ABSTRACT
Y‑autosome translocations are relatively uncommon in humans, with t(Y;1) stated to be even rarer. On the contrary, pericentric inversion 9 is the most commonly seen inversion of chromosome . Although considered to have no significant effect on male fertility, the literature reporting on reproductive risks for both aberrations remains controversial. We report here, as far as we know, the first case of a unique combination of balanced reciprocal translocation t(Y;1) with pericentric inversion of chromosome 9 in a patient with nonobstructive azoospermia (NOA) and an otherwise normal phenotype. Our patient was a 37-year-old Caucasian male sent to our Department due to azoospermia reported by semen analysis. The cytogenetic analysis revealed a balanced reciprocal translocation including chromosomes Y and 1 in all observed metaphases: 46, X,t(Y;1)(q12;q21) and a pericentric inversion of chromosome 9: inv(9)(p12q13). By performing metaphase FISH, the t(Y;1) translocation was confirmed. By means of multiplex-PCR, no Y-chromosome microdeletions were detected in the AZF regions. This report demonstrates a unique karyotype showing balanced reciprocal translocation t(Y;1)(q12;q21) with pericentric inversion 9: inv(9)(p12q13), in a patient with NOA, and highlights the importance of appropriate genetic counseling for patients with regard to the medical management of balanced chromosomal aberrations.
INTRODUCTION
Over the last few decades, male infertility and the declining trend of semen quality have been receiving increased attention. The social and economic burden of male infertility is enormous (Winters and Walsh Citation2014; Hauser et al. Citation2015). Nonobstructive azoospermia (NOA) is considered the most serious form of male infertility, affecting approximately 10% of infertile men worldwide (Cerván-Martín et al. Citation2020). It refers to a complete absence of sperm in the ejaculate and has an important genetic contribution (Cocuzza et al. Citation2013). It is well known that the cytogenetic abnormalities might interfere with the spermatogenesis process. The most common causes are sex chromosome aneuploidies, especially Klinefelter syndrome (47, XXY). Other structural chromosome aberrations, including rearrangements between sex chromosomes and autosomes, can also lead to NOA (Skakkebaek et al. Citation2016; Cerván-Martín et al. Citation2020).
Y‑autosome translocations are relatively uncommon in humans (~1 in 2000), with t(Y;1) stated to be even rarer (Powell Citation2005). Apart from some special cases correlated with (sub)fertile phenotypes, Y–autosome translocations are generally associated with male infertility (Harton and Tempest Citation2012). It has been hypothesized that males are infertile when the break occurs in the azoospermia factor (AZF) region of Yq11, while the breaks in the Yq12 heterochromatic region seemed to have no significant effect (Vogt and Fernandes Citation2003). Nevertheless, it has been reported that translocations including a breakpoint in the Yq12 heterochromatic region can also lead to male infertility (Delobel et al. Citation1998). Precisely, they are related to oligospermia and azoospermia, especially in Y:autosome translocations carriers (Braun-Falco et al. Citation2007; Carrell Citation2007). Besides, the integrity of chromosome 1 is equally important for male fertility (Bache et al. Citation2004). Interestingly, depending on the position of the breakpoint, two groups of infertility are distinguished: pre-gestational and gestational. The break at 1q21, reported in our case, is clinically manifested as pre-gestational infertility, characterized by the inability of the couple to conceive (Wang et al. Citation2016).
Regardless of being classified as a minor chromosomal rearrangement that carries no additional reproductive risk (Merrion and Maisenbacher Citation2019), many reports indicate that patients with pericentric inversion 9 may produce abnormal gametes, consequently leading to infertility (Sasagawa et al. Citation1998; Collodel et al. Citation2006; Mozdarani et al. Citation2007). This can be explained by disruptions in spermatogenesis that have arisen from the acentric fragments or loops created in meiosis (Mozdarani et al. Citation2007).
We report here, as far as we know, the first case of a unique combination of balanced reciprocal translocation t(Y;1)(q12;q21) with pericentric inversion of chromosome 9: inv(9)(p12q13) in a patient with NOA.
CASE REPORT
A 37-year-old Caucasian male, 185 cm in height, ABRh+, from the Southern Dalmatia region of Croatia, was sent to our Department because of azoospermia reported by semen analysis. Written informed consent and a detailed questionnaire were obtained from the patient. The patient’s family medical history included no cases of infertility. His history, physical examination, and habits, except smoking half a pack of cigarettes daily, were unremarkable. Ultrasound examination of the genital–urinary system showed normal results.
