ABSTRACT
The COVID-19 pandemic, caused by the SARS-CoV-2 virus, is an unprecedented global situation, and all countries have adopted their own measurements to mitigate the spread of the virus in the first as well as in the subsequent waves of infection. All measures, especially in the first wave of the pandemic, were in combination with recommendations provided by professional and scientific organizations. Similar measures were applied to specific procedures, such as the management of infertility, including in vitro fertilization-embryo transfer (IVF-ET) treatments. Although there is no clear scientific evidence yet that the SARS-CoV-2 may exert negative effects on IVF outcome, especially at the early stages, several clinical reports indicate that the virus may impact male fertility through specific receptors presented at the somatic cells of the testis and used by the virus in order to gain entry to the respective cells. Nevertheless, it is not unreasonable to suspect that the virus may affect sperm function as well as oocyte performance directly through specific receptors or indirectly through other signaling pathways. Despite the good practice of IVF laboratory techniques, culture media may also be contaminated during equilibration when airborne virus’s particles can contaminate culture media from an already infected embryology area or staff. Furthermore, although there is no clinical evidence, liquid nitrogen could be a route of infection for gametes and embryos when it has been contaminated during production or transportation. Therefore, cryopreservation of gametes and embryos must be virus-free. This communication aims to provide some aspects of the possible impact of the virus on gametes and embryos and how it may affect the cryopreservation procedures.
Abbreviations: ACE2: angiotensin- converting enzyme 2; ART: assisted reproductive technology; ASRM: American Society for Reproductive Medicine; CDC: Centers for Disease Control and Prevention; COVID-19: coronavirus disease 2019; ESHRE: European Society of Human Reproduction and Embryology; ET: embryo transfer; FSH: follicle stimulating hormone; IFFS: International Federation of Fertility Societies; IVF: in vitro fertilization; LH: luteinizing hormone; LN: liquid nitrogen; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; T: testosterone; WHO: World Health Organization.
Introduction
The novel acute ‘severe respiratory syndrome coronavirus 2’ (SARS-CoV-2), which causes the COVID-19 (Corona Virus Disease 2019), was declared by World Health Organization (WHO) on Mar 11, 2020, as a pandemic. Since then, every country has adopted specific risk-reduction measurements to flatten the growth curve of infected cases, to reduce the spread of the virus, and to help the Healthcare system to adopt the unrecorded challenge produced by the virus. As a result, restrictions on non-urgent medical care were initially proposed, which were applied in almost all countries. Infertility treatment may be considered non-urgent medical care. However, urgent assisted reproductive technologies (ART), such as cryopreservation (in oncological patients), is a process that is not exempt from the virus threat. Moreover, scientific committees, such as the European Society of Human Reproduction and Embryology (ESHRE) and the American Society for Reproductive Medicine (ASRM), in an attempt to mitigate the virus spread, initially recommended the cryopreservation of oocytes to be obtained from cycles still in the stage of ovarian stimulation or the resulting embryos, especially when the treatment is for fertility preservation, e.g., in oncological cases. Several reports favor continuing fertility treatments (Tesarik Citation2020), as it is believed that ART processes and the achieved pregnancy are not affected by the virus when appropriate precautions are taken. Other reports propose various guidelines during fertility treatments (La Marca et al. Citation2020; Monteleone et al. Citation2020; Souza et al. Citation2020; Requena et al. Citation2020), others proposed remedies to mitigate the consequences of a prolonged cessation of services by providing services only in relative ‘sensitive’ cases (Esteves et al. Citation2020; Alviggi et al. Citation2020), while others are trying to find some connection between ART and COVID-19 at the cellular level of gametes and embryos (Essahib et al. Citation2020; Rajput et al. Citation2020; Hickman et al. Citation2020). This variation in several theses may progress to become an ongoing and rather heated debate. Toward the end of the first wave, a joint statement of ASRM, ESHRE, and IFFS on COVID-19 and assisted reproduction provided new guidance on recommencing ART treatments and affirmed the importance of continued reproductive care during the COVID-19 pandemic (Veiga et al. Citation2020). As the second wave of infection covered all over the World, a new IFFS statement on COVID-19 was provided (Dec 16, 2020). Nevertheless, all relevant in vitro fertilization (IVF) procedures can commence, in line with local committees’ regulations and in conjunction with safe practice both before and during a treatment cycle as well as in the embryology laboratory, while in parallel research is needed to monitor the effects of COVID-19 on gametes and reproductive tissues.
Τhe crusade of vaccination has just begun. As women who want to get pregnant shortly, either spontaneously or through IVF, will not be vaccinated (due to the lack of evidence), these couples are remaining at risk of infection. Therefore, the possible infection route of either the semen or the oocyte or during cryopreservation and culture media will be discussed. Nevertheless, pregnant healthcare workers are recommended to be vaccinated because their level of risk is different from other people, which means that the advice on vaccination is changing. Last, a few days ago, ASRM (Dec 16, 2020), ESHRE (Jan 12, 2021), and a joint statement by IFFS/ESHRE (Veiga et al. Citation2021) published statements on COVID-19 and vaccination. The scientific committees seem to agree that there is a lack of information relative to vaccination and pregnancy. Couples that want to undergo IVF treatment should postpone treatment for several days after the completion of their vaccination because of the lack of information between vaccines and embryological parameters. Moreover, since the vaccines are primarily mRNA-based which is rapidly degraded and does not enter the cell’s nucleus, and do not contain live virus, it seems that there is no reason to delay IVF attempts because of vaccination administration.
Recently, mutations of the virus have been confirmed and have been embedded in the population (Guruprasad Citation2021). The D614G mutation (a domain of the receptor) seems to enhance the infectivity, possibly raising concerns about the effective implementation of vaccination (Gobeil et al. Citation2020). On the other hand, the role of new mutations in the reproductive process is entirely unknown. Therefore, under these conditions and with the COVID-19 pandemic not yet under control, the possible effects of the virus on embryological parameters should be analyzed more thoroughly.
