ABSTRACT
The study aimed to determine the variation of Y-chromosome-bearing sperm content among individual ejaculates. A real-time polymerase chain reaction (qPCR) with unique primers was developed and used to calculate the percentage of Y-chromosome-bearing sperm in individual ejaculates from 50 randomly selected men. There was a significant difference in the overall mean ± SD between the proportion of Y-chromosome-bearing sperm and X-chromosome-bearing sperm (45.36 ± 7.88 vs. 54.42 ± 7.88). Of the 50 ejaculates, 17 had more than, and 14 had less than the 99% confidence interval of the mean of the Y-chromosome-bearing sperm (45.58 ± 2.87). These results suggest that the inconsistency in sperm-based sex-selection outcomes appears to be a function of differences in the ejaculates and highlights the need for further study in environmental and genetic factors contributing to X or Y bearing spermatozoan instability.
Abbreviations: qPCR: real-time polymerase chain reaction; ROS: reactive oxygen species; DTT: dithiothreitol; SRY: sex-determining region Y.
KEYWORDS:
Introduction
The sex ratio of human spermatozoa (Chaudhary et al. Citation2014)is a topic that has been studied for multiple reasons, including family-balancing and medical concerns. A variety of techniques and procedures have been reported to promote fractions of sperm that are bearing either an X- or Y-chromosome (Oyeyipo et al. Citation2017; Umehara et al. Citation2019) with greater purity than the original sample. However, to date, none of these techniques or procedures have yielded reliable results in human subjects or anticipated clinical outcomes, and, for this reason, they have not been truly accepted by the scientific community. This inconsistency may be due to the intrinsic nature of the ejaculate X- and Y-chromosome-bearing sperm content. Thus, a clinically applicable approach to sex selection has remained elusive.
The presumption has long been that the percentages of Y- and X-bearing sperm in a given sample would be equal (Umehara et al. Citation2019). But a deeper review of the existing data, however, reveals wide variation in percentages among individual ejaculates among different species of animals, including human (Checa et al. Citation2002; Han et al. Citation1993; Chandler et al. Citation1998; Chaudhary et al. Citation2014; Rorie et al. Citation2014; Umehara et al. Citation2019). This variation has been attributed to several factors, including but not limited to genetic instability (Middelkamp et al. Citation2020), motility and swimming pattern (Check et al. Citation1989), pH susceptibility (Hamamah and Gatti Citation1998). Consequently, recognizing and assessing individual differences may provide a better path to successfully enriching the proportion of X- or Y-chromosome-bearing spermatozoa in an ejaculate as desired during sperm selection.
This study set out to conduct a blinded, randomized trial of the variation of Y-bearing sperm within human ejaculates. A real-time qPCR with unique primers was developed to assess the level of variation in the percentage of Y-chromosome-bearing sperm among individuals. The percentage of Y-chromosome-bearing sperm was calculated for the individual ejaculates of 50 randomly selected men.
Results and discussion
The mean percentage of Y-chromosome-bearing sperm for each sample was calculated. There was a significant difference in the overall mean ± SD between the proportion of Y-chromosome-bearing sperm and X-chromosome-bearing sperm (45.36 ± 7.88 vs. 54.42 ± 7.88). More importantly, a high level of variation in the percentages of Y-chromosome-bearing sperm was observed among the ejaculates of the 50 individuals. A total of 17 ejaculates had more than the 99% confidence interval of the overall mean of the Y-chromosome-bearing sperm (45.58 ± 2.87; ), and 14 ejaculates had less.
Figure 1. Randomly assayed human ejaculates demonstrate a high level of variability in percentage of Y bearing chromosome
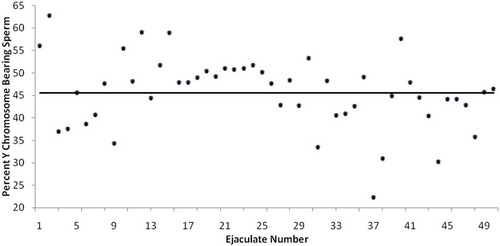
Our results indicate that utilizing a randomized approach, a basal level of variation exists in the percentage of Y-bearing sperm in human ejaculates. Based on these results, it appears that variation in the ratio of X- to Y-bearing sperm among the ejaculates of different individuals may be responsible for the reported inconsistency in sperm sex-selection outcomes. As revealed in , the level of such variation was relatively high. Interestingly, any single specimen is more likely to deviate from an expected 1:1 ratio than it is to conform to it.