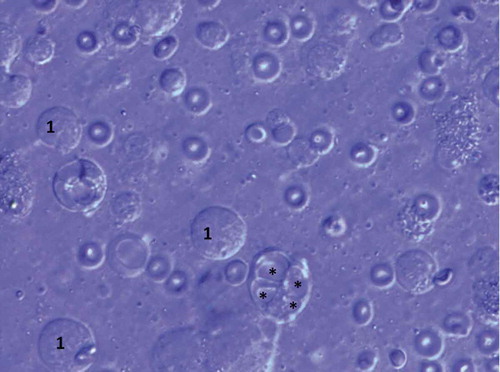
The spermiogram, natively and after centrifugation, revealed azoospermia, with regular semen volume and pH. The biochemical, serological, and hematological analyses were within expected values. Hormone serum levels were: FSH 11.5 IU/L (normal 1.3–8.1), LH 3.7 IU/L (1.0–5.3), testosterone 11.4 nmol/L (9.3–35.4), estradiol 0.112 nmol/L (0.03–0.24) and prolactin 130 mIU/L (53–462). The unilateral (right) diagnostic microdissection testicular sperm extraction (micro-TESE) was undertaken at 26x magnification and exhibited a homogenous picture of thick, opaque seminiferous tubules. After sampling, 53 specimens were macerated and scanned under magnification 400–1000x. All samples showed similar results: seminiferous tubules filled with immature germ cells degenerated by vacuolization and Sertoli cells. Various lipid inclusions were visible. No viable sperm were found ( and ).
Figure 1. Morphology of testicular germ cells showing a cluster of immature germ cells, Sertoli cells (S), and various lipid inclusions (*) (unstained). Olympus IX71 inverted microscopy (400X)
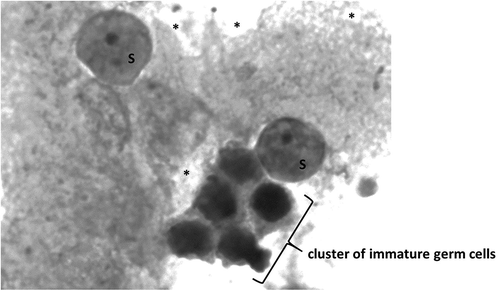
Figure 2. Morphology of testicular germ cells showing primary spermatocytes (1) and vacuolar degeneration (*) (stained with Giemsa). Olympus BX41 microscopy (1000X)
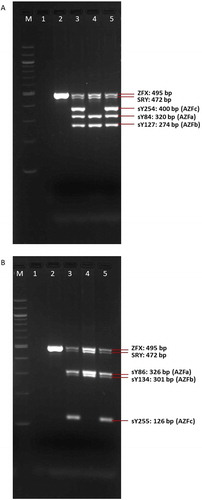
The cytogenetic analysis (G-banding) revealed a balanced reciprocal translocation between chromosomes Y and 1 in all observed metaphases: 46, X,t(Y;1)(q12;q21) and a pericentric inversion of chromosome 9: inv(9)(p12q13) (). By performing metaphase FISH, the t(Y;1) translocation was confirmed, including the integrity of telomeres and centromeres. Commercial TOTELVysionTM Multicolor DNA Probes for chromosomes 1, X and Y, CEP 18 SpectrumAqua/X SpectrumGreen/Y SpectrumOrange (Vysis®, Abbott Laboratories, Abbott Park, Illinois, U.S.A.), and Kreatech KBI-20,001 SE 1 (1qh) probe (Leica Biosystems Division of Leica Microsystems Inc., 1700 Leider Lane, Buffalo Grove, U.S.A.) were used ( and ). By means of multiplex-PCR, no Y-chromosome microdeletions were detected in the AZFa (sY84, sY86), AZFb (sY127, sY134), and AZFc (sY254, sY255) regions ().