The route of infection
The route of SARS-CoV-2 infection involves the presence of the ACE2 receptors in the host cells, followed by the priming and cleavage of the virus by the supplementary TMPRSS2 membrane receptors. Briefly, TMPRSS2 primes and cleaves the S1/S2 site on the SARS-CoV-2 spike protein, and subsequently, the S1 protein binds to ACE2 to enter into the host cell (Hoffmann et al. Citation2020; Wrapp et al. Citation2020). It is clear that as the number of ACE2 receptors increases in a host cell, the specific cell is more susceptible to viral entry.
Semen
Human spermatozoa may be contaminated by the virus either directly through the presence of the angiotensin-converting enzyme 2 (ACE2) receptor (Wang and Xu Citation2020; Younis et al. Citation2020) and the cellular protease transmembrane protease serine 2 (TMPRSS2) (Lukassen et al. Citation2020), or indirectly through the laboratory environment (Anifandis et al. Citation2020). The role of TMPRSS2 in virus spread was proposed some time ago (Iwata-Yoshikawa et al. Citation2019); however, the role of TMPRSS12, a member of the TMPRSS family, has been recently suggested to be essential for sperm motility and migration through the utero-tubal junction (Larasati et al. Citation2020).
Negative results in semen
It has been reported that SARS-CoV-2 was not detected in semen samples provided by patients who recovered from COVID-19 (Pan et al. Citation2020). In the same line, positive COVID-19 male patients did not display detectable virus levels in the respective semen samples, while abnormal sperm morphology was observed mainly due to the acute fever (Temiz et al. Citation2020; Ruan et al. Citation2021). Very recently, a relatively small population study reached the same conclusion (Song et al. Citation2020). In that study, it was observed that the SARS-CoV-2 was absent from both the semen and testis specimens of COVID-19 patients, proposing that the virus is highly unlikely to be sexually transmitted (Song et al. Citation2020). Similar findings were observed by Holtmann and colleagues (Holtmann et al. Citation2020). The limited expression of ACE2 and TMPRSS2 in the testis tissue, in combination with the fact that ACE2 is mainly expressed in Sertoli cells and TMPRSS2 in a subpopulation of different developmental stages of the germ cells, possible adverse downstream effects of the virus is still a chance (Wang et al. Citation2018; Anifandis et al. Citation2021). However, all the authors underlined that the presence of the virus could not be excluded in acutely infected COVID-19 patients, while Eisenberg commented that higher viral loads might have led to detectable levels (Eisenberg Citation2020). Importantly, ACE2 is expressed within the Leydig cells of the testis, implying that ACE2 might be important in spermatogenesis (Douglas et al. Citation2004) and for that reason, concerns regarding the infection of the testes and possible sexual transmission have been raised (VerVerma et al. Citation2020). In other words, it is hypothesized that positive SARS-CoV-2 infected patients may experience functional abnormalities in male germ cells leading to male infertility. A possible route of transmission has already been described for the Zika virus (Feldmann Citation2018; Cardona Maya et al. Citation2020). Nevertheless, the testicular immune privilege and the blood-testis barrier it is likely to protect the testicular cells unless a threshold level is achieved (Liu et al. Citation2020).
Positive results in semen
As testicular damage has been confirmed in COVID-19 patients (Pan et al. Citation2020), it is not unreasonable to speculate that this novel virus might be transmitted by semen which could potentially serve as a route of transmission from patients with mild symptoms or even during the incubation period of the virus (Paoli et al. Citation2021; Cardona Maya et al. Citation2016; De Toni et al. Citation2020). Indeed, recently it was found that SARS-CoV-2 was present in semen samples of patients with COVID-19 either during the acute or the recovering phase of infection (Li et al. Citation2020a). A small number of all COVID-19 positive males studied the presence of the virus was confirmed (Gonzalez et al. Citation2020). Despite the small number of cases studied, the authors declared that SARS-CoV-2 might be transmitted sexually, and future studies are required in regards to virus shedding and concentration in semen samples. Very recently, a novel experimental study by Maleki and Tartibian (Maleki and Tartibian Citation2021), investigating a number of seminal biomarkers in COVID-19 male patients, they observed higher levels of seminal plasma ACE2 enzymatic activity of inflammatory and apoptotic agents as well as a significant impairment of sperm properties in comparison to the control group, implying that the SARS-CoV-2 presents a serious threat for male reproduction. Moreover, attention should be paid to semen samples of asymptomatic patients since the virus infection rate and the transmission ability are relatively high (Qian et al. Citation2020). In a medRxiv245 unrefereed preprint study with 81 positive COVID-19 men, it was found that the serum LH levels were significantly higher compared to age-matched controls (Ma et al. Citation2020). This status of COVID-19 patients may lead to the suppression of the hypothalamic–pituitary–testicular Axis (Pal and Banerjee Citation2020) and subsequently to male infertility. Whatever happens, it is worrying that this novel coronavirus outbreak could exert serious effects on the male reproductive system (Massarotti et al. Citation2021; Fraietta et al. Citation2020; Perry et al. Citation2020). Apart from contaminated sperm samples, there is a negligible risk that SARS-CoV-2 particles might be present during cryopreservation and might survive after thawing as viruses are usually resistant to the freeze-thaw process (Adiga et al. Citation2020). Semen cryopreservation involves the dilution of nude sperm sample (which is not free from the virus) with an equal quantity of cryoprotectants. Therefore, sperm cryopreservation is an ART procedure that should account for virus-free since viruses have been found to retain their contamination ability even after 40 years of cryopreservation (Merrill et al. Citation2018). Alternatively, to bypass the possible contamination, cryopreservation of seminal plasma-free sperm samples (prepared without the seminal plasma) in vapor cryostorage tanks can be a good and safe approach for semen cryopreservation (Anifandis et al. Citation2020).
Follicles/oocytes
Although the preference of the virus to the male gender (Foresta et al. Citation2020; Anifandis et al. Citation2021), follicles and oocytes may also be infected. To date, there are limited data concerning COVID-19 and follicles/oocytes. Nevertheless, considerations are raised, and a hypothesis similar to that of semen may develop. The ACE family has been found to be present in mammalian ovaries (Pan et al. Citation2013), implying a role of ACEs in female fertility. Moreover, LH via gonadotrophin intermediacy has been found to upregulate the ACE2-Ang-(1-7)-Mas axis promoting finally meiotic resumption (Honorato-Sampaio et al. Citation2012; Santos et al. Citation2013). Whether the ACE2 or other homologous to ACE2 receptors are present on the follicle surface has been recently investigated (Essahib et al. Citation2020; Virant-Klun and Strle Citation2021). ACE2 expression has been confirmed in human ovaries, while angiotensin-(1-7) has been detected in measurable amounts in the follicular fluid (Reis et al. Citation2011). ACE2 is expressed in granulosa cells implying its involvement in follicular development (Barreta et al. Citation2015).