This variation is not surprising based on the results of related studies: Checa et al. (Citation2002) reported a variation in the X-chromosome-bearing sperm content in bulls ranging from 38.7 to 58.2%. Chandler et al. (Citation1998) stated that spermatozoa bearing X- and Y-chromosomes were unequally represented in the ejaculates of bulls and boars, with a range of 24% to 84%. Rorie et al. (Citation2014) studied this question in bulls with real-time PCR and came up with a range of X variation from 44% to 64%, which is much closer to the results reported by Checa et al. Rorie et al. also concluded that the ratio of X- to Y-bearing sperm differed between bulls but not between the ejaculates of individual bulls. Chaudhary et al. (Citation2014) published a study of a large number of ejaculates from different men utilizing FISH, a less precise method than qPCR. They concluded that there was a greater preponderance of X-bearing spermatozoa.
Interestingly, Umehara et al. in 2019 reported a novel method of sexing sperm utilizing Toll-Like Receptors 7 and 8 expressed on the X chromosome. Moreover, the authors identified the spatial expression of these receptors within the spermatozoa. They utilized a ligand-binding selection approach to reliably enrich selection for male or female pups in their mouse model. Taken together, the variation of X- to Y-bearing sperm continues to be an evolving field of study that alters our understanding of spermatogenesis.
Additionally, the mechanism of variation remains largely unknown. The entirety of factors that may impact the variation of X- to Y-bearing sperm is beyond the scope (Rahman and Pang Citation2020) of this report but still merits discussion. While our study was randomized in sample collection, one impacting factor that likely affects all males of reproductive age in Western society is stress (Ilacqua et al. Citation2018). Modern lifestyles and stressors are linked to reactive oxygen species (ROS) induction (Sharifi-Rad et al. Citation2020). ROS levels are correlated with increased infertility exemplified by defective sperm maturation (Agarwal et al. Citation2014; You et al. Citation2017).
Additionally, exposure to a variety of environmental chemicals have been shown to alter the sex ratio in addition to ROS induction (Van Larebeke et al. Citation2008). Finally, there may also be a disconnect between sampling the sex ratio of ejaculates versus the observed birth sex ratio. It has been shown that the uterus and oviduct can select only functional spermatozoa for fertilization (Holt and Fazeli Citation2010). These are just examples of factors that may be responsible for the variation in X- to Y- bearing sperm that we observed in this report.
The findings of the present study and those previously mentioned confirm the inconsistency in sex ratio of the spermatozoa in the ejaculates of different individuals. Additionally, as this study was a simple blinded and randomized assay of 50 ejaculates, more studies are needed to dissect the molecular mechanisms as to the source of the variation. However, this finding has particular relevance to sperm sex-selection procedures. For example, suppose an ejaculate with a greater preponderance of X-bearing sperm were to be subjected to a Y-enrichment procedure. In that case, it is unlikely that the probability of conceiving a male would increase beyond the normative, anticipated 50:50 level. Thus, it is advisable to determine the percentage of Y-bearing sperm in each ejaculate before any and every sex-selection procedure to assess the odds of increasing the desired outcome.
Materials and methods
Sample selection and DNA extraction
Ejaculates from 50 men were obtained by self-masturbation and were frozen following routine semen analysis. As this was a blinded study, the health and fertility status of these individuals was not made available to the authors. These 50 frozen sperm samples were thawed and extracted through a modification of the Qiagen Gentra Puregene Kit (Hilden, Germany) protocol for DNA purification from body fluids. Dithiothreitol (DTT) was added to the Cell Lysis Buffer provided in the kit to a final concentration of 80 mM. 250 µL of this modified buffer was then added to 50 µL of the thawed sperm sample. The rest of the protocol for DNA purification from body fluids was then followed as specified by the manufacturer.
