ABSTRACT
Recent evidence suggests that gamete-imprinted genes play a role in embryo and placenta development and growth. This systematic review aimed to evaluate whether altered methylation of sperm-imprinted genes associates with sperm DNA fragmentation (SDF), pregnancy loss rate and assisted reproductive technique (ART) outcome. To accomplish this, Pubmed, MEDLINE, Cochrane, Academic One Files, Google Scholar, and Scopus databases were used for search strategy from each database inception until December 2020. Specific keywords were used. Studies satisfying the PECOS (Population, Exposure, Comparison/Comparator, Outcomes, Study design) model were retrieved. Ten studies could be included in the qualitative analysis. A significant association was reported between increased SDF rate and aberrant methylation of H19/IGF2 and KCNQ1 genes by two studies. A significantly lower H19 methylation was found in patients with idiopathic recurrent pregnancy loss (RPL) and in infertile patients compared to fertile men. Methylation of GLT2, PEG1/MEST, and ZAC/PLACL1 were similar in patients with RPL and controls. The ART outcome was similar in patients with aberrant and normal methylation of H19, SNRPN, KCNQ1OT1, PEG1/MEST, LIT1, PEG3, NESPAS, and GLT2. By contrast, a study showed an association between altered GLT2 methylation and more inferior ART results. If further confirmed by well-sized studies, these data might be helpful to identify possible epigenetic predictors of ART outcome. Particularly, aberrant methylation of H19/IGF2 and KCNQ1 genes might represent interesting targets that deserve further investigation.
Introduction
In recent decades, the prevalence of male infertility is continuously increasing. Cross-sectional studies indicate that male infertility, defined as a history of infertility exceeding 12 months in the male partner, ranges from 9 to 16%, depending on the population studied (Geelhoed et al. Citation2002; Klemetti et al. Citation2010; Louis et al. Citation2013; Datta et al. Citation2016). Accordingly, several studies unanimously report a decline in sperm concentration and total sperm count over the past forty years (Levine et al. Citation2017; Yuan et al. Citation2018). This highlights the need to investigate the underlying causes.
Imprinting is defined as the expression of a specific gene by the maternal (maternally-expressed imprinted gene) or the paternal (paternally-expressed imprinted gene) allele, rendering the gene functionally haploid. Animal studies have provided evidence for the role of imprinted genes in embryo and placenta development and growth. For instance, in the mouse, the Paternally expressed 10 (Peg10) gene plays a role in placenta formation, the genes Insulin-like growth factor 2 (Igf2) and Growth factor-bound protein 10 (Grb10) in nutrient transport across the placenta, and the Igf2, Igf2 receptor (Igf2r), Grb10, and Cyclin-dependent kinase inhibitor 1 c (Cdkn1c) genes in fetal growth (Cassidy and Charalambous Citation2018).
Several human studies have reported the association between abnormal sperm parameters and altered methylation of sperm imprinted genes. For example, the abnormal methylation of the genes Zac tumor suppression (ZAC), Gene trap locus 2 (GTL2), and Deleted in azoospermia-like (DAZL) has been reported in patients with oligozoospermia (Hammoud et al. Citation2010; Navarro-Costa et al. Citation2010; Sato et al. Citation2011; Laurentino et al. Citation2015), whereas the altered methylation of Family with sequence similarity 50, member B (FAM50B) and Guanidine nucleotide-binding protein, alpha-stimulating activity polypeptide 1 (GNAS) occurs in patients with asthenozoospermia (Xu et al. Citation2016). A meta-analysis of twenty-four studies that included 1441 patients reported a 9.9-fold higher aberrant sperm DNA methylation of imprinted genes in patients with infertility compared with controls. Notably, statistical significance for H19, Mesoderm‐specific transcript (MEST), and SNRPN genes was achieved (Santi et al. Citation2017). The majority of the studies included did not report the pregnancy rate (Santi et al. Citation2017). They were based on conventional sperm parameters with infertility intended as a condition of abnormal sperm parameters (mainly, oligozoospermia). Despite this meta-analysis proving that the altered methylation of sperm imprinted genes is associated with a decreased sperm number, no information on the impact of aberrant methylation of imprinted genes on pregnancy-related outcomes was provided. This end-point is worthy of investigation because of the relevance of these genes in placenta formation, nutrient transport, and fetal growth in mammals (Cassidy and Charalambous Citation2018). Indeed, it may be hypothesized that the aberrant methylation of imprinted genes may interfere with these processes, thus impacting fertilization or embryo growth, since the pattern of methylation of imprinted genes in the gametes is transmitted to the offspring (Cassidy and Charalambous Citation2018).