Moreover, it is possible that the virus may be present in the follicular fluid since the Center for Diseases Control and Prevention (CDC) has argued that non-respiratory body fluids from infected women can contain viable, infectious viruses. This may happen through mechanisms and pathways that involve receptors or ACE2-like receptors. Whether the virus can survive in the follicular fluid destroying any interaction between follicles and oocytes should be investigated given the course of oocyte turnover and the contamination ability of the virus for several days. Given the hormonal and cellular interaction and the connection between granulosa cells and the growing oocyte (Alam and Miyano Citation2020), it can be easily assumed that every negative signal to the immediate or supporting environment of the oocyte may negatively affect oocyte performance. Therefore, it is not unreasonable to assume that SARS-COV-2 might negatively affect follicular development, which inevitably may lead to incomplete oocyte maturation and subsequently a further compromise regarding female infertility. That means that acute or even asymptomatic to COVID-19 women might display poor oocyte quality, which is unlikely to be detected by any morphological assessment. Nevertheless, recently, non-human primate ovarian tissue was found to co-express ACE2 and TMPRSS2, while the degree of ACE2 and TMPRSS2 co-expression increased in relation to oocyte maturity (from primordial to antral stage) (Stanley et al. Citation2020). On the other hand, the co-expression of ACE2 and TMPRSS2 was absent from both non-human and human cumulus cells (Stanley et al. Citation2020), implying that cumulus cells may serve as a physical barrier to SARS-CoV-2 infection. Indeed, SARS-CoV-2 positive women did not display any detectable viral RNA levels in the respective oocytes (Barragan et al., Citation2021). Likewise, endometrial tissue seems to be safe from SARS-CoV-2 since the ACE2 and TMPRSS2 expression is relatively low, but susceptibility to SARS-CoV-2 increases with age (Henarejos-Castillo et al. Citation2020). Oocyte cryopreservation occurs following the denudation of the oocyte (removal of the cumulus cells). Virus load seems to play an important role (in positive to the virus women or asymptomatic women) as well as the resistance to the virus of the interaction between somatic and germ cells. Bearing in mind that cryopreservation may further interrupt or destroy cellular or biochemical structures, it can be assumed that oocyte cryopreservation, during and perhaps after the pandemic, should be performed but with extra caution in case where a woman is asymptomatic or has not yet undergone the antigen test or has not yet completed the vaccination process.
Embryos
In line with spermatozoa and oocytes, any manipulation of embryos during the IVF process should be performed with caution. In detail, the use of various denudated agents to remove the cumulus cells from the oocyte, the swim-up or the gradient method followed by multiple washing steps to separate the viable from the non-viable spermatozoa, the injection of the spermatozoon into the oocyte (the ICSI procedure), the culture of fertilized oocytes, the addition of cryoprotectants in gamete or embryo samples during vitrification, the use of LN2, are procedures that extra caution should be paid. General measures to protect against the virus have already been proposed with respect to the area of the ART unit and in the embryology laboratory (Anifandis et al. Citation2020; Arav Citation2020). The presence of zona pellucida eliminates the possibility of the virus affecting the embryo. However, the possibility of infected spermatozoa (or merely of oocytes) to transmit any virus load during IVF insemination (and not ICSI) cannot be excluded due to the reasons described above. Nevertheless, this seems rather unlikely since the preparation of both sperm and oocytes for fertilization entails numerous steps that may ultimately eliminate any viral load. Although the expression of ACE2, TMPRSS2, and CD147 has been documented in human blastocysts (Essahib et al. Citation2020; Rajput et al. Citation2020) and the pre-implantation embryos (Chen et al. Citation2020), blastocyst embryo transfer may appear to be the best choice. The extra time of culture in the IVF laboratory may enable the possibility of taking the embryos through a daily routine of culture media change, ‘washing the embryos’ and promoting a less-likely infected culture environment. This practice may further eliminate the possibility of embryo contamination. The similarity of SARS-CoV-2 with other viruses regarding contamination and dispersal dynamics, such as the Zika virus, leads to the hypothesis that the early stages of embryo development are probably susceptible to virus infection. For instance, the trophectoderm cells of human and mouse pre-implantation embryos have been shown to be the target cells of the Zika virus leading to neural progenitor cell death, which causes microcephaly and early pregnancy loss (Tan et al. Citation2019).
Similarly, chicken embryos inoculated with Zika virus at the yolk sac developmental stage displayed delayed hatching and demonstrated neurological signs immediately after hatching (Ambagala et al. Citation2020). Exposure of gametes at the time of fertilization does not compromise blastocyst formation. However, Zika virus exposure severely impacts the zona-free blastocyst through embryonic degeneration at the very early stages of development, leading to early pregnancy loss, as shown in an experimental study (Block et al. Citation2020). Although the potential mother-to-child transmission of SARS-CoV-2 after childbirth is still worrying, the effect of this virus in the early stages of pregnancy should not be underestimated. However, the potential vertical transmission of SARS-CoV-2 from mother to fetus during pregnancy due to the limited expression of both receptors in follicles strengthens the above assumption (Lü et al. Citation2020). Moreover, the vertical transmission of SARS-CoV-2 at a theoretical level has been recently provided (Chen et al. Citation2020).
