ABSTRACT
The aim of our study was to investigate the effect of maternal and embryo MTHFR C677T and A1298C polymorphisms on embryo aneuploidies and mosaicism and the correlation between these genetic variants in transferred euploid embryos and IVF outcomes. MTHFR genotype was analyzed in 77 women who performed an IVF/ICSI cycle with PGT-A. Moreover, to evaluate the effect of embryo MTHFR polymorphisms on embryo aneuploidies and mosaicism, the MTHFR genotype was analyzed in 191 biopsied embryos from the PGT-A cycles of these patients. Additionally, 218 DNA samples from trophectoderm biopsies belonging to a different group of patients were also genotyped. MTHFR polymorphisms were analyzed in a total amount of 409 trophectoderm samples. The main parameters analyzed were embryo aneuploidy and mosaicism rates. Finally, the IVF outcomes of 241 single euploid embryo transfers were assessed and compared between different MTHFR embryo genotypes. The aneuploidy rates were similar in embryos from homozygous normal women and women with at least one mutated allele (54.7% vs. 30.2% in 677C>T and 37.8% vs. 42.7% in 1298A>C). Furthermore, no differences were observed in the mosaicism rate (24.0% vs. 13.8% in 677C>T and 17.1% vs. 17.3% in 1298A>C). A similar analysis was performed, taking into account the embryo genotype results. No differences in aneuploidy rate were observed between the study groups. The only significant difference was the mosaicism rate among 677C>T genotype (13.5% in 677CC group vs. 5.4% in 677CT/TT; p = 0.019). Implantation rate, biochemical and clinical miscarriage rates, and ongoing pregnancy rate were compared between different embryo genotypes, and no statistically significant differences were found. In conclusion, the maternal MTHFR genotype did not influence embryo chromosomal abnormalities. Moreover, the embryo MTHFR genotype was not associated with embryo aneuploidy or IVF outcomes such as implantation, pregnancy loss, and ongoing pregnancy when euploid embryos were transferred.
Abbreviations: MTHFR: methylenetetrahydrofolate reductase; IVF: in vitro fertilization; PGT-A: preimplantation genetic testing for aneuploidies; SAM: S-adenosyl methionine; SNP: single nucleotide polymorphism; SPSS: Statistical Package for Social Sciences; RIF: recurrent implantation failure; RPL: recurrent pregnancy loss; hCG: human chorionic gonadotropin; PBS: phosphate buffered saline; CGH: comparative genomic hybridization; NGS: next generation sequencing
Introduction
Folic acid and folates, present in certain foods, are essential nutrients for several biological functions since they participate in the synthesis of methionine and S-adenosyl methionine (SAM). The SAM, in turn, contributes to donating methyl groups in cellular processes such as DNA and protein synthesis and epigenetic mechanisms. Folate deficiency, therefore, can affect different biological processes, and it has been related to several pathologies and reproductive problems (Forges et al. Citation2007; Thaler Citation2014). Among all the genes involved in folate metabolism, one of the most important from the biological point of view is methylenetetrahydrofolate reductase, or MTHFR. The enzyme MTHFR catalyzes the conversion of 5,10-methylene-tetrahydrofolate to 5-methyl-tetrahydrofolate, the predominant circulating form of folate. 5-Methyl-tetrahydrofolate is the primary methyl donor for converting homocysteine to methionine (essential amino acid) and S-adenosyl-methionine (SAM), required for nucleotide and protein synthesis, as well as for DNA methylation. Regulation of MTHFR activity is crucial in maintaining cellular concentrations of methionine and SAM. Many MTHFR sequence variants have been described (Goyette et al. Citation1995). However, two single nucleotide polymorphisms (SNPs) located at positions 677 and 1298 have been the most studied (Frosst et al. Citation1995; Van Der Put et al. Citation1998). At position 677 of the gene, a change from cytosine to thymine (C677T) can occur, causing the replacement of alanine by valine in the protein (p.Ala222Val). This change results in a reduction in enzyme activity. Heterozygous (CT) and homozygous (TT) mutant individuals show a decline in the in vitro enzyme activity of 35% and 70%, respectively (Frosst et al. Citation1995). In comparison at position 1298, a change from adenine to cytosine (A1298C) may occur, leading to a substitution of glutamate for alanine (p.Glu429Ala) (Van Der Put et al. Citation1998). Homozygous mutant (CC) individuals exhibit a 40% decrease in enzyme function (Van Der Put et al. Citation1998; Weisberg et al. Citation1998). Moreover, those individuals heterozygous for the two polymorphisms (677CT and 1298AC) have 50% of normal enzyme activity and the same biochemical parameters as homozygous individuals 677TT (Van Der Put et al. Citation1998).
Deficiency of MTHFR may lead to an increase in plasma homocysteine levels (Jacques et al. Citation1996), a vasculotoxic, embryotoxic and neurotoxic molecule (Lucock Citation2006). In some individuals, the concentration of circulating homocysteine is so high that they develop hyperhomocysteinemia (Frosst et al. Citation1995; Van Der Put et al. Citation1998). This is a risk factor for myocardial infarction, venous thrombosis, recurrent miscarriage, and other problems during pregnancy (Wouters et al. Citation1993; Frosst et al. Citation1995; Nelen et al. Citation1997, Citation1998; Quere et al. Citation1998; Van Der Put et al. Citation1998; Alam et al. Citation2008). In comparison, the presence of MTHFR mutations, especially if there is an additional folate deficiency, causes a decrease in S-adenosyl-methionine level leading to DNA hypomethylation, which could affect embryo development. Moreover, MTHFR mutations have also been associated with chromosomal non-disjunction and fetal aneuploidies (Melnyk et al. Citation2000; Hassold et al. Citation2001).