This systematic review aimed to assess whether altered methylation of imprinted genes at the sperm level is associated with pregnancy loss, and assisted reproductive technique (ART) outcome. Furthermore, since sperm DNA fragmentation (SDF) is known to impact pregnancy-related outcomes (McQueen et al. Citation2019; Deng et al. Citation2019), we aimed at investigating whether a correlation between aberrant methylation of sperm imprinted genes and SDF exist. With the use of cryopreserved sperm for ART, we investigated the impact of cryopreservation on the methylation pattern of imprinted genes. This study’s novelty lies in the possibility of identifying the epigenetic predictors of ART outcomes and pregnancy loss, as the conventional sperm parameters are not entirely informative on sperm quality.
Results
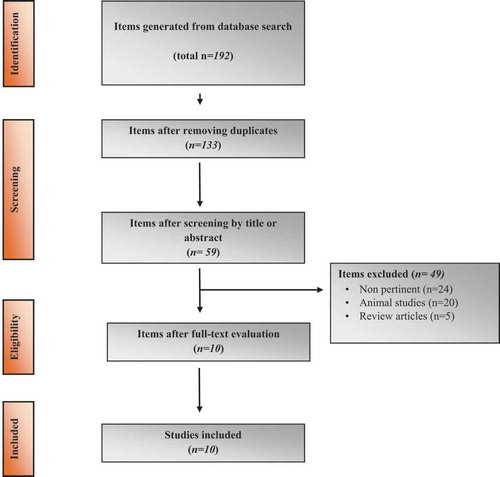
Using the strategy outlined in the methods, 192 articles were retrieved. After the exclusion of 133 duplicate records, 59 articles were screened. Of these, 24 were judged not pertinent upon reading their abstracts or full texts. Moreover, 20 studies using experimental animals and 5 review articles were excluded. The remaining 10 studies were carefully read and included in the analysis (). A summary of the studies included is reported in . summarizes the imprinted genes that have been investigated in this study.
Table 1. Summary of the studies included in this review
Table 2. Summary of imprinted genes evaluated in the present study
Imprinted gene methylation and sperm DNA fragmentation
To date, only one study has evaluated the association between SDF and the methylation level of imprinted genes. The authors analyzed the methylation status of 16 CpGs of the differentially methylated regions (DMRs) of the following 7 imprinted genes: H19, INS‐IGF2, KCNQ1, GTL2, MEST, PEG3, and SNRPN from 66 male patients with idiopathic infertility. The authors reported a significantly lower methylation level at the CpG cg17037101 of the IGF2 gene in patients with SDF ≥25% compared to those with SDF <25%. The degree of methylation of the other CpGs did not differ between the two groups. The authors also demonstrated a correlation between the altered methylation of IGF2 (cg17037101) and KCNQ1 (cg24932449) and SDF rate. The correlation analysis showed that IGF2 methylation (cg17037101) was negatively associated with SDF, as opposed to the level of KCNQ1 methylation (cg24932449) that was positively correlated (Ni et al. Citation2019).
The association between altered methylation levels of the IGF2-H19 loci and SDF rate was indirectly assessed by Dandi et al., 2019. This study used 151 semen samples from normozoospermic patients divided into 4 groups according to the level of reactive oxygen species (ROS). The group with a higher level of ROS had higher IGF2 methylation and higher DFI. These authors speculated that alterations might mediate deterioration of sperm DNA integrity in presence of moderate/high ROS levels in the methylation status of H19-IGF2 genes (Darbandi et al. Citation2019).
Imprinted gene methylation and pregnancy loss
The altered methylation of imprinted genes has been correlated with the risk of abortion. Akolkar and colleagues showed hypomethylation of CpG at the H19 imprinting control region (ICR) in 26 normozoospermic men whose wives had a history positive for recurrent pregnancy loss compared to 26 fertile controls. In particular, they reported a lower percentage of methylated CpGs in the clones analyzed, a lower percentage of complete H19 ICR methylation, and a lower percentage of methylation in the CTCF6-binding region (Ankolkar et al. Citation2012). CTCF is a vertebrate enhancer blocking protein. Hypomethylation of its binding site facilitates its adhesion to DNA with consequent silencing of the expression of the IGF2 gene. This, in turn, would lead to embryo loss. Global hypomethylation seems to be associated with increased fertilization by ‘epigenetically abnormal’ spermatozoa with a consequent increased risk of abortion (Ankolkar et al. Citation2012). The same authors, in another study involving 23 patient partners of women with a history of recurrent pregnancy loss (RPL) and 23 fertile controls, showed how the altered methylation of imprinted control regions (ICRs) of other paternally-expressed imprinted genes, such as DLK1-GTL2, MEST, PLAGL1, and global methylation of LINE1 elements, unlike to H19 ICR (Ankolkar et al. Citation2013), was not associated with idiopathic RPL (Ankolkar et al. Citation2013).