In contrast to the Zika virus, a similar experimental study in chickens in relation to SARS-CoV-2 infection that was conducted failed to support the previous result, meaning that the virus does not pose any infection risk in such embryos (Berhane et al. Citation2020). Recently, a case of a second-trimester miscarriage in a pregnant woman with SARS-CoV-2 infection has been reported (Baud et al. Citation2020). Moreover, ACE2 is highly expressed in human germ cells and pre-implantation embryos (Yan et al. Citation2013; Li et al. Citation2020c), while the nucleocapsid protein of SARS-CoV-2 abolishes the pluripotency of human-induced pluripotent stem cells (Lin et al. Citation2020). Since SARS-CoV-2 has been found to increase ACE2 expression by inhibiting or silencing the expression of specific embryo methylation patterns (Li et al. Citation2020d), it may be possible that SARS-CoV-2 may epigenetically adversely affect the early stages of embryo development. The possible impact of SARS-CoV-2 on fetuses is something that worth attention (Dang et al. Citation2020). Although COVID-19 is unlikely to cause any birth defects (Zhang et al. Citation2021), why then the doctors await the births to fall? (Wadman Citation2020). On the other hand, in vitro fertilization (IVF) may represent a safer reproductive strategy than natural conception at this time of the pandemic, while the embryos produced during ART manipulations appear to pose little risk (Stanley et al. Citation2020) but potential (Li et al. Citation2020d).
Culture media and liquid nitrogen
Gametes and embryos may be exposed to SARS-CoV-2 during preparation and cryopreservation. Culture media and the LN2 might be contaminated during equilibration when airborne SARS-CoV-2 particles from an infected embryology area or staff have infected the culture droplets and LN2 (Anifandis et al. Citation2020; De Ziegler et al. Citation2020) (). Recommendations for infection control in reproductive departments and embryo laboratories have been proposed (Maggiulli et al. Citation2020; Li et al. Citation2020b). Moreover, the use of laminar flows, prevents the contamination of the air in the working area during gamete manipulation, controlling the air quality by eliminating any viral particles and further mitigating the viral presence in the embryology area. Nevertheless, data from an infectious Zika virus experimental study, have shown increasing virus levels in the culture media following exposure (Block et al. Citation2020). Given the possible presence of the virus in the culture media, it will be wise to culture the embryos in separate microdroplets to reduce the risk of potential cross-contamination and encompassing the measure of ‘social distancing’ in embryo culture. Furthermore, the everyday refreshment of media in combination with non-sequential culture media seems to be a good option for eliminating the risk of culture media infection. Although the everyday refreshment of media may not be the optimal option due to time-lapse implementation in most laboratories, it seems that during the treatment of COVID-19 patients, this is the best option. Mineral oil is a prerequisite for most cultures. Its physicochemical properties may serve as a physical barrier protecting the embryo against the virus and symbolically ‘masking’ the embryo. However, mineral oil is not employed in cryopreservation procedures. Most steps in cryopreservation are taking place at room temperature or on a heated plate (37°C), where the virus may be present. To date, there are no data concerning the infection of culture media. Still, it is recommended that a declaration of virus-free media accompany its supply. As far as LN2 is concerned, SARS-CoV-2 may survive in such low, ultra-cooling temperatures. The Zika virus, for example, remains in the semen of symptom-free men for up to 1-year post-recovery (Kurscheidt et al. Citation2019), while the influenza virus can remain infectious even after 40 years in cryopreservation (Merrill et al. Citation2018). Therefore, the genetic similarity of SARS-CoV-2 with other viruses could explain a possible resistance of SAS-CoV-2 to LN2, especially when the virus load is relatively high. Although the direct or cross-contamination of human samples by viruses has not yet been recorded during cryopreservation, precautions through the use of highly secured closed devices and segregated cryovessels, should be taken during and after this pandemic (Yakass and Woodward Citation2020). Alternatively, vitrification of selected spermatozoa without the seminal plasma in combination with the addition of non-permeable cryoprotectants seem to be an effective method of maintaining sperm function without the possible presence of the virus (Schulz et al. Citation2020) because this vitrification method discards the seminal plasma and is followed by numerous washing steps.
Figure 1. A schematic presentation of the possible infection phases during embryological procedures by SARS-CoV-2. The virus through the ACE2 and TMPRSS2 receptors may gain entry in both male and female reproductive cells. During the IVF process, either with the typical insemination method or the ICSI procedure, the embryo might be infected when one of the two gametes have been infected. Alternatively, culture media used for the culture purposes of the embryos or LN2 might be infected either during production or transportation and subsequently may infect the cryopreservation procedure. High security straws may eliminate the possible risk of contamination
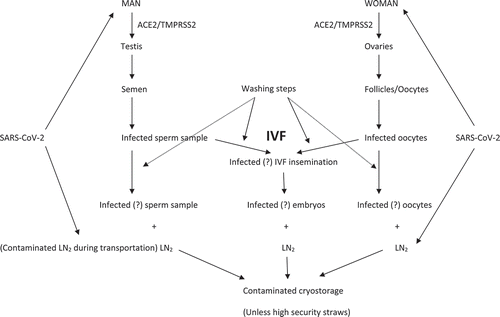
The addition of millimolar concentrations of BHT in the cryopreservation medium seems to maintain sperm parameters during vitrification, while in parallel, its antioxidant and antiviral properties further protects the sample from any viral infection (Merino et al. Citation2015). Alternatively, the use of sterile nitrogen to wash the devices during the thawing and warming phases may be advisable (Parmegiani et al. Citation2012). Larger studies with infected subjects should be done either to confirm or to exclude the presence of risks for male gametes that are destined for cryopreservation in liquid nitrogen (Corona et al. Citation2020; Scarica et al. Citation2021). Contamination of LN2 during production and transportation has not been reported in a clinical context. However, it is well known that the production and transportation of LN2 involves multiple steps that are not accomplished in a completely sterile environment. Therefore, sterilization or filtration of LN2 before any cryopreservation procedure appears to be a wise to eliminate any viral presence.
Conclusions
SARS-CoV-2 is currently a significant public health risk posing a serious threat for healthcare systems. Although vaccination has begun, COVID-19 seems to be a pandemic that packs a serious punch (Anifandis et al. Citation2021). Semen samples from COVID-19 patients may possess adequate levels of the virus. Since virus survival at cooling temperatures is not excluded, sperm parameters of the infected samples post-warming may be shifted. Oocyte performance as well as oocyte cryopreservation could be compromised during fertility treatments when a patient is virus positive. Similarly, embryo cryopreservation at the blastocyst stage may by far present as the optimal choice, because any possible contamination during fertilization may be diminished. Culture media and LN2 should be SARS-CoV-2 free, while the use of high security closed straws eliminates any possible contamination by LN2. As the pandemic continues to evolve as variants of the virus emerge that seem to reduce vaccine effectiveness, vaccines can prevent virus transmission but vaccines alone will notend the pandemic. . New generations of COVID-19 vaccines might be enough to control all the strains of the virus, but governments and social beliefs will affect the outcome of the pandemic.