Nevertheless, the relationship of the MTHFR 677T and 1298C polymorphisms with reproductive problems is still a controversial issue. Some studies suggest that these MTHFR mutations do not constitute a risk factor for infertility, even when the mutation is homozygous (Ni et al. Citation2015; Soligo et al. Citation2017). According to these publications, Dobson et al. (Citation2007) showed that these polymorphisms were not associated with embryo quality, pregnancy, or miscarriage rates in the IVF population. Eliminate, Patounakis et al. (Citation2016) examined 1717 female patients attempting their first cycle of IVF with a total of 4169 blastocysts transferred (mean of 2.4 transferred embryos). Regardless of MTHFR genotype, they did not observe any statistically significant differences in positive pregnancy test results, clinical pregnancy, embryo implantation, live birth, or pregnancy loss rates. In contrast to the previous studies, Safdarian et al. (Citation2014) considered the homozygote form of MTHFR C677T mutation a risk factor for recurrent IVF failure, and Enciso et al. (Citation2016) concluded that maternal MTHFR 1298C genotype incidence is increased in women with several unsuccessful IVF cycles.
The relationship between these polymorphisms involved in folate metabolism and chromosomal non‑disjunction leading to embryo aneuploidy has been investigated in recent years. Bae et al. (Citation2007), comparing the MTHFR genotype distribution between euploid and aneuploid spontaneously aborted embryos, showed that the MTHFR genotypes’ frequency did not affect the chromosomal status of the abortus. Furthermore, Kaur (Citation2013) found no association between C677T polymorphism and risk of non‑disjunction in women having Down syndrome children. However, Guo et al. (Citation2015) observed that MTHFR 677 T genotype was associated with a higher occurrence of trisomies on chromosomes 18 and 21. The 1298C allele was also significantly associated with the risk of fetal chromosomal aneuploidies leading to spontaneous miscarriages (Kim et al. Citation2011).
Others (Enciso et al. Citation2016), who studied the incidence of MTHFR polymorphisms in male and female infertile patients and embryos, concluded that maternal MTHFR genotype affects the embryo chromosomal status. However, they did not observe a relationship between embryo MTHFR genotype and aneuploidies. Surprisingly, they also described a negative association between the MTHFR 677T allele and the implantation rate of euploid embryos.
Considering the existing controversy, the objective of this study was first to evaluate the effect of maternal and embryo MTHFR gene polymorphisms on embryo aneuploidies and the correlation between embryo polymorphisms and IVF outcomes in euploid embryos. The incidence of MTHFR polymorphisms between our RPL and RIF female patients and a European population was then determined. This permitted us to investigate a possible relationship between MTHFR gene polymorphisms and embryo mosaicism, a hypothesis that has not been studied to date.
Results
Seventy-seven patients underwent ovarian stimulation followed by ICSI-PGT-A were included to investigate a possible effect of maternal MTHFR polymorphisms (C677T and A1298C) on embryo chromosomal abnormalities (aneuploidy and mosaicism) as summarized in . The MTHFR polymorphisms tests were performed within the inherited thrombophilia analysis, before the IVF cycle, for two main indications: recurrent implantation failure (RIF, n = 40) and recurrent pregnancy loss (RPL, n = 37).
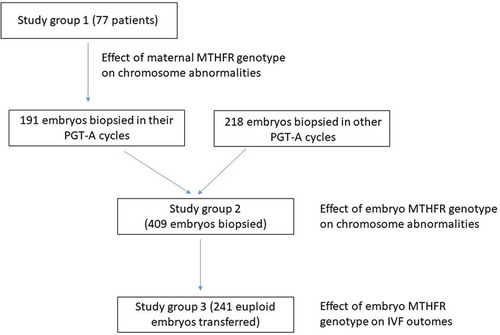
Figure 1. Study design. Flowchart describing the number of samples in the different groups included in the study. As shown, the study group 3 is a subset of the study group 2
Their biochemical and stimulation characteristics are summarized in . All variables were similar among different genotype groups (data are not shown). Homocysteine levels were 7.50 ± 1.70 mmol/L in MTHFR 677CC genotype vs. 7.6 ± 2.6 mmol/L in MTHFR 677CT/TT genotypes (p = 0.724), and 7.6 ± 1.7 mmol/L in 1298AA vs. 7.6 ± 2.7 mmol/L in MTHFR 1298AC/CC (p = 0.820).
Table 1. Patient characteristics and biochemical and stimulation variables
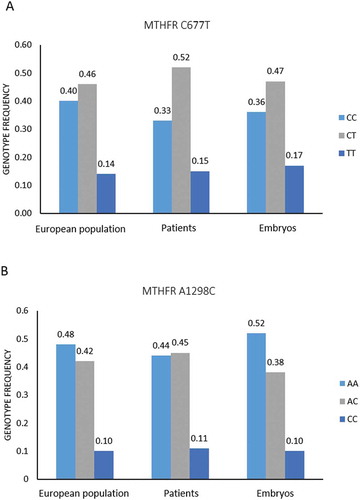
The genotype distribution of the different MTHFR polymorphisms is shown in . We compared the frequencies observed in our population with the 1000 Genomes Project (Phase 3) genotype frequencies, and we did not observe statistically significant differences between the two populations ().