Figure 3. Karyotype of the patient showing 46,X,t(Y;1)(q12;q21) and a pericentric inversion of chromosome 9: inv (9)(p12;q13)
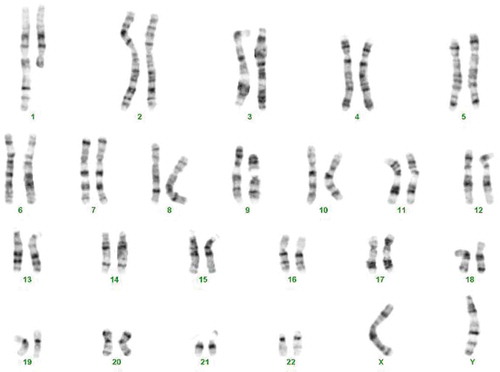
Figure 4. Metaphase FISH showing: der(1) with an intact 1p short arm and a positive signal for the 1p telomere (green); der(Y) with positive signals for the Yp telomere (yellow) and Y centromere (red), followed by the 1q long arm containing the 1q telomere (red); 1 with positive signals for the 1p (green) and 1q telomeres (red); X with positive signals for the Xp telomere (green) and X centromere (green); two chromosomes 18 with positive signals for centromere (aqua). DNA is stained blue with DAPI
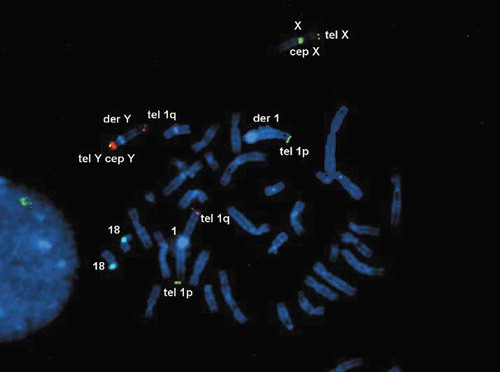
Figure 5. Metaphase FISH showing: der(1) with a positive signal for the 1 centromere (red); 1 with a positive signal for the 1 centromere (red). DNA is stained blue with DAPI
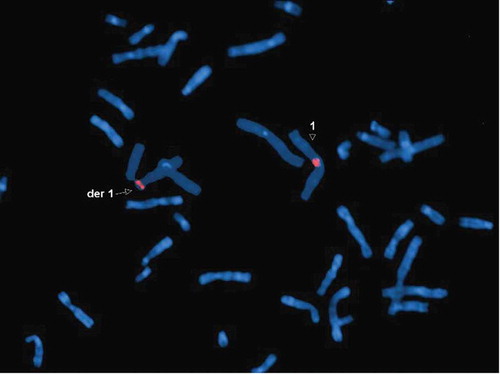
Figure 6. The results of multiplex-PCR-A (A) and multiplex-PCR-B (B) in control subjects and the patient showing intactness of Yp (SRY) and absence of Yq11.2 microdeletions in the AZFa, AZFb, and AZFc regions. M: marker (100 bp); 1: negative control; 2: control female; 3: control fertile male; 4: control infertile male (microdeletions in region AZFc – negative STSs sY254 and sY255); 5: patient
In accordance with the results of the tests, the couple was counseled to undergo assisted reproductive treatment to conceive using a sperm donor as the wife was found to be healthy.
DISCUSSION
Our case is, as far as we know, the first reported case of balanced reciprocal translocation t(Y;1)(q12;q21) combined with pericentric inversion 9: inv(9)(p12q13) in an infertile male presenting with NOA. Among previously described cases with a t(Y;1) translocation related to male infertility (Aftab et al. Citation2019), none included the Yq12 and 1q21 bands. Moreover, a patient’s karyotype showed pericentric inversion on human chromosome 9 that can also produce gametes with chromosomal imbalance (Muthuvel et al. Citation2016; McKinlay Gardner et al. Citation2018). Nevertheless, no microdeletions were detected in the AZFa, AZFb, and AZFc regions, and SRY in Yp was intact. On this basis, the authors propose that NOA reflects the combination of reciprocal t(Y;1) translocation and pericentric inversion 9.
In the general population, reciprocal translocations between the Y chromosome and non-acrocentrics, including chromosome 1, are rarely observed. However, they are more common in infertile men (Delobel et al. Citation1998). Besides, the reported rate of balanced reciprocal translocation in couples with reproductive failure is the highest on chromosome 1 (Wang et al. Citation2016). This is supported by an analysis of published literature that shows a strong correlation between chromosome 1 translocation carriers and reproductive failure, azoospermia, and male infertility (Bache et al. Citation2004). The detected translocation of the 1q segment to the Yq may disrupt the process of X–der(Y) and 1-der(1) chromosome pairing, crossing over and segregation, thereby leading to impaired sperm production and degeneration of spermatocytes by apoptosis (Pinho et al. Citation2005).