Imprinted gene methylation and assisted reproductive technique (ART) outcomes
There are contrasting evidence on methylation of imprinted genes and the results of ART. A study has evaluated the degree of methylation of 7 imprinted genes, two paternally-expressed (H19 and GTL2), and 5 maternally-expressed (LIT1, MEST, NESPAS, PEG3, and SNRPN) in 141 seminal fluid samples used for ART. The samples were divided into three groups. The first group contained 106 subjects with abnormal sperm parameters [oligo-astheno-teratozoospermia (OAT)], while the second group of 28 patients had normal sperm parameters, but infertility was due to female factors. The remaining seven subjects were excluded because neither a female nor a male infertility factor could be identified. Analysis of the samples showed lower methylation levels of two of the 7 genes analyzed (GTL2 and SNRPN) in patients with OAT compared to normozoospermic controls. No difference was found in the methylation rate of the other 5 imprinted genes between the two groups. Similarly, when the data were analyzed based on the ART outcomes (pregnancy and live-birth rate), the degree of methylation did not differ for any of the 7 genes investigated (El Hajj et al. Citation2011). Montjean and colleagues did not find any difference in the pregnancy rate of oligozoospermic patients with altered methylation profiles when compared to oligozoospermic patients with normal methylation profiles, irrespective of the ART procedure (Montjean et al. Citation2013). This was independently supported by a study of 97 infertile patients that assessed the methylation pattern of one paternally-expressed H19 and two maternally-expressed (SNRPN and KCNQ1OT1) imprinted genes. Infertile patients had a significantly lower level of H19 methylation compared to controls . A group with aberrant methylation (24 patients) and a group with normal methylation (69 patients) was identified. The results of 112 patients who underwent in vitro fertilization (IVF) treatment at the same time were recorded as used as the control. No significant differences were found for fertilization rate, good quality embryos, clinical pregnancy, or childbirth parameters (including birth weight and the week of delivery) between the three groups. Furthermore, they showed that in the cord blood samples of 13 children conceived by intracytoplasmic sperm injection (ICSI) from infertile patients, the methylation levels of these 3 genes were not significantly different from those of 30 naturally conceived children. This suggested that increased imprinting errors in spermatozoa of infertile patients do not have any influence on the imprinting of offspring (Tang et al. Citation2017). In contrast, another study analyzed the outcome of ART in 54 patients with normal sperm parameters or OAT. About half of the female partners achieved pregnancy and childbirth in both groups, whereas the other half did not. Interstingly, the percentage of abnormally demethylated GTL2 alleles was significantly higher in spermatozoa from patients who did not attain a favorable ART outcome. This negative association was even stronger in patients with OAT (Kuhtz et al. Citation2014).
Imprinted gene methylation and cryopreservation
Evidence from the literature suggests that sperm cryopreservation is associated with increased ROS production and DNA fragmentation (Hezavehei et al. Citation2018). Given the correlation above between ROS, SDF, and sperm DNA methylation (Darbandi et al. Citation2019), some studies have attempted to evaluate the relationship between altered DNA methylation and cryopreservation, suggesting a probable safety of the procedure on the appropriate methylation process of imprinted genes (Kläver et al. Citation2012; Khosravizadeh et al. Citation2020). In fact, a study conducted on sperm samples from 10 normozoospermic men divided into 4 aliquots (fresh, diluted in cryoprotectant, diluted in cryoprotectant and cryopreserved for 1 month, and incubated with H2O2) showed that although in the cryopreserved samples there was an increase in ROS production and SDF compared with the fresh group, there was no difference in the methylation levels of the two imprinted genes examined (SNRPN and UBE3A) (Khosravizadeh et al. Citation2020). Similarly, another study conducted on 10 semen samples showed no difference in the degree of methylation of 3 maternally imprinted genes (LIT1, SNRPN, MEST), two paternally imprinted genes (GTL2, H19), two repetitive elements (ALU, LINE1), one spermatogenesis-specific gene (VASA), and one gene associated with male infertility (MTHFR) between short- and medium-term cryopreserved and fresh sample aliquots (Kläver et al. Citation2012).
Discussion
This systematic review was undertaken to understand whether aberrant methylation of imprinted genes at the sperm level is associated with higher SDF, pregnancy rate, and unfavorable ART results. The impact of cryopreservation on methylation of imprinted genes was also evaluated. By carefully reviewing the literature, using a systematic search strategy, 10 articles were finally included. An association between aberrant methylation of specific imprinted genes (mainly, H19/IGF2 genes) and high SDF or pregnancy loss rates was reported. In contrast, the impact on the ART outcome is a more controversial issue since the majority of data did not find any effect. However, contrasting results have been reported for the GTL2/DLK1 methylation rate. Cryopreservation does not appear to alter the methylation process of sperm imprinted genes. Overall, the number of studies exploring this essential aspect of reproductive medicine is scanty. Indeed, only 10 studies were identified that could be included in this systematic review, and, thus, the first consideration is there is a need for further research exploring this topic.