Table 2. Genotype frequencies compared with 1000 Genomes Project (Phase 3) European population
To explore the effect of different maternal MTHFR genotypes on embryo chromosomal abnormalities, we compared aneuploidy and mosaicism rates between different C677T and A1298C genotypes. In the 191 biopsied embryos from the PGT-A cycles, the mean aneuploidy rate was 38.8%, and the mosaicism rate was 17.3%. According to the MTHFR polymorphisms, the aneuploidy rate obtained was comparable in embryos from patients with a homozygous normal genotype and those embryos from women with at least one mutant allele (54.7% in 677CC vs. 30.2% in 677CT/TT, p = 0.058 and 37.8% in 1298AA vs. 42.7% in 1298AC/CC, p = 0.212). Furthermore, no differences were observed in the mosaicism rate (24.0% in 677CC vs. 13.8% in 677CT/TT, p = 0.345 and 17.1% in 1298AA vs. 17.3% in 1298AC/CC, p = 0.865) ().
Table 3. Comparison of aneuploidy and mosaicism rates in embryos (n = 191) according to maternal MTHFR genotype
To investigate the effect of the embryo MTHFR genotypes on chromosomal alterations with a wide number of samples, we genotyped 409 DNA samples from trophectoderm biopsies (study group 2). shows the C677T and A1298C genotype distribution in these embryos, together with those observed in female patients (study group 1) and the European population. Fisher’s Exact test was used to compare embryo genotype frequencies with the frequencies in the European population and female patients, and no significant differences were observed (p > 0.05). With regard to chromosomal alterations, the aneuploidy rate was 18.1%, and the mosaicism rate was 8.1%, being the average maternal age 32.6 ± 6.7 years. If we consider the embryo MTHFR genotype, the aneuploidy rate was 22.3% in 677CC blastocysts vs. 15.7% in blastocysts with at least one mutated allele (p = 0.464), and 17.9% in embryos 1298AA vs. 18.3% in embryos 1298AC/CC (p = 0.988). In comparison, the incidence of embryo mosaicism was 13.5% in 677CC group vs. 5.4% in 677CT/TT group (p = 0.019) and 7.5% in 1298AA vs. 9.1% in 1298AC/CC group (p = 0.817). The only significant difference was observed in mosaicism rate among 677C>T genotype ().
Table 4. Comparison of aneuploidy and mosaicism rates in embryos (n = 409) according to their MTHFR genotype
Figure 2. C677T and A1298C MTHFR genotype frequencies in the 1000 Genomes Project (Phase 3) European population, female patients and embryos. A) C677T genotypes. B) A1298C genotypes. Fisher’s exact test was used to compare genotype frequencies in patients and embryos with the European population frequencies
The different individual genotypes and genotype and haplotype combinations were analyzed to examine the relationship among the embryo C677T/A1298C MTHFR genotypes and the IVF outcomes. The pregnancy rate, implantation rate, biochemical and clinical miscarriage rates, and the ongoing pregnancy rate for 241 single euploid blastocyst transfer cycles were assessed (). There were no significant differences in any of the outcomes, for 677CC vs. 677CT/677TT genotypes (analyzed individually or combined), 1298AA vs. 1298AC/1298CC (analyzed individually or combined), and 677CC/1298AA haplotype vs. 677CT/TT/1298AC/CC (those haplotypes with at least one 677 and/or 1298 mutated allele), except for the pregnancy rate in the 1298AC genotype. The pregnancy rate was 60.0% in 1298AC embryos vs. 47.3% in 1298AA embryos (p = 0.023).
Table 5. IVF outcomes for single euploid embryo transfers according to different C677T/A1298C MTHFR genotypes in transferred embryos (n = 241)
Discussion
The results of this study show that frequencies of different C677T and A1298C MTHFR genotypes in our population of 77 infertile women (RIF or RPL) do not differ from the genotype frequencies described in the general population within the same ethnic group. This suggests that these polymorphisms are not associated with infertility problems such as recurrent pregnancy loss and recurrent implantation failure in this population. According to these data, the meta-analysis published by Di Nisio et al. (Citation2011) demonstrated that the percentage of women MTHFR heterozygous or homozygous for C677T/A1298C was, overall, similar among the patients with ART failures and controls. These results agree with Patounakis et al. (Citation2016), who genotyped 1717 female IVF patients. No significant differences in genotype frequencies between their study group and a similar population were found. The meta-analysis by Wu et al. (Citation2012) showed that the C677T MTHFR polymorphism was not associated with RPL in the Caucasian population. Rai (Citation2014) reported similar results with respect to the A1298C mutation.
Nevertheless, Enciso et al. (Citation2016), who analyzed these genotypes among 92 female infertile patients, showed a higher frequency of MTHFR 1298CC genotype in patients suffering from RIF than fertile controls. However, the number of RIF patients was low. The low frequency of the 1298CC genotype in their control group may reconcile the reported differences.
