) curves by calculating the area under the curves with a 95% confidence interval (CI).
Ethics approval
This study was approved by the Ethics Committee in Human Research from Universidade Federal de Minas Gerais (nº ETIC 032/2004) and a written informed consent was obtained from all participants. The investigation was conducted in accordance with the Declaration of Helsinki of 1975.
Authors’ Contributions
Designed the study and revised the manuscript: HCG, EST selected and operated the patients : ABR, RM performed the histological diagnoses: ABR, PGS; analyzed the images and produced the figures: FRCLA, WAA, ALCB analyzed the quantitative results and drafted the manuscript: ABR, FMR. All authors reviewed and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Agarwal A, Baskaran S, Parekh N, Cho CL, Henkel R, Vij S, … Shah R. 2020. Male infertility. Lancet. doi:10.1016/s0140-6736(20)32667-2
- Amaral D, Chiarini-Garcia H, Vale Filho VR, Allen WR. 2004. Effect of formalin and bouin fixation upon the mare’s endometrial biopsies embedded in plastic resin. Arquivo Brasileiro De Medicina Veterinária E Zootecnia. 56:07–12.
- Bennett HS, Wyrick AD, Lee SW, McNeil JH. 1976. Science and art in preparing tissues embedded in plastic for light microscopy, with special reference to glycol methacrylate, glass knives and simple stains. Stain Technol. 51(2):71–97. doi:10.3109/10520297609116677.
- Bergmann M, Behre HM, Nieschlag E. 1994. Serum FSH and testicular morphology in male infertility. Clin Endocrinol (Oxf). 40(1):133–136. doi:10.1111/j.1365-2265.1994.tb02455.x.
- Chiarini-Garcia H, Lima MY, Reis AB, Martello R, Nihi F, Gomes ML, Almeida FR. 2017. Influence of three different histological methods on the morphology and morphometrical data in human testis. Histol Histopathol. 32(1):27–34. doi:10.14670/HH-11-765.
- Chiarini-Garcia H, Meistrich ML. 2008. High-resolution light microscopic characterization of spermatogonia. Methods Mol Biol. 450:95–107. doi:10.1007/978-1-60327-214-8_6
- Chiarini-Garcia H, Parreira GG, Almeida FR. 2011. Glycol methacrylate embedding for improved morphological, morphometrical, and immunohistochemical investigations under light microscopy: testes as a model. Methods Mol Biol. 689:3–18. doi:10.1007/978-1-60761-950-5_1
- Chiarini-Garcia H, Raymer AM, Russell LD. 2003. Non-random distribution of spermatogonia in rats: evidence of niches in the seminiferous tubules. Reproduction. 126(5):669–680. doi:10.1530/rep.0.1260669.
- Chiarini-Garcia H, Russell LD. 2001. High-resolution light microscopic characterization of mouse spermatogonia. Biol Reprod. 65(4):1170–1178. doi:10.1095/biolreprod65.4.1170.
- Cito G, Coccia ME, Dabizzi S, Morselli S, Della Camera PA, Cocci A, … Natali A. 2018. Relevance of testicular histopathology on prediction of sperm retrieval rates in case of non-obstructive and obstructive azoospermia. Urologia. 85(2):60–67. doi:10.1177/0391560318758940.
- Cole MB Jr., Sykes SM. 1974. Glycol methacrylate in light microscopy: a routine method for embedding and sectioning animal tissues. Stain Technol. 49(6):387–400. doi:10.3109/10520297409117016.
- Corona G, Minhas S, Giwercman A, Bettocchi C, Dinkelman-Smit M, Dohle G, … Sofikitis N. 2019. Sperm recovery and ICSI outcomes in men with non-obstructive azoospermia: a systematic review and meta-analysis. Hum Reprod Update. 25(6):733–757. doi:10.1093/humupd/dmz028.
- Dajani YF. 1998. Testicular biopsy: an update. Curr Diagn Pathol. 5(1):17–22. doi:10.1016/S0968-6053(98)80030-6.
- Ehmcke J, Schlatt S. 2006. A revised model for spermatogonial expansion in man: lessons from non-human primates. Reproduction. 132(5):673–680. doi:10.1530/rep.1.01081.
- Eken A, Gulec F. 2018. Microdissection testicular sperm extraction (micro-TESE): predictive value of preoperative hormonal levels and pathology in non-obstructive azoospermia. Kaohsiung J Med Sci. 34(2):103–108. doi:10.1016/j.kjms.2017.08.010.
- Eliveld J, Van Wely M, Meer A, Repping S, Van Der Veen F, Van Pelt AMM. 2018. The risk of TESE-induced hypogonadism: a systematic review and meta-analysis. Hum Reprod Update. 24(4):442–454. doi:10.1093/humupd/dmy015.