Overall, the studies included in this qualitative review evaluated methylation of the following genes at the sperm level: H19/IGF2, GTL2/DLK1, PEG1/MEST, PLACL1, LIT1, NESPAS, PEG3, SNRPN, UBE3A, KCNQ1, and KCNQ1OT1. All of them are paternally-expressed imprinted genes except for H19, GTL2, UBE3A, and KCNQ1 that are maternally-expressed imprinted genes (http://www.geneimprint.com/site/genes-by-species). Imprinting is a mechanism that allows monoallelic and parent-specific gene expression since the gene in the other allele is silenced by methylation (Bajrami and Spiroski Citation2016). Hypermethylation leads to gene silencing, whereas hypomethylation allows gene expression (Bajrami & Spiroski et al., Citation2016). It has been estimated that ~1% of the human genome has a monoallelic and parent-specific expression (Elbracht et al. Citation2020). Errors of genomic imprinting have classically been recognized in the pathogenesis of some neurodevelopmental disorders, such as Angelman or Prader–Willi syndrome, which represent the consequence of the loss of paternal or maternal gene expression, respectively (Bajrami and Spiroski Citation2016).
Imprinted genes play a role in mammalian reproduction since many of them encode for proteins or transcription factors that are involved in embryo growth and development (Anifandis et al. Citation2015). As a consequence, aberrant methylation of imprinted genes in the gametes can affect the pattern of methylation in the embryo, leading to pregnancy loss, or the development of imprinting disorders in the offspring (Elbracht et al. Citation2020). In particular, H19 and GTL2 methylation seem to play a key role in embryo growth and development (Li et al. Citation2020).
H19/IGF2 that map in the chromosome 11p15.5 is the first described couple of imprinted genes (De Chiara et al. Citation1991). H19 is a maternally-expressed imprinted gene that encodes a non-coding RNA suppressing the IGF1R gene expression (Gao et al. Citation2012). IGF2 is a paternally-expressed imprinted gene encoding for the homonymous protein (Gao et al. Citation2012). The expression of these genes is regulated by the level of methylation of their common enhancer, which maps upstream H19 gene in the maternal allele. This enhancer is physiologically non-methylated, thus allowing the transcription of H19 and inhibiting the expression of IGF2. On the contrary, it is hypermethylated in the paternal allele, and this promotes IGF2 gene expression and the silencing of the H19 gene. Both IGF2 and IGF1R mRNA have been described in human spermatozoa (Cannarella et al. Citation2020b). Moreover, increasing evidence addresses sperm-carried RNA’s role in fertilization and embryo growth (Guo et al. Citation2017; Cannarella et al. Citation2020a). Hypomethylation of spermatozoal H19 may promote H19 expression, decreasing the transcription of the IGF2 growth factor, thus affecting embryo growth. Accordingly, some studies suggest a critical role for H19 methylation, as shown in mice before 12.5 days of gestation (Li et al. Citation2020). This can reasonably justify the significantly reduced H19 gene methylation at the sperm level reported in couples with RPL (Ankolkar et al. Citation2012) as well as in infertile patients (El Hajj et al. Citation2011; Tang et al. Citation2017). Also, low H19 methylation may be involved in ROS- and SDF-induced infertility. Accordingly, spermatozoa are highly susceptible to ROS-induced damage due to their low scavenger activity (Showell et al. Citation2014). A high level of ROS is known to negatively affect fertility and ART outcome, mainly by inducing SDF (Lanzafame et al. Citation2009; Showell et al. Citation2014). The levels of ROS in spermatozoa have been positively associated with SDF rate and negatively with H19 methylation rate (Darbandi et al. Citation2019), which may hypothetically lead to decreased IGF2 gene expression in spermatozoa exposed to high ROS levels or with a high SDF rate.
GTL2, also known as GTL2, and DLK1 genes are reciprocally imprinted genes. GTL2 encodes a maternally expressed lncRNA, whereas the DLK1 gene encodes the paternal complement (Movahed et al. Citation2020). These imprints are maintained in the preimplantation embryo until the second cleavage, suggesting a role for the DLK1/GTL2 imprinting in embryo development and growth (Geuns et al. Citation2007). Particularly, Gtl2 methylation seems to play a key role in the late-gestation embryo (Li et al. Citation2020). This may explain the finding of aberrant GTL2 methylation in spermatozoa of negative ART outcome (Kuhtz et al. Citation2014), although another study did not confirm this (El Hajj et al. Citation2011).