One of our study’s main objectives was to investigate the effect of these maternal polymorphisms on embryo aneuploidy and mosaicism. It was reasoned that even though no differences were observed in MTHFR genotype distribution between our infertile female patients and the general population, the controversy of their implication in chromosomal non-disjunction and alterations in the offspring could be resolved. No differences were found in chromosome abnormalities among different genotype groups comparing the embryo aneuploidy and mosaicism rates among patients with homozygous normal genotype (CC in C677T or AA in A1298C) and those with at least one mutated allele (CT/TT or AC/CC). Kaur (Citation2013) also observed no effect of MTHFR C677T polymorphism on chromosomal alterations in the offspring. However, our results disagree with other publications that showed an association between 677T or 1298C alleles and embryo or fetal aneuploidies (Kim et al. Citation2011; Guo et al. Citation2015; Enciso et al. Citation2016). These studies did not consider homocysteine levels in the patients. However, in our study, homocysteine levels were similar among the genotype groups. Perhaps through an adequate folate status, the mother’s vitamin supplementation or diet may have balanced the MTHFR mutations’ effects. Previous publications have shown a decrease in homocysteine levels in women taking folic acid, regardless of the MTHFR genotype (Brönstrup et al. Citation1998; Fohr et al. Citation2002), associated with a higher number of mature and better-quality oocytes (Szymański and Kazdepka-Ziemińska Citation2003). Therefore, as long as the folate/folic acid intake in the female patient is sufficient, there seems to be no impact of these MTHFR mutations on meiosis during folliculogenesis. This is consistent with no effect on chromosomal abnormalities in the embryos were found.
When we analyzed the embryo MTHFR C677T/A1298C genotypes, no differences among the genotype frequencies compared with the frequencies observed in female patients and in the 1000 Genomes Project (Phase 3) European population were detected. No significant differences in chromosomal aneuploidy rate were apparent when embryos with homozygous normal genotype and those with at least one mutated allele (CC vs. CT/TT in C677T or AA vs. AC/CC in A1298C) were compared. Similar conclusions were reached by Enciso et al. (Citation2016), who observed no differences in genotype frequencies between euploid and aneuploid embryos. However, in our study, the mosaicism rate was higher in the 677CC group than the 677CT/TT group (13.5% vs. 5.4%, p = 0.019), while in the 1298AA group, it was similar to the 1298AC/CC group (7.5 vs. 9.1%, p = 0.817). It is difficult to explain the statistically significant difference in the mosaicism rate in C677T polymorphism. Additional studies are required to clarify this genotype’s role in the processes that lead to mosaicism in the embryo, considering the still unknown mechanisms of mosaicism and biological limitations in the diagnosis.
On one hand, Patounakis et al. (Citation2016) did not find any differences in the pregancy rate, clinical pregnancy rate, implantation rate, biochemical or clinical pregnancy loss, and the live birth rate for MTHFR C677T and A1298C polymorphisms after the transfer of 4169 blastocysts. However, they tested these single nucleotide polymorphisms (SNPs) in the female patients, not in the embryos. On the other hand, other authors who compared the genotype of euploid embryos that implanted with those that did not implant described differences in MTHFR C677T genotypes (Enciso et al. Citation2016). Their results indicated that the percentage of embryos with the MTHFR 677TT genotype was greater in embryos that did not implant vs. those that had implanted. Our study analyzed all the IVF clinical outcomes (pregnancy rate, implantation rate, biochemical and clinical miscarriage rates, and ongoing pregnancy rate) after single euploid embryo transfer in a much larger number of samples (euploid embryos) than the previous study. We also compared these outcomes in individual MTHFR genotypes and, additionally, in different genotype and haplotype combinations. The data showed no significant differences in any of the analyzed outcomes, for different C677T/A1298C genotypes (analyzed individually or combined) and for different haplotypes, with the logistic regression test using as confounding factors the maternal age and the embryo quality, except for the pregnancy rate in the 1298AC genotype. The pregnancy rate was higher in 1298AC embryos vs. 1298AA embryos (60.0% vs. 47.3%, p = 0.023). Since implantation and ongoing pregnancy rates were similar among these genotypes (47.4% in 1298AC vs. 39.7% in 1298AA for implantation rate, and 42.1% in 1298AC vs. 37.4% in 1298AA for ongoing pregnancy rate), we did not give greater clinical relevance to the higher pregnancy rate in the MTHFR 1298AC genotype.
In conclusion, the distribution of MTHFR C677T and A1298C genotypes was similar in our RIF/RPL infertile patients compared with the European population. In this population of infertile women, maternal genotype did not affect embryo chromosomal abnormalities. No differences among homocysteine levels in different genotype groups were found. The folate levels in these women were assumed to be normal, even though they were not measured, suggesting an addition to future studies. Although it seems that female MTHFR polymorphisms do not affect non-disjunction events, the limited sample analyzed precludes definitive conclusions. Therefore, larger sample size studies are required to confirm our findings.
A recent meta-analysis has reported that the MTHFR A1298C is not associated with male infertility, and the MTHFR C677T is associated with male infertility in Asians (Han et al. Citation2020). Conversely, Enciso et al. (Citation2016) showed no increase in the frequency of these paternal MTHFR SNPs in the group of RIF/RPL patients than controls, also no significant effect in embryo aneuploidy. However, in infertile males, an increase in sperm chromosomal abnormalities have been described (Silber et al. Citation2003; Rodrigo et al. Citation2011), and the paternal contribution to RIF or RPL is controversial. In the last 20 years, several studies have shown a high incidence of sperm aneuploidy in RIF and RPL patients (Al-Hassan et al. Citation2005; Petit et al. Citation2005; Vialard et al. Citation2008; Ramasamy et al. Citation2015), whereas some recent have not reported an association (Rodrigo et al. Citation2019). Therefore, given the current controversy, the paternal effect of MTHFR polymorphisms on embryo aneuploidy should be assessed in couples with implantation failure or recurrent pregnancy loss.