- Ellenburg JL, Kolettis P, Drwiega JC, Posey AM, Goldberg M, Mehrad M, … Gordetsky J. 2020. Formalin versus bouin solution for testis biopsies: which is the better fixative? Clin Pathol. 13:2632010X19897262. doi:10.1177/2632010X19897262
- Fox CH, Johnson FB, Whiting J, Roller PP. 1985. Formaldehyde fixation. J Histochem Cytochem. 33(8):845–853. doi:10.1177/33.8.3894502.
- Gnessi L, Scarselli F, Minasi MG, Mariani S, Lubrano C, Basciani S, … Greco E. 2018. Testicular histopathology, semen analysis and FSH, predictive value of sperm retrieval: supportive counseling in case of reoperation after testicular sperm extraction (TESE). BMC Urol. 18(1):63. doi:10.1186/s12894-018-0379-7.
- Gul M, Hildorf S, Dong L, Thorup J, Hoffmann ER, Jensen CFS, … Goossens E. 2020. Review of injection techniques for spermatogonial stem cell transplantation. Hum Reprod Update. 26(3):368–391. doi:10.1093/humupd/dmaa003.
- Howroyd P, Hoyle-Thacker R, Lyght O, Williams D, Kleymenova E. 2005. Morphology of the fetal rat testis preserved in different fixatives. Toxicol Pathol. 33(2):300–304. doi:10.1080/01926230590896145.
- Janosek-Albright KJC, Schlegel PN, Dabaja AA. 2015. Testis sperm extraction. Asian J Urol. 2(2):79–84. doi:10.1016/j.ajur.2015.04.018.
- Latendresse JR, Warbrittion AR, Jonassen H, Creasy DM. 2002. Fixation of testes and eyes using a modified davidson’s fluid: comparison with bouin’s fluid and conventional davidson’s fluid. Toxicol Pathol. 30(4):524–533. doi:10.1080/01926230290105721.
- Levin HS. 1979. Testicular biopsy in the study of male infertility: its current usefulness, histologic techniques, and prospects for the future. Hum Pathol. 10(5):569–584. doi:10.1016/s0046-8177(79)80100-8.
- McLachlan RI, Rajpert-De Meyts E, Hoei-Hansen CE, De Kretser DM, Skakkebaek NE. 2007. Histological evaluation of the human testis–approaches to optimizing the clinical value of the assessment: mini review. Hum Reprod. 22(1):2–16. doi:10.1093/humrep/del279.
- Practice Committee of the American Society for Reproductive, M. 2015. Diagnostic evaluation of the infertile male: a committee opinion. Fertil Steril. 103(3):e18–25. doi:10.1016/j.fertnstert.2014.12.103.
- Puhse G, Hense J, Bergmann M, Kliesch S. 2011. Bilateral histological evaluation of exocrine testicular function in men with obstructive azoospermia: condition of spermatogenesis and andrological implications? Hum Reprod. 26(10):2606–2612. doi:10.1093/humrep/der257.
- Sato T, Katagiri K, Gohbara A, Inoue K, Ogonuki N, Ogura A, … Ogawa T. 2011. In vitro production of functional sperm in cultured neonatal mouse testes. Nature. 471(7339):504–507. doi:10.1038/nature09850.
- Schoysman R, Vanderzwalmen P, Nijs M, Segal L, Segal-Bertin G, Geerts L, … Schoysman D. 1993. Pregnancy after fertilisation with human testicular spermatozoa. Lancet. 342(8881):1237. doi:10.1016/0140-6736(93)92217-h.
- Sharma S, Wistuba J, Pock T, Schlatt S, Neuhaus N. 2019. Spermatogonial stem cells: updates from specification to clinical relevance. Hum Reprod Update. 25(3):275–297. doi:10.1093/humupd/dmz006.
- Singh H, Bishen KA, Garg D, Sukhija H, Sharma D, Tomar U. 2019. Fixation and fixatives: roles and functions—a short review. Dental J Adv Stud. 7(2):51–55. doi:10.1055/s-0039-1693098.
- Toksoz S, Kizilkan Y. 2019. Comparison of the histopathological findings of testis tissues of non-obstructive azoospermia with the findings after microscopic testicular sperm extraction. Urol J. 16(2):212–215. doi:10.22037/uj.v16i2.4756.
- Woodruff JM, Greenfield SA. 1979. Advantages of glycol methacrylate embedding systems for light microscopy. J Histotechnol. 2(4):164–167. doi:10.1179/his.1979.2.4.164.
- Xiao X, Hu R, Deng FM, Shen SS, Yang XJ, Wu CL. 2017. Practical applications of immunohistochemistry in the diagnosis of genitourinary tumors. Arch Pathol Lab Med. 141(9):1181–1194. doi:10.5858/arpa.2016-0530-RA.
- Zitzmann M, Nordhoff V, Von Schonfeld V, Nordsiek-Mengede A, Kliesch S, Schuring AN, … Nieschlag E. 2006. Elevated follicle-stimulating hormone levels and the chances for azoospermic men to become fathers after retrieval of elongated spermatids from cryopreserved testicular tissue. Fertil Steril. 86(2):339–347. doi:10.1016/j.fertnstert.2005.12.058.