In the current case, the breakpoint occurred in the 1q21, which represents a significant cluster on chromosome 1. Also, this band is associated with pre-gestational infertility, along with the breaks at 1p13 and 1q12 (Wang et al. Citation2016). According to the Ensembl database and available literature, HORMAD1 (HORMA domain containing 1) gene, located on 1q21.3, is listed as a prime candidate toward a marker panel for male fertility impairment (class A candidate marker) (Ensembl Citation2020; Greither et al. Citation2020). Interestingly, HORMAD1 plays a key role in meiotic prophase quality control in both sexes. It regulates three different functions during meiosis: (I) ensures availability of sufficient numbers of DNA double-strand breaks, (II) promotes synaptonemal-complex formation, and (III) plays a key role in meiotic silencing of unsynapsed chromatin (MSUC) (GeneCards Citation1996). Taking into consideration the important role of other genes in 1q21 for normal cell functioning, altered gene expression could occur due to repositioning, which may lead to aberrant fertility. Altered expression of a gene when its chromosomal location is changed though retaining an intact transcription unit is known as the term ‘position effect.’ In this case, several mechanisms could explain gene malfunctioning: (I) inappropriate activation or silencing the gene caused by the separation of the promoter/transcription unit apart from acting regulatory elements, (II) inadequate gene expression caused by the juxtaposition of the gene with an enhancer from another gene, (III) inadequate locus shutting caused by elimination of a long-range insulator; (IV) reduction in gene expression caused by gene competition for the regulatory element; (V) silencing the gene by the juxtaposition of a euchromatic with a heterochromatic region (classical position effect variegation) (Kleinjan and Van Heyningen Citation1998; Wang et al. Citation2020).
Moreover, Y breakpoints, even the ones that occur outside the AZF region, are known to cause male infertility. According to the earlier reports on Y-autosome translocations involving the breakpoint in the observed Yq12, it is suggested that break in this heterochromatic region leads to oligospermia and azoospermia, irrespective of the autosome included in the translocation (Matsuda et al. Citation1989; Pinho et al. Citation2005; Braun-Falco et al. Citation2007; Yumura et al. Citation2012; Ghevaria et al. Citation2017).
Furthermore, pericentric inversion 9 is the most common inversion seen in humans, with an overall frequency of approximately 1.6% (Šípek et al. Citation2015). Although this region is inert in nature, reproductive disorders in some carriers have been reported. This can be explained by a disruption in meiosis I that may result in unbalanced gametes (Pinho et al. Citation2005). Disruptions arise during crossing over from the formation of recombinant chromosomes between the normal and inverted homolog of chromosome 9 (McKinlay Gardner et al. Citation2018), leading to early miscarriages or live births with various anomalies. However, literature reporting on reproductive risks associated with this population variant is controversial (Merrion and Maisenbacher Citation2019). Regardless, when combined with the aforementioned translocation, it should be considered as an additional predisposing factor for infertility.
For such patients, it is of high importance to receive appropriate genetic counseling with regard to the medical management of the aforementioned types of chromosomal aberrations. After receiving genetic counseling, donor spermatozoon with in vitro fertilization is a recommended technique.
In conclusion, we have demonstrated a unique karyotype showing balanced reciprocal translocation t(Y;1)(q12;q21) with pericentric inversion 9: inv(9)(p12q13) in a patient with NOA and an otherwise normal phenotype. Authors propose that NOA, as a clinical manifestation of spermatogenesis arrest at meiosis I, is effectively caused by the combination of these two balanced chromosomal rearrangements. Moreover, the importance of appropriate genetic counseling for patients regarding the medical management of balanced chromosomal aberrations is highlighted.
Ethical approval
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from the patient before inclusion in the study.
Guidelines
Our report is in accordance with the CARE guideline available through the EQUATOR network (http://www.equator-network.org/) and COPE guidelines (http://publicationethics.org/).