Disclosure of potential conflicts of interest
No potential conflict of interest was reported by the authors.
Authors’ contributions
Conceptualization: GA, IM; writing—original draft preparation: GA, CM, MS, GS, AG; writing—review and editing: AD; supervision: IM. All authors have read and agreed to the version of the manuscript.
Additional information
Funding
References
- Adiga SK, Tholeti P, Uppangala S, Kalthur G, Gualtieri R, Talevi R. 2020. Fertility preservation during the COVID-19 pandemic: mitigating the viral contamination risk to reproductive cells in cryostorage. Reprod Biomed Online. 41(6):991–997. doi:10.1016/j.rbmo.2020.09.013.
- Alam MH, Miyano T. 2020. Interaction between growing oocytes and granulosa cells in vitro. Reprod Med Biol. 19(1):13–23. doi:10.1002/rmb2.12292.
- Alviggi C, Esteves SC, Orvieto R, Conforti A, La Marca A, Fischer R, Andersen CY, Bühler K, Sunkara SK, Polyzos NP, et al. 2020. POSEIDON (Patient-Oriented Strategies Encompassing IndividualizeD Oocyte Number) group 2020. COVID-19 and assisted reproductive technology services: repercussions for patients and proposal for individualized clinical management. Reprod Biol Endocrinol. 18(1):45. doi:10.1186/s12958-020-00605-z.
- Ambagala A, Truong T, Cottam-Birt C, Berhane Y, Gerdts V, Karniychuk U, Safronetz D, Babiuk S. 2020. Susceptibility of Chicken Embryos, Sheep, Cattle, Pigs, and Chickens to Zika Virus Infection. Front Vet Sci. 7:23. doi:10.3389/fvets.2020.00023
- Anifandis G, Messini CI, Daponte A, Messinis IE. 2020. COVID-19 and fertility: a virtual reality. Reprod Biomed Online. 41(2):157–159. doi:10.1016/j.rbmo.2020.05.001.
- Anifandis G, Tempest H, Oliva R, Swanson G, Simopoulou M, Easley C, Primig M, Messini C, Turek P, Sutovsky P, et al. 2021. COVID-19 and human reproduction: a pandemic that packs a serious punch. Syst Biol Reprod Med. 67(1):3–23. doi:10.1080/19396368.2020.1855271
- Arav A. 2020. A recommendation for IVF lab practice in light of the current COVID-19 pandemic. J Assist Reprod Genet. 37(7):1543. doi:10.1007/s10815-020-01841-3.
- Barragan M, Guillén JJ, Martin-Palomino N, Rodriguez A, Vassena R. 2021. Undetectable viral RNA in oocytes from SARS-CoV-2 positive women. Hum Reprod. 36(2):390–394. doi:10.1093/humrep/deaa284.
- Barreta MH, Gasperin BG, Ferreira R, Rovani M, Pereira GR, Bohrer RC, De Oliveira JF, Gonçalves PB. 2015. The components of the angiotensin-(1-7) system are differentially expressed during follicular wave in cattle. J Renin Angiotensin Aldosterone Syst. 16(2):275–283. doi:10.1177/1470320313491996.
- Baud D, Greub G, Favre G, Gengler C, Jaton K, Dubruc E, Pomar L. 2020. Second-trimester miscarriage in a pregnant woman with SARS-CoV-2 infection. JAMA. 323(21):2198–2200. doi:10.1001/jama.2020.7233.
- Berhane Y, Suderman M, Babiuk S, Pickering B. 2020. Susceptibility of turkeys, chickens and chicken embryos to SARS-CoV-2. Transbound Emerg Dis. Dec 29 . doi: 10.1111/tbed.13970.
- Block LN, Aliota MT, Friedrich TC, Chotzko ML, Mean KD, Wiepz GJ, Golos TG, Schmidt JK. 2020. Embryotoxic impact of Zika virus in a rhesus macaque in vitro implantation model. Biol Reprod. 102(4):806‐16. doi:10.1093/biolre/ioz236.
- Cardona Maya WD, Du Plessis SS, Velilla PA. 2016. Semen as virus reservoir? J Assist Reprod Genet. 33(9):1255–1256. doi:10.1007/s10815-016-0747-8.
- Cardona Maya WD, Du Plessis SS, Velilla PA. 2020. SARS-CoV-2 and the testis: similarities to other viruses and routes of infection. Reprod Biomed Online. 40(6):763–764. doi:10.1016/j.rbmo.2020.04.009.
- Chen W, Yuan P, Yang M, Yan Z, Kong S, Yan J, Liu X, Chen Y, Qiao J, Yan L. 2020. SARS-CoV-2 entry factors: ACE2 and TMPRSS2 are expressed in peri-implantation embryos and the maternal-fetal interface. Engineering (Beijing). 6(10):1162–1169. doi:10.1016/j.eng.2020.07.013.
- Corona G, Baldi E, Isidori AM, Paoli D, Pallotti F, De Santis L, Francavilla F, La Vignera S, Selice R, Caponecchia L, et al. 2020. SARS-CoV-2 infection, male fertility and sperm cryopreservation: a position statement of the Italian Society of Andrology and Sexual Medicine (SIAMS) (Società Italiana di Andrologia e Medicina della Sessualità). J Endocrinol Invest. 43(8):1153–1157. doi:10.1007/s40618-020-01290-w.
- Dang D, Wang L, Zhang C, Li Z, Wu H. 2020. Potential effects of SARS-CoV-2 infection during pregnancy on fetuses and newborns are worthy of attention. J Obstet Gynaecol Res. 46(10):1951–1957. doi:10.1111/jog.14406.
- De Toni L, Garolla A, Di Nisio A, Rocca MS, Foresta C. 2020. Caution in the management of SARS-CoV-2 infection in males. Andrology. 41(5):967–968. doi:10.1016/j.rbmo.2020.08.016.
- De Ziegler D, Pirtea P, Scott RT, Rienzi L, Poulain M, Pharm D, Jean Marc Ayoubi JM. 2020. ART during the SARS-CoV-2 pandemic: a new context, new challenges, and new recommendations. Fertil Steril (Fertility and Sterility Dialogue).