The novelty of this study lies in the attempt to identify possible epigenetic sperm markers which may be used in the future and, if further confirmed by well-sized studies, as predictors of ART outcome and pregnancy loss, as the conventional sperm parameters not completely informative of the sperm quality. Particularly, according to the evidence of the present study, aberrant methylation of H19/IGF2 and KCNQ1 genes might represent attractive targets, which deserve further investigation.
Notably, environmental factors such as cigarette smoke and nutrition can impact sperm epigenetics (Dunford et al., Citation2017; Åsenius et al. Citation2020). Similarly, maternal pressure can affect embryo imprints (Petry et al. Citation2016). This may provide another molecular mechanism underlying human infertility or genomic imprinting disorders.
Finally, these results must be interpreted with caution because of the limited number of patients enrolled in each study and the very low number of studies that could be included in this systematic review. Furthermore, we included studies assessing the impact of sperm epigenetics, and not embryo epigenetics [notheworthy, even culture conditions can impact embryo imprints (Simopoulou et al. Citation2018)], on ART outcomes.
In conclusion, the present systematic review reports an association between increased SDF rate and aberrant methylation of H19/IGF2 and KCNQ1 genes (Ni et al. Citation2019; Darbandi et al. Citation2019). Aberrant methylation of H19 was also observed in patients with idiopathic RPL (Ankolkar et al. Citation2012). In contrast, no difference in the degree of methylation has been noted in these patients for GTL2, PEG1/MEST, and PLACL1 (Ankolkar et al. Citation2013). Lower H19 (El Hajj et al. Citation2011; Tang et al. Citation2017) and higher SNRPN (El Hajj et al. Citation2011) methylation levels in infertile patients compared to fertile men has been reported, although this does not impact ART (El Hajj et al. Citation2011; Montjean et al. Citation2013; Tang et al. Citation2017). Similarly, aberrant methylation of LIT1 (Tang et al. Citation2017), PEG1/MEST (El Hajj et al. Citation2011; Montjean et al. Citation2013), LIT1, PEG3, NESPAS, and GTL2 (El Hajj et al. Citation2011) does not appear to influence ART. By contrast, (Kuhtz et al. Citation2014), showed an association between altered GTL2 gene methylation and poorer ART outcome. Finally, methylation imprints do not seem to be altered by cryopreservation (Kläver et al. Citation2012; Khosravizadeh et al. Citation2020). If confirmed by further studies, these data might help to predict ART outcomes. However, the findings summarized in this review derive from scant literature suggesting the need for further studies on this central issue in reproductive medicine.
Methods
Search strategy
This study was performed collecting data through extensive research in the Pubmed, MEDLINE, Cochrane, Academic One Files, Google Scholar, and Scopus databases from each database inception to December 2020. The search strategy used the following combination of MeSH terms and keywords: ‘H19’, ‘MEST’, ‘LINE1’, ‘SNRPN’, ‘UBE3A’, ‘BRDT’, ‘CFTC6’, ‘CREM’, ‘DAZL’, ‘FAM50B’, ‘GNAS’, ‘GTL2’, ‘IGF2’, ‘PRDM16’, ‘CLF4’, ‘BRUNOL4’, ‘KCNQ’, ‘LIT1’, ‘MEG3’, ‘PEG3’, ‘RHOX’, ‘DLK’, ‘DIO’, ‘NESP’, ‘ZAC’, ‘gene methylation’, ‘fertilization rate’, ‘sperm DNA fragmentation’, ‘assisted reproductive technique’, ‘pregnancy rate’, ‘abortion’, ‘miscarriage’. Additional manual searches were done using the reference lists of relevant studies. The search strategy was restricted to English-language studies enrolling human participants. Identification of eligible studies was independently performed by two different authors (A.C. and R.A.C.). Disagreements were resolved by a third investigator (R.C.). Definition of the outcome measures is reported in the Supplementary Table 1.
Inclusion and exclusion criteria
Eligible studies were identified according to the PECOS (Population, Exposure, Comparison/Comparator, Outcomes, Study design) model (Supplementary Table 2). Particularly, studies evaluating methylation of sperm imprinted genes of patients undergoing ART, SDF assessment, or cryopreservation were included. Observational studies (case-control, cross-sectional, prospective, and case-series) were assessed for eligibility. Intervention studies, as well as commentaries, letters, case-reports, reviews, studies with incomplete or unsuitable data, were excluded. Duplicates were checked and removed.