Author's contributions
Conceptualization, methodology, critical review and editing of the draft, supervision: JV. Performing the experiments, data analysis: AB, NSČ, PR, participating in study design, validation, and revising the manuscript: ABT, SO. Writing – original draft: AB. All authors read and approved the final manuscript.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
The authors are thankful for the cooperation of the patient and his family. Besides, they would like to express sincere gratitude to all laboratory technicians at the Department of Medical Biology and Genetics for their technical support.
Disclosure statement
The authors declare that they have no conflict of interest.
Additional information
Funding
References
- Aftab A, T R V, Kar B. 2019. A rare case of de novo balanced reciprocal Y:1 chromosomal translocation in patient presenting with azoospermia. Andrologia 51(4):e13246. doi:10.1111/and.13246.
- Bache I, Assche EV, Cingoz S, Bugge M, Tümer Z, Hjorth M, Lundsteen C, Lespinasse J, Winther K, Niebuhr A, et al. 2004. An excess of chromosome 1 breakpoints in male infertility. Eur J Hum Genet. 12(12):993–1000. doi:10.1038/sj.ejhg.5201263
- Braun-Falco M, Schempp W, Nevinny-Stickel-Hinzpeter C, Köhn FM. 2007. Azoospermia due to a unique de novo balanced reciprocal translocation (Y;1) (q12;q25). J Androl. 28(5):647–651.
- Carrell DT. 2007. The genetics of male infertility. In. In: Carrell DT, editor. The genetics of male infertility. 1st ed ed. New York (NY): Humana Press; p. 3–27.
- Cerván-Martín M, Castilla C, Palomino-Morales P-M, Carmona C. 2020. Genetic Landscape of Nonobstructive Azoospermia and New Perspectives for the Clinic. Journal of Clinical Medicine. 9(2):300. doi:10.3390/jcm9020300.
- Cocuzza M, Alvarenga C, Pagani R 2013. The epidemiology and etiology of azoospermia. Clinics (Sao Paulo) 68 Suppl 68 Suppl S1:15–26. 10.6061/clinics/2013(Sup01)03
- Collodel G, Moretti E, Capitani S, Piomboni P, Anichini C, Estenoz M, Baccetti B. 2006. TEM, FISH and molecular studies in infertile men with pericentric inversion of chromosome 9. Andrologia 38(4):122–127. doi:10.1111/j.1439-0272.2006.00725.x.
- Delobel B, Djlelati R, Gabriel-Robez O, Croquette M-F, Rousseaux-Prevost R, Rousseaux J, Rigot J-M, Rumpler Y. 1998. Y-autosome translocation and infertility: usefulness of molecular, cytogenetic and meiotic studies. Human Genetics. 102(1):98–102. doi:10.1007/s004390050660.
- Ensembl. 2020. EMBL-EBI; [ accessed 2021 Jan 11]. https://www.ensembl.org/index.html.
- The GeneCards human gene database. 1996-. Release 5.0. Rehovot (Israel): Weizmann Institute of Science; [ accessed 2021 Jan 11]. https://www.genecards.org/
- Ghevaria H, Naja R, SenGupta S, Serhal P, Delhanty J. 2017. Meiotic outcome in two carriers of Y autosome reciprocal translocations: selective elimination of certain segregants. Molecular Cytogenetics. 10(1):1. doi:10.1186/s13039-017-0303-y.
- Greither T, Schumacher J, Dejung M, Behre H, Zischler H H, Butter F, Herlyn H. 2020. Fertility relevance probability analysis shortlists genetic markers for male fertility impairment. Cytogenetic and Genome Research. 160(9):506–522. doi:10.1159/000511117.
- Harton GL, Tempest HG. 2012. Chromosomal disorders and male infertility. Asian Journal of Andrology. 14(1):32–39. doi:10.1038/aja.2011.66.
- Hauser R, Skakkebaek NE, Hass U, Toppari J, Juul A, Andersson AM, Kortenkamp A, Heindel JJ, Trasande L. 2015. Male reproductive disorders, diseases, and costs of exposure to endocrine-disrupting chemicals in the European Union. The Journal of Clinical Endocrinology & Metabolism. 100(4):1267–1277. doi:10.1210/jc.2014-4325.
- Kleinjan D, Van Heyningen V. 1998. Position Effect in Human Genetic Disease. In: Human Molecular Genetics. 7(10):1611–1618.