- Douglas GC, O’Bryan MK, Hedger MP, Lee DK, Yarski MA, Smith A, Lew RA. 2004. The novel angiotensin-converting enzyme (ACE) homolog, ACE2, is selectively expressed by adult Leydig cells of the testis. Endocrinology. 145(10):4703–4711. doi:10.1210/en.2004-0443.
- Eisenberg ML. 2020. Coronavirus Disease 2019 (COVID-19) and men’s reproductive health. Fertil Steril. 113(6):1154. doi:10.1016/j.fertnstert.2020.04.039.
- Essahib W, Verheyen G, Tournaye H, Van De Velde H. 2020. SARS-CoV-2 host receptors ACE2 and CD147 (BSG) are present on human oocytes and blastocysts. J Assist Reprod Genet. 37(11):2657–2660. doi:10.1007/s10815-020-01952-x.
- Esteves SC, Lombardo F, Garrido N, Alvarez J, Zini A, Colpi GM, Kirkman-Brown J, Lewis SE, Björndahl L, Majzoub A, et al. 2020. SARS-CoV-2 pandemic and repercussions for male infertility patients: a proposal for the individualized provision of andrological services. Andrology. 18(1):45. doi:10.1186/s12958-020-00605-z.
- Feldmann H. 2018. Virus in semen and the risk of sexual transmission. N Eng J Med. 378(15):1440–1441. doi:10.1056/NEJMe1803212.
- Foresta C, Rocca MS, Di Nisio A. 2020. Gender susceptibility to COVID-19: a review of the putative role of sex hormones and X chromosome. J Endocrinol Invest. 16:1–6. doi:10.1007/s40618-020-01383-6
- Fraietta R, Pasqualotto FF, Roque M, Taitson PF. 2020. SARS-COV-2 and male reproductive health. JBRA Assist Reprod. 24(3):347–350. doi:10.5935/1518-0557.20200047.
- Gobeil SM, Janowska K, McDowell S, Mansouri K, Parks R, Manne K, Stalls V, Kopp MF, Henderson R, Edwards RJ, et al. 2020. D614G mutation alters SARS-CoV-2 spike conformation and enhances protease cleavage at the S1/S2 junction. Cell Rep. 108630. doi:10.1016/j.celrep.2020.108630
- Gonzalez DC, Khodamoradi K, Pai R, Guarch K, Connelly ZM, Ibrahim E, Arora H, Ramasamy R. 2020. A systematic review on the investigation of SARS-CoV-2 in semen. Res Rep Urol. 12:615–621. doi:10.2147/RRU.S277679
- Guruprasad L. 2021. Human SARS CoV-2 spike protein mutations. Proteins. 89(5):569–576. doi:10.1002/prot.26042.
- Henarejos-Castillo I, Sebastian-Leon P, Devesa-Peiro A, Pellicer A, Diaz-Gimeno P. 2020. SARS-CoV-2 infection risk assessment in the endometrium: viral infection-related gene expression across the menstrual cycle. Fertil Steril. 114(2):223–232. doi:10.1016/j.fertnstert.2020.06.026.
- Hickman C, Rogers S, Huang G, MacArthur S, Meseguer M, Nogueira D, Portela R, Rienzi L, Sharp T, Ye H. 2020. Managing the IVF laboratory during a pandemic: international perspectives from laboratory managers. Reprod Biomed Online. 41(2):141–150. doi:10.1016/j.rbmo.2020.05.006.
- Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, et al. 2020. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 181(2):271–280.e8. doi:10.1016/j.cell.2020.02.052.
- Holtmann N, Edimiris P, Andree M, Doehmen C, Baston-Buest D, Adams O, Kruessel J-S, Bielfeld A-P. 2020. Assessment of SARS-CoV-2 in human semen - a cohort study. Fertil Steril. 114(2):233–238. doi:10.1016/j.fertnstert.2020.05.028.
- Honorato-Sampaio K, Pereira VM, Santos RA, Reis AM. 2012. Evidence that angiotensin-(1-7) is an intermediate of gonadotrophin-induced oocyte maturation in the rat preovulatory follicle. Exp Physiol. 97(5):642–650. 107:83-88. doi:10.1016/j.peptides.2018.08.007.
- IFFS: COVID-19 task force statements. 2020. https://www.iffsreproduction.org/page/COVID-Statements.
- Iwata-Yoshikawa N, Okamura T, Shimizu Y, Hasegawa H, Takeda M, Nagata N. 2019. TMPRSS2 contributes to virus spread and immunopathology in the airways of murine models after coronavirus infection. J Virol. 93(6):e01815–18. doi:10.1128/JVI.01815-18.
- Kurscheidt FA, Mesquita CSS, Damke GMZF, Damke E, Carvalho ARBA, Suehiro TT, Teixeira JJV, Da Silva VRS, Souza RP, Consolaro MEL. 2019. Persistence and clinical relevance of Zika virus in the male genital tract. Nat Rev Urol. 16(4):211–230. doi:10.1038/s41585-019-0149-7.
- La Marca A, Niederberger C, Pellicer A, Nelson SM. 2020. COVID-19: lessons from the Italian reproductive medical experience. Fertil Steril. 113(5):920–922. doi:10.1016/j.fertnstert.2020.03.021.
- Larasati T, Noda T, Fujihara Y, Shimada K, Tobita T, Yu Z, Matzuk MM, Ikawa M. 2020. Tmprss12 is required for sperm motility and uterotubal junction migration in mice. Biol Reprod. 103(2):254–263. doi:10.1093/biolre/ioaa060.
- Li D, Jin M, BaO P, Zhao W, Zhang S. 2020a. Clinical characteristics and results of semen tests among men with coronavirus disease 2019. JAMA. 3(5):e208292.
- Li G, Li W, Song B, Wu H, Tang D, Wang C, He X, Cao Y. 2020b. SARS-CoV-2 and the reproductive system: assessment of risk and recommendations for infection control in reproductive departments. Syst Biol Reprod Med. 66(6):343–346. doi:10.1080/19396368.2020.1817627.
- Li R, Yin T, Fang F, Li Q, Chen J, Wang Y, Hao Y, Wu G, Duan P, Wang Y, et al. 2020c. Potential risks of SARS-CoV-2 infection on reproductive health. Reprod Biomed Online. 41(1):89–95. doi:10.1016/j.rbmo.2020.04.018.