Our analysis also suggests that the embryo MTHFR genotype does not influence the embryo aneuploidy rate. It does not seem to affect embryo implantation or pregnancy through any other mechanism since the IVF outcomes are similar among genotypes in euploid embryo transfers. The only correlation observed was in the mosaicism rate among C677T embryo genotypes. Given these results, future studies are necessary to investigate the role of MTHFR in mosaicism development in the embryo. A possible influencing factor not considered in this study is the composition of the culture medium in terms of folate and other related metabolites, which are necessary for the cellular processes mentioned above, so this should be considered in future studies.
Materials and methods
Study design and population
To investigate a possible effect of maternal MTHFR polymorphisms (C677T and A1298C) on embryo chromosomal abnormalities (aneuploidy and mosaicism), we included in the study 77 women who underwent an IVF/ICSI cycle with their oocytes and who also carried out a preimplantation genetic testing for aneuploidies (PGT-A) from January 2016 until December 2018 (study group 1). The patients underwent ovarian stimulation following a personalized protocol designed by a specialized clinician, according to their characteristics and their clinical history. The MTHFR polymorphisms tests were performed within the inherited thrombophilia analysis, before the IVF cycle, for two main indications: recurrent implantation failure (RIF, n = 40) and recurrent pregnancy loss (RPL, n = 37). When the couple had four or more good quality embryos transferred (according to Gardner and Schoolcraft scoring), it was considered RIF and RPL when the couple had suffered two or more consecutive miscarriages. The MTHFR genotype frequencies of this study group were compared to the 1000 Genomes Project (Phase 3) genotype frequencies for the European population (Auton et al., Citation2015) to determine any differences between the populations.
Moreover, for this study, we analyzed the C677T and A1298C MTHFR genotypes in the 191 biopsied embryos from the PGT-A cycles of these patients (mean of 2.5 biopsied embryos per patient) to evaluate the effect of embryo MTHFR polymorphisms on embryo aneuploidies and mosaicism. In addition, to increase the number of analyzed embryos, 218 DNA samples from trophectoderm biopsies belonging to a different group of patients were also genotyped for these polymorphisms. Fifty-eight embryos came from cycles with patients’ own oocytes and 160 from cycles with donated oocytes. These patients underwent a PGT-A cycle for different indications (RIF, RPL, previous chromosomopathies, and advanced maternal age). The total number of embryos included to compare the chromosomal alterations between different embryo MTHFR genotypes was 409 (forming the study group 2).
To evaluate the impact of the embryo C677T/A1298C MTHFR genotypes on IVF outcomes, we compared the pregnancy rate, implantation rate, biochemical and clinical miscarriage rates, and the ongoing pregnancy rate between different embryo genotypes. These parameters were analyzed in 241 frozen-thawed embryo transfer cycles. Single euploid (and no mosaic) blastocysts were transferred (study group 3) to eliminate the bias of chromosomal abnormalities in the embryo. Out of the 241 cycles, 91 were from patients’ oocytes and 150 from donated oocytes. All the embryos were vitrified after the biopsy, and they were thawed and transferred in a subsequent cycle. The study design is shown schematically in .
A chorionic gonadotropin (hCG) blood test performed 8 to 10 days after the embryo transfer greater than 2 mIU/mL was considered a positive pregnancy test. Pregnancy rate was calculated by dividing the number of hCG positive tests by the number of embryos transferred. Implantation rate was defined as the number of gestational sacs observed by transvaginal ultrasound at 6 weeks of pregnancy divided by the number of embryos transferred. The biochemical miscarriage rate was computed by dividing the cases where the hCG was positive, and no sac was visualized in ultrasound by the embryos transferred. The ongoing pregnancy rate was defined as the number of fetuses with heart activity at 12 weeks of pregnancy divided per transferred embryo. The clinical miscarriage rate was obtained by dividing the cases where no heart activity was visualized after implantation by the number of embryos implanted.
MTHFR genotyping
To carry out the MTHFR genotyping, the patients’ lymphocyte genomic DNA was extracted from a peripheral blood sample, using the MagMAX DNA Multi-Sample Ultra 2.0 kit (Thermo Fisher Scientific, Colchester, UK) on a KingFisher™ Duo Prime system (Thermo Fisher Scientific, Colchester, UK), following the manufacturer’s instructions. DNA was quantified using Qubit™ dsDNA HS Assay Kit with the Qubit Fluorometer (Thermo Fisher Scientific, Colchester, UK). On the other hand, the DNA from the biopsied embryos in the PGT-A cycles was obtained from trophectoderm cells (5–10) of day 5–6 blastocysts and amplified using the SurePlex DNA Amplification System (Illumina®, San Diego, CA, USA) to whole genome amplification (WGA).
MTHFR was genotyped with TRF-plus Thrombosis Risk Panel (Elucigene Diagnostics, Manchester, UK). The Veriti 96 Well Thermal Cycler (Applied Biosystems™, UK) was used to perform the PCR. The reaction was carried out in a final volume of 3.5 µL containing 2.5 µL of the Reaction Mix (TA) and 0.75 µL of the diluted genomic DNA (0.5–10 ng/ul) in the case of patients or 0.75 ul of the amplified DNA from trophectoderm biopsies. The reaction conditions were as follows: one cycle of 20 min at 94°C, followed by 30 cycles of 1 minute at 94°C, 2 minutes at 58°C and 1 minute at 72°C and finally 20 minutes at 72°C. The amplification product (0.5 ul) was then injected into the capillary electrophoresis system Applied Biosystems SeqStudio (Thermo Fisher Scientific, Colchester, UK), mixed with 10.25 ul Hi-Di™ Formamide (Thermo Fisher Scientific, Colchester, UK) and 0.25 ul GeneScan™ 500 LIZ™ dye Size Standard (Thermo Fisher Scientific, Colchester, UK), previously denatured at 95°C for 4 min. The results were analyzed using GeneMapper™ 5.0 software (Thermo Fisher Scientific, Colchester, UK).