Risk of bias evaluation
The quality of the studies was evaluated using the ‘Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies’ and the ‘Quality Assessment of Case-Control Studies’ provided by the National Institute of Health (https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools). The potential risk for selection bias, information bias, measure bias, or confounding bias were evaluated (Supplementary Tables 3 and 4). Studies were rated as good, fair, and of poor quality, where a high risk of bias was translated into a rating of poor quality (‘-’), and low risk of bias was translated into a rating of good quality (‘+’).
Disclosure of interest
The authors report no conflict of interest.
Authors’ contributions
Conceptualization: RC, AEC; methodology: RC, AC; formal analysis: AC, RAC; investigation: LMM, SLV;data curation: RC, AC; writing—original draftpreparation: RC, AC; writing—review and editing: RC, AEC; visualization: RAC, LMM; supervision: SLV; project administration: RC, AEC. All authors have read and agreed to the published version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Anifandis G, Messini CI, Dafopoulos K, Messinis IE. 2015. Genes and conditions controlling mammalian pre- and post-implantation embryo development. Curr Genomics. 16(1):32–46. doi:10.2174/1389202916666141224205025.
- Ankolkar M, Patil A, Warke H, Salvi V, Kedia Mokashi N, Pathak S, Balasinor NH. 2012. Methylation analysis of idiopathic recurrent spontaneous miscarriage cases reveals aberrant imprinting at H19 ICR in normozoospermic individuals. Fertil Steril. 98(5):1186–1192. doi:10.1016/j.fertnstert.2012.07.1143.
- Ankolkar M, Salvi V, Warke H, Vundinti BR, Balasinor NH. 2013. Methylation status of imprinted genes DLK1-GTL2, MEST (PEG1), ZAC (PLAGL1), and LINE-1 elements in spermatozoa of normozoospermic men, unlike H19 imprinting control regions, is not associated with idiopathic recurrent spontaneous miscarriages. Fertil Steril. 99(6):1668–1673. doi:10.1016/j.fertnstert.2013.01.107.
- Åsenius F, Danson AF, Marzi SJ. 2020. DNA methylation in human sperm: a systematic review. Hum Reprod Update. 26(6):841–873. doi:10.1093/humupd/dmaa025.
- Bajrami E, Spiroski M. 2016. Genomic imprinting. Open Access Maced J Med Sci. 4(1):181–184. doi:10.3889/oamjms.2016.028.
- Cannarella R, Barbagallo F, Crafa A, La Vignera S, Condorelli RA, Calogero AE. 2020a. Seminal plasma transcriptome and proteome: towards a molecular approach in the diagnosis of idiopathic male infertility. Int J Mol Sci. 21(19):7308. doi:10.3390/ijms21197308.
- Cannarella R, Condorelli RA, La Vignera S, Bellucci C, Luca G, Calafiore R, Calogero AE. 2020b. IGF2 and IGF1R mRNAs are detectable in human spermatozoa. World J Mens Health. 38(4):545–551. doi:10.5534/wjmh.190070.
- Cassidy FC, Charalambous M. 2018. Genomic imprinting, growth and maternal-fetal interactions. J Exp Biol. 221(PtSuppl 1):jeb164517. doi:10.1242/jeb.164517.
- Darbandi M, Darbandi S, Agarwal A, Baskaran S, Dutta S, Sengupta P, Khorram Khorshid HR, Esteves S, Gilany K, Hedayati M, et al. 2019. Reactive oxygen species-induced alterations in H19-Igf2 methylation patterns, seminal plasma metabolites, and semen quality. J Assist Reprod Genet. 36(2):241–253. doi:10.1007/s10815-018-1350-y
- Datta J, Palmer MJ, Tanton C, Gibson LJ, Jones KG, Macdowall W, Glasier A, Sonnenberg P, Field N, Mercer CH, et al. 2016. Prevalence of infertility and help seeking among 15000 women and men. Hum Reprod. 31(9):2108–2118. doi:10.1093/humrep/dew123
- De Chiara TM, Robertson EJ, Efstratiadis A. 1991. Parental imprinting of the mouse insulin-like growth factor II gene. Cell. 64(4):849–859. doi:10.1016/0092-8674(91)90513-X.
- Deng C, Li T, Xie Y, Guo Y, Yang QY, Liang X, Deng CH, Liu GH. 2019. Sperm DNA fragmentation index influences assisted reproductive technology outcome: a systematic review and meta-analysis combined with a retrospective cohort study. Andrologia. 51(6):e13263. doi:10.1111/and.13263.
- Dunford AR, Sangster JM. 2017. Maternal and paternal periconceptional nutrition as an indicator of offspring metabolic syndrome risk in later life through epigenetic imprinting: a systematic review. Diabetes Metab Syndr Suppl. 2:S655–S662. doi:10.1016/j.dsx.2017.04.021.