- Li Y, Li H, Zhou L. 2020d. EZH2-mediated H3K27me3 inhibits ACE2 expression. Biochem Biophys Res Commun. 526(4):947–952. doi:10.1016/j.bbrc.2020.04.010.
- Lin Z, Fang Q, Mai J, Zhou L, Qian Y, Cai T, Chen Z, Wang P, Lin B 2020. The nucleocapsid protein of SARS-CoV-2 abolished pluripotency in human induced pluripotent stem cells. bioRxiv 2020–2023.
- Liu Y, Yan LM, Wan L, Xiang TX, Le A, Liu JM, Peiris M, Poon LLM, Zhang W. 2020. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis. 20(6):656–657. doi:10.1016/S1473-3099(20)30232-2.
- Lü M, Qiu L, Jia G, Guo R, Leng Q. 2020. Single-cell expression profiles of ACE2 and TMPRSS2 reveals potential vertical transmission and fetus infection of SARS-CoV-2. Aging (Albany NY). 12(20):19880–19897. doi:10.18632/aging.104015.
- Lukassen S, Chua RL, Trefzer T, Kahn NC, Schneider MA, Muley T, Winter H, Meister M, Veith C, Boots AW, et al. 2020. SARS-CoV-2 receptor ACE2 and TMPRSS2 are primarily expressed in bronchial transient secretory cells. EMBO J. 39(10):e105114. doi:10.15252/embj.20105114.
- Ma L, Xie W, Li D, Shi L, Mao Y, Xiong Y, Zhang Y, Zhang M 2020. Effect of SARS-CoV-2 infection upon male gonadal function: a single center-based study. medRxiv.2020.2003.2021.20037267.
- Maggiulli R, Giancani A, Fabozzi G, Dovere L, Tacconi L, Amendola MG, Cimadomo D, Ubaldi FM, Rienzi L. 2020. Assessment and management of the risk of SARS-CoV-2 infection in an IVF laboratory. Reprod Biomed Online. 41(3):385–394. doi:10.1016/j.rbmo.2020.06.017.
- Maleki BH, Tartibian B. 2021. COVID-19 and male reproductive function: a prospective, longitudinal cohort study. Reproduction. 161(3):319–331. doi:10.1530/REP-20-0382.
- Massarotti C, Garolla A, Maccarini E, Scaruffi P, Stigliani S, Anserini P, Foresta C. 2021. SARS-CoV-2 in the semen: where does it come from? Andrology. 9(1):39–41. doi:10.1111/andr.12839.
- Merino O, Aguagüiña WE, Esponda P, Risopatrón J, Isachenko E, Isachenko V, Sánchez R. 2015. Protective effect of butylated hydroxytoluene on sperm function in human spermatozoa cryopreserved by vitrification technique. Andrologia. 47(2):186–193. doi:10.1111/and.12246.
- Merrill DR, Wade CD, Fahnestock P, Baker RO 2018. Long-term and short-term stability of viruses depend on storage temperature and preservation method. Beiresources poster.
- Monteleone PA, Nakano M, Lazar V, Gomes AP, Martin H, Bonetti TC. 2020. A review of initial data on pregnancy during the COVID-19 outbreak: implications for assisted reproductive treatments. JBRA Assist Reprod. 24(2):219–225. doi:10.5935/1518-0557.20200030.
- Pal R, Banerjee M. 2020. COVID-19 and the endocrine system: exploring the unexplored. J Endocrinol Invest. 43(7):1027–1031. doi:10.1007/s40618-020-01276-8.
- Pan F, Xiao X, Guo J, Song Y, Li H, Patel DP, Spivak AM, Alukal JP, Zhang X, Xiong C, et al. 2020. No evidence of SARS-CoV-2 in semen of males recovering from COVID-19. Fertil Steril. 13(6):1135–1139. doi:10.1016/j.fertnstert.2020.04.024.
- Pan PP, Zhan QT, Le F, Zheng YM, Jin F. 2013. Angiotensin-converting enzymes play a dominant role in fertility. Int J Mol Sci. 14(10):21071–21086. doi:10.3390/ijms141021071.
- Paoli D, Pallotti F, Turriziani O, Mazzuti L, Antonelli G, Lenzi A, Lombardo F. 2021. SARS-CoV-2 presence in seminal fluid: myth or reality. Andrology. 9(1):23–26. doi:10.1111/andr.12825.
- Parmegiani L, Accorsi A, Bernardi S, Arnone A, Cognigni GE, Filicori MA. 2012. Reliable procedure for decontamination before thawing of human specimens cryostored in liquid nitrogen: three washes with sterile liquid nitrogen (SLN2). Fertil Steril. 98(4):870–875. doi:10.1016/j.fertnstert.2012.06.028.
- Perry MJ, Arrington S, Neumann LM, Carrell D, Mores CN. 2020. It is currently unknown whether SARS-CoV-2 is viable in semen or whether COVID-19 damages spermatozoa. Andrology. 9(1):30–32. doi:10.1111/andr.12831.
- Qian G, Yang N, Ma AHY, Wang L, Li G, Chen X, Chen X. 2020. A COVID-19 Transmission within a family cluster by presymptomatic infectors in China. Clin Infect Dis. 71(15):861–862. doi:10.1093/cid/ciaa316.
- Rajput SK, Logsdon DM, Kile B, Engelhorn HJ, Goheen B, Khan S, Swain J, McCormick S, Schoolcraft WB, Yuan Y, et al. 2020. Human eggs, zygotes, and embryos express the receptor ACE2 and protease TMPRSS2 protein necessary for SARS-CoV-2 infection. F S Sci. 10.1016/j.xfss.2020.12.005.
- Reis FM, Bouissou DR, Pereira VM, Camargos AF, Dos Reis AM, Santos RA. 2011. Angiotensin-(1-7), its receptor Mas, and the angiotensin-converting enzyme type 2 are expressed in the human ovary. Fertil Steril. 95(1):176–181. doi:10.1016/j.fertnstert.2010.06.060.