Trophectoderm biopsy
All oocytes were fertilized by ICSI using standard IVF protocols. The generated embryos were cultured in groups under low oxygen conditions in 50 µl microdrops of continuous media (Global® Total® LP, CooperSurgical®). Assisted hatching was performed on day 3. Trophectoderm biopsy was performed on day 5 or 6 on good-quality expanded blastocysts using a 200 mW diode laser (Saturn, Research Instruments Ltd, Cornwall, UK) (Hamilton Thorne, Beverly, USA). The biopsied cells (5–10) were transferred to 200ul PCR tubes with 1 µl of PBS (phosphate-buffered saline) 1X, ph 7.4, until further analysis. Embryos were vitrified according the protocol of Vitrification Freeze Kit (Vit Kit® Freeze, IrvineScientific, California, USA).
Embryo chromosomal analysis
In order to select euploid embryos in PGT-A cycles, embryo analysis was performed using SurePrint G3-8x60K-Human CGH microarrays (Agilent Technologies®, Santa Clara, CA, USA) or Veriseq-NGS (Illumina®, San Diego, CA, USA), with previous whole genome amplification using SurePlex DNA Amplification System (Illumina®, San Diego, CA, USA), according to the manufacturer’s protocols. In Veriseq protocol, the sequencing platform used was the MiSeq System (Illumina®, San Diego, CA, USA). For chromosome analysis, the Cytogenomics v2.5 software (Agilent Technologies®, Santa Clara, CA, USA) and the BlueFuse Multi software Illumina®, San Diego, CA, USA) were used for each corresponding technique. Embryos were reported as euploid if the analyzed sample contained less than 25% of aneuploid cells, mosaic if it contained between 25% and 50% of aneuploid cells in one or more chromosomes, and aneuploid if the percentage of aneuploidy was over 50%. The detection limit for the segmental aneuploidies was 8 Mb (previously validated in our laboratory in embryos from parents carrying chromosome translocations).
Statistical analysis
Statistical analysis was performed with Statistical Package for Social Sciences software, version 20.0 (SPSS; Chicago, IL, USA). For continuous variables, descriptive analysis was done using the mean and standard deviation. Univariate analysis to study the differences between the different genotypes with respect to continuous variables (biochemical and stimulation variables) was carried out by analyzing variance ANOVA. We determined the differences between groups using Fisher’s exact test (two-sided) for genotype frequency. The embryo aneuploidy and mosaicism rates, with regard to different patient and embryo MTHFR genotypes, were compared with a multivariate analysis, through a binary logistic regression statistical test, using as confounding variables: maternal age, PGT-A technique and embryo quality. In the case of IVF outcomes in euploid embryos, pregnancy rate, implantation rate, biochemical and miscarriage rate, and ongoing pregnancy rate, were compared among different embryo MTHFR genotypes, employing a logistic regression statistical test and using as confounding factors embryo quality and oocyte origin. In all analysis, statistical significance was defined as p < 0.05.
Ethical approval
All work was conducted with the formal approval of the Instituto Bernabeu Institutional Review Board, and it follows the principles of the Declaration of Helsinki. Informed consent was obtained from all patients prior to the study. The trophectoderm DNA samples used for the purpose of this study were the surplus amplification products following PGT-A analysis.
Authors’ contributions
Supervised and supported the study: RM, BL; designed and wrote the manuscript: RM; revised the manuscript: BL, JAO; data collection: RM, AC, HC; embryo genotyping: AC; statistical analysis: JAO. All authors read and approved the final manuscript.
Acknowledgments
The authors would like to thank Anastasia Utkina and Virginia Alarcon the revision and correction of the language.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Alam MA, Husain SA, Narang R, Chauhan SS, Kabra M, Vasisht S. 2008. Association of polymorphism in the thermolabile 5, 10-methylene tetrahydrofolate reductase gene and hyperhomocysteinemia with coronary artery disease. Mol Cell Biochem. 310(1–2):111–117. doi:10.1007/s11010-007-9671-7.
- Al-Hassan S, Hellani A, Al-Shahrani A, Al-Deery M, Jaroudi K, Coskun S. 2005. Sperm chromosomal abnormalities in patients with unexplained recurrent abortions. Arch Androl. 51(1):69–76. doi:10.1080/014850190518062.
- Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, Korbel JO, Marchini JL, McCarthy S, McVean GA, Abecasis GR, et al. 2015. A global reference for human genetic variation. Nature. 526(7571):68–74.
- Bae J, Shin SJ, Cha SH, Choi DH, Lee S, Kim NK. 2007. Prevalent genotypes of methylenetetrahydrofolate reductase (mthfr c677t and a1298c) in spontaneously aborted embryos. Fertil Steril. 87(2):351–355. doi:10.1016/j.fertnstert.2006.06.027.
- Brönstrup A, Hages M, Prinz-Langenohl R, Pietrzik K. 1998. Effects of folic acid and combinations of folic acid and vitamin b-12 on plasma homocysteine concentrations in healthy, young women. Am J Clin Nutr. 68(5):1104–1110. doi:10.1093/ajcn/68.5.1104.
- Di Nisio M, Rutjes AW, Ferrante N, Tiboni GM, Cuccurullo F, Porreca E. 2011. Thrombophilia and outcomes of assisted reproduction technologies: a systematic review and meta-analysis. Blood. 118(10):2670–2678. doi:10.1182/blood-2011-03-340216.