- El Hajj N, Zechner U, Schneider E, Tresch A, Gromoll J, Hahn T, Schorsch M, Haaf T. 2011. Methylation status of imprinted genes and repetitive elements in sperm DNA from infertile males. Sex Dev. 5(2):60–69. doi:10.1159/000323806.
- Elbracht M, Mackay D, Begemann M, Kagan KO, Eggermann T. 2020. Disturbed genomic imprinting and its relevance for human reproduction: causes and clinical consequences. Hum Reprod Update. 26(2):197–213. doi:10.1093/humupd/dmz045.
- Gao WL, Liu M, Yang Y, Yang H, Liao Q, Bai Y, Li YX, Li D, Peng C, Wang YL. 2012. The imprinted H19 gene regulates human placental trophoblast cell proliferation via encoding miR-675 that targets nodal modulator 1 (NOMO1). RNA Biol. 9(7):1002e1010. doi:10.4161/rna.20807.
- Geelhoed DW, Nayembil D, Asare K, Schagen Van Leeuwen JH, Van Roosmalen J. 2002. Infertility in rural Ghana. Int J Gynaecol Obstet. 79(2):137–142. doi:10.1016/S0020-7292(02)00237-0.
- Geuns E, De Temmerman N, Hilven P, Van Steirteghem A, Liebaers I, De Rycke M. 2007. Methylation analysis of the intergenic differentially methylated region of DLK1-GTL2 in human. Eur J Hum Genet. 15(3):352–361. doi:10.1038/sj.ejhg.5201759.
- Guo L, Chao SB, Xiao L, Wang ZB, Meng TG, Li YY, Han ZM, Ouyang YC, Hou Y, Sun QY, et al. 2017. H. Sperm-carried RNAs play critical roles in mouse embryonic development. Oncotarget. 8(40):67394–67405. doi:10.18632/oncotarget.18672
- Hammoud SS, Purwar J, Pflueger C, Cairns BR, Carrell DT. 2010. Alterations in sperm DNA methylation patterns at imprinted loci in two classes of infertility. Fertil Steril. 94(5):1728–1733. doi:10.1016/j.fertnstert.2009.09.010.
- Hezavehei M, Sharafi M, Kouchesfahani HM, Henkel R, Agarwal A, Esmaeili V, Shahverdi A. 2018. Sperm cryopreservation: a review on current molecular cryobiology and advanced approaches. Reprod Biomed Online. 37(3):327–339. doi:10.1016/j.rbmo.2018.05.012.
- Khosravizadeh Z, Hassanzadeh G, Tavakkoly Bazzaz J, Alizadeh F, Totonchi M, Salehi E, Khodamoradi K, Khanehzad M, Hosseini SR, et al. 2020. The effect of cryopreservation on DNA methylation patterns of the chromosome 15q11-q13 region in human spermatozoa. Cell Tissue Bank. 21(3):433–445. doi:10.1007/s10561-020-09828-1
- Kläver R, Bleiziffer A, Redmann K, Mallidis C, Kliesch S, Gromoll J. 2012. Routine cryopreservation of spermatozoa is safe–evidence from the DNA methylation pattern of nine spermatozoa genes. J Assist Reprod Genet. 29(9):943–950. doi:10.1007/s10815-012-9813-z.
- Klemetti R, Raitanen J, Sihvo S, Saarni S, Koponen P. 2010. Infertility, mental disorders and well-being–a nationwide survey. Acta Obstet Gynecol Scand. 89(5):677–682. doi:10.3109/00016341003623746.
- Kuhtz J, Schneider E, El Hajj N, Zimmermann L, Fust O, Linek B, Seufert R, Hahn T, Schorsch M, Haaf T. 2014. Epigenetic heterogeneity of developmentally important genes in human sperm: implications for assisted reproduction outcome. Epigenetics. 9(12):1648–1658. doi:10.4161/15592294.2014.988063.
- Lanzafame FM, La Vignera S, Vicari E, Calogero AE. 2009. Oxidative stress and medical antioxidant treatment in male infertility. Reprod Biomed Online. 19(5):638–659. doi:10.1016/j.rbmo.2009.09.014.
- Laurentino S, Beygo J, Nordhoff V, Kliesch S, Wistuba J, Borgmann J, Buiting K, Horsthemke B, Gromoll J. 2015. Epigenetic germline mosaicism in infertile men. Hum Mol Genet. 24(5):1295–1304. doi:10.1093/hmg/ddu540.
- Levine H, Jørgensen N, Martino-Andrade A, Mendiola J, Weksler-Derri D, Mindlis I, Pinotti R, Swan SH. 2017. Temporal trends in sperm count: a systematic review and meta-regression analysis. Hum Reprod Update. 23(6):646–659. doi:10.1093/humupd/dmx022.