- Requena A, Cruz M, Vergara V, Prados N, Galliano D, Pellicer A. 2020. A picture of the covid-19 impact on IVIRMA fertility treatment clinics in Spain and Italy. Reprod BioMed Online. 41(1):1–5. doi:10.1016/j.rbmo.2020.04.015.
- Ruan Y, Hu B, Liu Z, Liu K, Jiang H, Li H, Li R, Luan Y, Liu X, Yu G, et al. 2021. No detection of SARS-CoV-2 from urine, expressed prostatic secretions, and semen in 74 recovered COVID-19 male patients: a perspective and urogenital evaluation. Andrology. 9(1):99–106. doi:10.1111/andr.12939.
- Santos RA, Ferreira AJ, Verano-Braga T, Bader M. 2013. Angiotensin-converting enzyme 2, angiotensin-(1-7) and Mas: new players of the renin-angiotensin system. J Endocrinol. 216(2):R1–R17. doi:10.1530/JOE-12-0341.
- Scarica C, Parmegiani L, Rienzi L, Anastasi A, Cimadomo D, Klinger FG, Licata E, Sosa Fernandez L, De Santis L. 2021. SARS-CoV-2 persistence at subzero temperatures. J Assist Reprod Genet. 5:1–3. doi:10.1007/s10815-021-02094-4
- Schulz M, Risopatrón J, Uribe P, Isachenko E, Isachenko V, Sánchez R. 2020. Human sperm vitrification: a scientific report. Andrology. 8(6):1642–1650. doi:10.1111/andr.12847.
- Song C, Wang Y, Li W, Hu B, Chen G, Xia P, Wang W, Li C, Diao F, Hu Z, et al. 2020. Absence of 2019 novel coronavirus in semen and testes of COVID-19 patients. Biol Reprod. 103(1):4–6. doi:10.1093/biolre/ioaa050.
- Souza MD, Nakagawa H, Taitson PF, Cordts EB, Antunes RA. 2020. Management of ART and COVID-19: infertility in times of pan- demic. What now? JBRA Assist Reprod. 24(3):231–232. doi:10.5935/1518-0557.20200031.
- Stanley KE, Thomas E, Leaver M, Wells D. 2020. Coronavirus disease (COVID-19) and fertility: viral host entry protein expression in male and female reproductive tissues. Fertil Steril. 114(1):33–43. doi:10.1016/j.fertnstert.2020.05.001.
- Tan L, Lacko LA, Zhou T, Tomoiaga D, Hurtado R, Zhang T, Sevilla A, Zhong A, Mason CE, Noggle S, et al. 2019. Pre- and peri-implantation Zika virus infection impairs fetal development by targeting trophectoderm cells. Nat Commun. 10(1):4155. doi:10.1038/s41467-019-12063-2.
- Temiz MZ, Dincer MM, Hacibey I, Yazar RO, Celik C, Kucuk SH, Alkurt G, Doganay L, Yuruk E, Muslumanoglu AY. 2020. Investigation of SARS-CoV-2 in semen samples and the effects of COVID-19 on male sexual health by using semen analysis and serum male hormone profile: a cross-sectional, pilot study. Andrologia. e13912. doi:10.1111/and.13912
- Tesarik J. 2020. After corona: there is life after the pandemic. Reprod BioMed Online. 40(6):760–762. doi:10.1016/j.rbmo.2020.04.002.
- Veiga A, Gianaroli L, Ory S, Horton M 2021. Joint IFFS/ESHRE statement on COVID-19 vaccination for pregnant women and those considering pregnancy.
- Veiga A, Gianaroli L, Ory S, Horton M, Feinberg E, Penzias A. 2020. Assisted reproduction and COVID-19: a joint statement of ASRM, ESHRE and IFFS. Hum Reprod Open. 2020(3):hoaa033. doi:10.1093/hropen/hoaa033.
- Verma S, Saksena S, Sadri-Ardekani H. 2020. ACE2 receptor expression in testes: implications in COVID-19 pathogenesis. Biol Reprod. 40(6):760–762. doi:10.1016/j.rbmo.2020.04.002.
- Virant-Klun I, Strle F. 2021. Human oocytes express both ACE2 and BSG genes and corresponding proteins: is SARS-CoV-2 infection possible? Stem Cell Rev Rep. 5:1–7. doi:10.1007/s12015-020-10101-x
- Wadman M. 2020. COVID-19 unlikely to cause birth defects, but doctors await fall births. Science. 369(6504):607. doi:10.1126/science.369.6504.607.
- Wang M, Liu X, Chang G, Chen Y, An G, Yan L, Gao S, Xu Y, Cui Y, Dong J, et al. 2018. Single-cell RNA sequencing analysis reveals sequential cell fate transition during human spermatogenesis. Cell Stem Cell. 23(4):599–614.e4. doi:10.1016/j.stem.2018.08.007.
- Wang Z, Xu X. 2020. scRNA-seq profiling of human testes reveals the presence of the ACE2 receptor, a target for SARS-CoV-2 infection in spermatogonia, leydig and sertoli cells. Cells. 9(4):920. doi:10.3390/cells9040920.
- Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, Graham BS, McLellan JS. 2020. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 367(6483):1260–1263. doi:10.1126/science.abb2507.
- Yakass MB, Woodward B. 2020. COVID-19: should we continue to cryopreserve sperm during the pandemic? Reprod BioMed Online. 40(6):905. doi:10.1016/j.rbmo.2020.04.004.
- Yan L, Yang M, Guo H, Yang L, Wu J, Li R, Liu P, Lian Y, Zheng X, Yan J, et al. 2013. Single-cell RNA-Seq profiling of human pre-implantation embryos and embryonic stem cells. Nat Struct Mol Biol. 20(9):1131–1139. doi:10.1038/nsmb.2660.
- Younis JS, Abassi Z, Skorecki K. 2020. Is there an impact of the COVID-19 pandemic on male fertility? The ACE2 connection. Am J Physiol Endocrinol Metab. 318(6):E878–E880. doi:10.1152/ajpendo.00183.2020.
- Zhang P, Heyman T, Greechan M, Dygulska B, Al Sayyed F, Narula P, Lederman S. 2021. Maternal, neonatal and placental characteristics of SARS-CoV-2 positive mothers. J Matern Fetal Neonatal Med. 28:1–9. doi:10.1080/14767058.2021.1892637