- Dobson AT, Davis RM, Rosen MP, Shen S, Rinaudo PF, Chan J, Cedars MI. 2007. Methylenetetrahydrofolate reductase c677t and a1298c variants do not affect ongoing pregnancy rates following ivf. Hum Reprod. 22(2):450–456. doi:10.1093/humrep/del396.
- Enciso M, Sarasa J, Xanthopoulou L, Bristow S, Bowles M, Fragouli E, Delhanty J, Wells D. 2016. Polymorphisms in the mthfr gene influence embryo viability and the incidence of aneuploidy. Hum Genet. 135(5):555–568. doi:10.1007/s00439-016-1652-z.
- Fohr IP, Prinz-Langenohl R, Brönstrup A, Bohlmann AM, Nau H, Berthold HK, Pietrzik K. 2002. 5,10-methylenetetrahydrofolate reductase genotype determines the plasma homocysteine-lowering effect of supplementation with 5-methyltetrahydrofolate or folic acid in healthy young women. Am J Clin Nutr. 75(2):275–282. doi:10.1093/ajcn/75.2.275.
- Forges T, Monnier-Barbarino P, Alberto JM, Guéant-Rodriguez RM, Daval JL, Guéant JL. 2007. Impact of folate and homocysteine metabolism on human reproductive health. Hum Reprod Update. 13(3):225–238. doi:10.1093/humupd/dml063.
- Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, Matthews RG, Boers GJ, Den Heijer M, Kluijtmans LA, Van Den Heuvel LP. 1995. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet. 10(1):111–113. doi:10.1038/ng0595-111.
- Goyette P, Frosst P, Rosenblatt DS, Rozen R. 1995. Seven novel mutations in the methylenetetrahydrofolate reductase gene and genotype/phenotype correlations in severe methylenetetrahydrofolate reductase deficiency. Am J Hum Genet. 56(5):1052–1059.
- Guo Q, Wang H, Yang K, Zhang B, Li T, Liao S. 2015. [association of mthfr and mtrr genes polymorphisms with non-disjunctions of chromosomes 18 and 21]. Zhonghua Yi Xue Yi Chuan Xue Za Zhi = Zhonghua Yixue Yichuanxue Zazhi = Chinese Journal of Medical Genetics. 32(3):395–399. doi:10.3760/cma.j..1003-9406.2015.03.021.
- Han LJ, He XF, Ye XH. 2020. Methylenetetrahydrofolate reductase C677T and A1298C polymorphisms and male infertility risk: an updated meta-analysis. Med (Baltimore). 99(51):e23662. doi:10.1097/MD.0000000000023662.
- Hassold TJ, Burrage LC, Chan ER, Judis LM, Schwartz S, James SJ, Jacobs PA, Thomas NS. 2001. Maternal folate polymorphisms and the etiology of human nondisjunction. Am J Hum Genet. 69(2):434–439. doi:10.1086/321971.
- Jacques PF, Bostom AG, Williams RR, Ellison RC, Eckfeldt JH, Rosenberg IH, Selhub J, Rozen R. 1996. Relation between folate status, a common mutation in methylenetetrahydrofolate reductase, and plasma homocysteine concentrations. Circulation. 93(1):7–9. doi:10.1161/01.CIR.93.1.7.
- Kaur A. 2013. Prevalence of methylenetetrahydrofolate reductase 677 c-t polymorphism among mothers of down syndrome children. Indian J Hum Genet. 19(4):412–414. doi:10.4103/0971-6866.124368.
- Kim SY, Park SY, Choi JW, Kim DJ, Lee SY, Lim JH, Han JY, Ryu HM, Kim MH. 2011. Association between mthfr 1298a>c polymorphism and spontaneous abortion with fetal chromosomal aneuploidy. Am J Reprod Immunol. 66(4):252–258. doi:10.1111/j.1600-0897.2011.00996.x.
- Lucock MD. 2006. Synergy of genes and nutrients: the case of homocysteine. Curr Opin Clin Nutr Metab Care. 9(6):748–756. doi:10.1097/01.mco.0000247468.18790.1e.
- Melnyk S, Pogribna M, Pogribny IP, Yi P, James SJ. 2000. Measurement of plasma and intracellular s-adenosylmethionine and s-adenosylhomocysteine utilizing coulometric electrochemical detection: alterations with plasma homocysteine and pyridoxal 5ʹ-phosphate concentrations. Clin Chem. 46(2):265–272. doi:10.1093/clinchem/46.2.265.
- Nelen WL, Blom HJ, Thomas CM, Steegers EA, Boers GH, Eskes TK. 1998. Methylenetetrahydrofolate reductase polymorphism affects the change in homocysteine and folate concentrations resulting from low dose folic acid supplementation in women with unexplained recurrent miscarriages. J Nutr. 128(8):1336–1341. doi:10.1093/jn/128.8.1336.
- Nelen WL, Steegers EA, Eskes TK, Blom HJ. 1997. Genetic risk factor for unexplained recurrent early pregnancy loss. Lancet. 350(9081):861. doi:10.1016/S0140-6736(97)24038-9.
- Ni W, Li H, Wu A, Zhang P, Yang H, Yang X, Huang X, Jiang L. 2015. Lack of association between genetic polymorphisms in three folate-related enzyme genes and male infertility in the chinese population. J Assist Reprod Genet. 32(3):369–374. doi:10.1007/s10815-014-0423-9.