- Matsuda T, Hayashi K, Nonomura M, Yamamoto S, Yoshida O. 1989. Azoospermic male with a balanced Y-autosome translocation. Urologia Internationalis. 44(1):43–46. doi:10.1159/000281450.
- McKinlay Gardner RJ, Amor DJ, McKinlay Gardner RJ, Amor DJ. 2018. Inversions. In: McKinlay Gardner RJ, Amor DJ, editors. Gardner and ’ ‘Sutherland’s Chromosome Abnormalities and Genetic Counseling. 5 ed ed. Oxford (UK: UK): Oxford University Press; p. 164–170.
- Merrion K, Maisenbacher M. 2019. Pericentric inversion (Inv) 9 variant—reproductive risk factor or benign finding? Journal of Assisted Reproduction and Genetics. 36(12):2557–2561. doi:10.1007/s10815-019-01601-y.
- Mozdarani H, Meybodi AM, Karimi H. 2007. Impact of pericentric inversion of Chromosome 9 [inv (9) (p11q12)] on infertility. Indian Journal of Human Genetics. 13(1):26–29. doi:10.4103/0971-6866.32031.
- Muthuvel A, Ravindran M, Chander A, Subbian C. 2016. Pericentric inversion of chromosome 9 causing infertility and subsequent successful in vitro fertilization. Nigerian Medical Journal. 57(2):142–144. doi:10.4103/0300-1652.182080.
- Pinho MJ, Neves R, Costa P, Ferrás C, Sousa M, Alves C, Almeida C, Fernandes S, Silva J, Ferrás L, et al. 2005. Unique t(Y;1)(q12;q12) reciprocal translocation with loss of the heterochromatic region of chromosome 1 in a male with azoospermia due to meiotic arrest: a case report. Human Reproduction. 20(3):689–696. doi:10.1093/humrep/deh653
- Powell CM. 2005. Sex Chromosomes and Sex Chromosome Abnormalities. In. In: Gersen SL, Keagle MB, editors. The Principles of Clinical Cytogenetics. 2nd ed ed. New York NY: Humana Press; p. 207–208.
- Sasagawa I, Ishigooka M, Kubota Y, Tomaru M, Hashimoto T, Nakada T. 1998. Pericentric inversion of chromosome 9 in infertile men. International Urology and Nephrology. 30(2):203–207. doi:10.1007/BF02550578.
- Šípek ŠA, Panczak A, Mihalová R, Hrčková L, Suttrová E, Sobotka V, Lonský P, Kaspříková N, Gregor V. 2015. Pericentric Inversion of Human Chromosome 9 Epidemiology Study in Czech Males and Females. Folia Biologica. 61(4):140–146.
- Skakkebaek NE, Rajpert-De Meyts E, Buck Louis GM, Toppari J, Andersson AM, Eisenberg ML, Jensen TK, Jørgensen N, Swan SH, Sapra KJ, et al. 2016. Male Reproductive Disorders and Fertility Trends: influences of Environment and Genetic Susceptibility. Physiol Rev. 96(1):55–97. doi:10.1152/physrev.00017.2015
- Vogt PH, Fernandes S. 2003. Polymorphic DAZ gene family in polymorphic structure of AZFc locus: artwork or functional for human spermatogenesis? APMIS 111(1):115–126. doi:10.1034/j.1600-0463.2003.11101161.x.
- Wang H, Jia Z, Mao A, Xu B, Wang S, Wang L, Liu S, Zhang H, Zhang X, Yu T, et al. 2020. Analysis of balanced reciprocal translocations in patients with subfertility using single-molecule optical mapping. J Assist Reprod Genet. 37(3):509–516. doi:10.1007/s10815-020-01702-z
- Wang RX, Zhang HG, Pan Y, Chen S, Yue FG, Zhu DL, Liu RZ. 2016. Translocation breakpoints of chromosome 1 in male carriers: clinical features and implications for genetic counseling. Genet Mol Res. 15:4.
- Winters BR, Walsh TJ. 2014. The epidemiology of male infertility. Urol Clin North Am. 41(1):195–204. doi:10.1016/j.ucl.2013.08.006.
- Yumura Y, Murase M, Katayama K, Segino M, Aizawa Y, Kuroda SN, Noguchi K. 2012. Y-autosome translocationassociated with male infertility: a case report. Hinyokika Kiyo. 58(6):307–310.