- Li Q, Li Y, Yin Q, Huang S, Wang K, Zhuo L, Li W, Chang B, Li J. 2020. Temporal regulation of prenatal embryonic development by paternal imprinted loci. Sci China Life Sci. 63(1):1–17. doi:10.1007/s11427-019-9817-6.
- Louis JF, Thoma ME, Sørensen DN, McLain AC, King RB, Sundaram R, Keiding N, Buck Louis GM. 2013. The prevalence of couple infertility in the United States from a male perspective: evidence from a nationally representative sample. Andrology. 1(5):741–748. doi:10.1111/j.2047-2927.2013.00110.x.
- McQueen DB, Zhang J, Robins JC. 2019. Sperm DNA fragmentation and recurrent pregnancy loss: a systematic review and meta-analysis. Fertil Steril. 112(1):54–60.e3. doi:10.1016/j.fertnstert.2019.03.003.
- Montjean D, Ravel C, Benkhalifa M, Cohen-Bacrie P, Berthaut I, Bashamboo A, McElreavey K. 2013. Methylation changes in mature sperm deoxyribonucleic acid from oligozoospermic men: assessment of genetic variants and assisted reproductive technology outcome. Fertil Steril. 100(5):1241–1247. doi:10.1016/j.fertnstert.2013.06.047.
- Movahed E, Shabani R, Hosseini S, Shahidi S, Salehi M. 2020. Interfering effects of in vitro fertilization and vitrification on expression of Gtl2 and Dlk1 in mouse blastocysts. Int J Fertil Steril. 14(2):110–115. doi:10.22074/ijfs.2020.5984.
- Navarro-Costa P, Nogueira P, Carvalho M, Leal F, Cordeiro I, Calhaz-Jorge C, Gonçalves J, Plancha CE. 2010. Incorrect DNA methylation of the DAZL promoter CpG island associates with defective human sperm. Hum Reprod. 25(10):2647–2654. doi:10.1093/humrep/deq200.
- Ni W, Pan C, Pan Q, Fei Q, Huang X, Zhang C. 2019. Methylation levels of IGF2 and KCNQ1 in spermatozoa from infertile men are associated with sperm DNA damage. Andrologia. 51(5):e13239. doi:10.1111/and.13239.
- Petry CJ, Sanz Marcos N, Pimentel G, Hayes MG, Nodzenski M, Scholtens DM, Hughes IA, Acerini CL, Ong KK, Lowe WL, et al. 2016. Associations between fetal imprinted genes and maternal blood pressure in pregnancy. Hypertension. 68(6):1459–1466. doi:10.1161/HYPERTENSIONAHA.116.08261
- Santi D, De Vincentis S, Magnani E, Spaggiari G. 2017. Impairment of sperm DNA methylation in male infertility: a meta-analytic study. Andrology. 5(4):695–703. doi:10.1111/andr.12379.
- Sato A, Hiura H, Okae H, Miyauchi N, Abe Y, Utsunomiya T, Yaegashi N, Arima T. 2011. Assessing loss of imprint methylation in sperm from subfertile men using novel methylation polymerase chain reaction luminex analysis. Fertil Steril. 95(1):129–134, 134.e1–4. doi:10.1016/j.fertnstert.2010.06.076.
- Showell MG, Mackenzie-Proctor R, Brown J, Yazdani A, Stankiewicz MT, Hart RJ. 2014. Antioxidants for male subfertility. Cochrane Database Syst Rev. (12):CD007411. doi:10.1002/14651858.CD007411.pub3.
- Simopoulou M, Sfakianoudis K, Rapani A, Giannelou P, Anifandis G, Bolaris S, Pantou A, Lambropoulou M, Pappas A, Deligeoroglou E, et al. 2018. Considerations regarding embryo culture conditions: from media to epigenetics. Vivo. 32(3):451–460.
- Tang L, Liu Z, Zhang R, Su C, Yang W, Yao Y, Zhao S, Yu Y. 2017. Imprinting alterations in sperm may not significantly influence ART outcomes and imprinting patterns in the cord blood of offspring. PLoS One. 12(11):e0187869. doi:10.1371/journal.pone.0187869.
- Xu J, Zhang A, Zhang Z, Wang P, Qian Y, He L, Shi H, Xing Q, Du J. 2016. DNA methylation levels of imprinted and nonimprinted genes DMRs associated with defective human spermatozoa. Andrologia. 48(9):939–947. doi:10.1111/and.12535.
- Yuan HF, Shangguan HF, Zheng Y, Meng TQ, Xiong CL, Guan HT. 2018. Decline in semen concentration of healthy Chinese adults: evidence from 9357 participants from 2010 to 2015. Asian J Androl. 20(4):379–384. doi:10.4103/aja.aja_80_17.