- Patounakis G, Bergh E, Forman EJ, Tao X, Lonczak A, Franasiak JM, Treff N, Scott RT. 2016. Multiple thrombophilic single nucleotide polymorphisms lack a significant effect on outcomes in fresh ivf cycles: an analysis of 1717 patients. J Assist Reprod Genet. 33(1):67–73. doi:10.1007/s10815-015-0606-z.
- Petit FM, Frydman N, Benkhalifa M, Le Du A, Aboura A, Fanchin R, Frydman R, Tachdjian G. 2005. Could sperm aneuploidy rate determination be used as a predictive test before intracytoplasmic sperm injection? J Androl. 26(2):235–241. doi:10.1002/j.1939-4640.2005.tb01090.x.
- Quere I, Bellet H, Hoffet M, Janbon C, Mares P, Gris JC. 1998. A woman with five consecutive fetal deaths: case report and retrospective analysis of hyperhomocysteinemia prevalence in 100 consecutive women with recurrent miscarriages. Fertil Steril. 69(1):152–154. doi:10.1016/S0015-0282(97)00451-2.
- Rai V. 2014. Methylenetetrahydrofolate reductase gene a1298c polymorphism and susceptibility to recurrent pregnancy loss: a meta-analysis. Cell Mol Biol (Noisy-le-grand). 60(2):27–34.
- Ramasamy R, Scovell JM, Kovac JR, Cook PJ, Lamb DJ, Lipshultz LI. 2015. Fluorescence in situ hybridization detects increased sperm aneuploidy in men with recurrent pregnancy loss. Fertil Steril. 103(4):906–909.e901. doi:10.1016/j.fertnstert.2015.01.029.
- Rodrigo L, Meseguer M, Mateu E, Mercader A, Peinado V, Bori L, Campos-Galindo I, Milán M, García-Herrero S, Simón C, et al. 2019. Sperm chromosomal abnormalities and their contribution to human embryo aneuploidy. Biol Reprod. 101(6):1091–1101. doi:10.1093/biolre/ioz125
- Rodrigo L, Rubio C, Peinado V, Villamón R, Al-Asmar N, Remohí J, Pellicer A, Simón C, Gil-Salom M. 2011. Testicular sperm from patients with obstructive and nonobstructive azoospermia: aneuploidy risk and reproductive prognosis using testicular sperm from fertile donors as control samples. Fertil Steril. 95(3):1005–1012. doi:10.1016/j.fertnstert.2010.10.022.
- Safdarian L, Najmi Z, Aleyasin A, Aghahosseini M, Rashidi M, Asadollah S. 2014. Recurrent ivf failure and hereditary thrombophilia. Iran J Reprod Med. 12(7):467–470.
- Silber S, Escudero T, Lenahan K, Abdelhadi I, Kilani Z, Munné S. 2003. Chromosomal abnormalities in embryos derived from testicular sperm extraction. Fertil Steril. 79(1):30–38. doi:10.1016/S0015-0282(02)04407-2.
- Soligo AG, Barini R, Annichino-Bizzacchi JM. 2017. Prevalence of the mthfr c677t mutation in fertile and infertile women. Rev Bras Ginecol Obstet. 39(12):659–662. doi:10.1055/s-0037-1606289.
- Szymański W, Kazdepka-Ziemińska A. 2003. [effect of homocysteine concentration in follicular fluid on a degree of oocyte maturity]. Ginekol Pol. 74(10):1392–1396.
- Thaler CJ. 2014. Folate metabolism and human reproduction. Geburtshilfe Frauenheilkd. 74(9):845–851. doi:10.1055/s-0034-1383058.
- Van Der Put NM, Gabreëls F, Stevens EM, Smeitink JA, Trijbels FJ, Eskes TK, Van Den Heuvel LP, Blom HJ. 1998. A second common mutation in the methylenetetrahydrofolate reductase gene: an additional risk factor for neural-tube defects? Am J Hum Genet. 62(5):1044–1051. doi:10.1086/301825.
- Vialard F, Hammoud I, Molina-Gomes D, Wainer R, Bergere M, Albert M, Bailly M, De Mazancourt P, Selva J. 2008. Gamete cytogenetic study in couples with implantation failure: aneuploidy rate is increased in both couple members. J Assist Reprod Genet. 25(11–12):539–545. doi:10.1007/s10815-008-9258-6.
- Weisberg I, Tran P, Christensen B, Sibani S, Rozen R. 1998. A second genetic polymorphism in methylenetetrahydrofolate reductase (mthfr) associated with decreased enzyme activity. Mol Genet Metab. 64(3):169–172. doi:10.1006/mgme.1998.2714.
- Wouters MG, Boers GH, Blom HJ, Trijbels FJ, Thomas CM, Borm GF, Steegers-Theunissen RP, Eskes TK. 1993. Hyperhomocysteinemia: a risk factor in women with unexplained recurrent early pregnancy loss *†*Supported by grant number 28.1006.1 from Praeventiefonds, The Hague, The Netherlands.†presented at the 40th annual meeting of society for gynecologic investigation, Toronto, Canada, March 31 to April 3, 1993. Fertil Steril. 60(5):820–825. doi:10.1016/S0015-0282(16)56282-7.
- Wu X, Zhao L, Zhu H, He D, Tang W, Luo Y. 2012. Association between the mthfr c677t polymorphism and recurrent pregnancy loss: a meta-analysis. Genet Test Mol Biomarkers. 16(7):806–811. doi:10.1089/gtmb.2011.0318.