Quantitative PCR
To determine the percent of Y-chromosome-bearing sperm in a sample, a quantitative PCR was developed using sex-determining region Y (SRY) on the Y-chromosome as the target, and the CFTR primer pair or PPIH as the reference. The primer sequences for both SRY and PPIH were obtained from Integrated DNA Technologies (Coralville, IA): SRY – F: TGGCGATTAAGTCAAATTCGC; R: CCCCCTAGTACCCTGACAATGTATT (amplicon = 137 bp); CFTR – F: GAAGAGAACAAAGTGCGGCAG; R: TTGCCGGAAGAGGCTCCT (amplicon = 69 bp); and PPIH – F: CAGTGCATGGTAAACTGGAAAG; R: AGGTGCTTCCTTTGTATCCTATT (amplicon = 109 bp). All reactions were performed on Applied Biosystem’s (Foster City, CA) ViiA 7 Real-Time Thermal Cycler in triplicate at 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 seconds and then 60°C for one minute, followed by melt curve analysis. All reactions were performed with 20 ng of sample or control DNA, 200 nM primer concentrations, and SYBR Green 2X master mix chemistry reagents. Data were analyzed on the same instrument. The ratio of Y-bearing sperm was calculated using the 2−ΔΔCt relative quantification method with a DNA sample of 30 random pooled healthy male sperm samples as the control. SRY was the target gene while CFTR was chosen as the reference gene for quantification as it has been validated and used routinely in our laboratory for patient testing in similar assays. To ensure there were no differences in CFTR expression in our experimental samples, PPIH, a common reference gene, was used as a second reference control.
Statistics and data reproducibility
All samples were assayed in triplicate. For each sample, the standard deviation was calculated between replicates and 99% confidence interval constructed. Any samples showing high standard deviation were repeated. Additionally, 9 samples showing the highest variation from normal were repeated, ultimately demonstrating consistency in these high-variation specimens, and supporting the validity of the method. All data was analyzed with Microsoft Excel (Redmond, WA).
Ethics approval
The Institutional Review Board exempted this study under 45 CFR 46.101(b) (4). Informed consent was subsequently obtained from each participant.
Authors' contributions
Data collection: MI, ST, JG; manuscript preparation, analysis and interpretation: JG, MBF, RSJ; manuscript discussion and review: RSJ, JG, SL, MBF, HMO; statistical setup and final version of manuscript writing: RSJ, JG, MBF.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Agarwal A, Sharma RK, Sharma R, Assidi M, Abuzenadah AM, Alshahrani S, Durairajanayagam D, Sabanegh E. 2014. Characterizing semen parameters and their association with reactive oxygen species in infertile men. Reprod Biol Endocrinol. 12(1):33. Published 2014 May 7. doi:https://doi.org/10.1186/1477-7827-12-33.
- Chandler JE, Steinholt-Chenevert HC, Adkinson RW, Moser EB. 1998. Sex ratio variation between ejaculates within sire evaluated by polymerase chain reaction, calving and farrowing records. J Dairy Sci. 81:1855–1867. doi:https://doi.org/10.3168/jds.S0022-0302(98)75756-X.
- Chaudhary I, Jain M, Halder A. 2014. Sperm sex ratio (X: y ratio) and its variations. Austin J Reprod Med Infertil. 1(1):7.
- Checa ML, Dunner S, Cañón J. 2002. Prediction of X- and Y-chromosome content in bovine sperm by using DNA pools through capillary electrophoresis. Theriogenology. 58:1579–1586. doi:https://doi.org/10.1016/S0093-691X(02)01077-4.
- Check JH, Shanis BS, Cooper SO, Bollendorf A. 1989. Male sex preselection: swim-up technique and insemination of women after ovulation induction. Arch Androl. 23:165–166. doi:https://doi.org/10.3109/01485018908986839.
- Hamamah S, Gatti JL. 1998 Dec. Role of the ionic environment and internal pH on sperm activity. Hum Reprod. 13(Suppl 4):20–30. PMID: 10091055. doi:https://doi.org/10.1093/humrep/13.suppl_4.20.
- Han TL, Ford JH, Webb GC, Flaherty SP, Correll A, Matthews CD. 1993. Simultaneous detection of X- and Y-bearing human sperm by double fluorescence in situ hybridization. Mol Reprod Dev. 34:308–313. doi:https://doi.org/10.1002/mrd.1080340311.
- Holt WV, Fazeli A. 2010 Nov. The oviduct as a complex mediator of mammalian sperm function and selection. Mol Reprod Dev. 77(11):934–943. Epub 2010 Sep 30. PMID: 20886635. doi:https://doi.org/10.1002/mrd.21234.
- Ilacqua A, Izzo G, Emerenziani GP, Baldari C, Aversa A. 2018. Lifestyle and fertility: the influence of stress and quality of life on male fertility. Reprod Biol Endocrinol. Nov26 16(1):115. PMID: 30474562; PMCID: PMC6260894 doi:https://doi.org/10.1186/s12958-018-0436-9.
- Middelkamp S, van Tol HTA, Spierings DCJ, Boymans S, Guryev V, Roelen BAJ, Lansdorp PM, Cuppen E, Kuijk EW. 2020. Sperm DNA damage causes genomic instability in early embryonic development. Sci Adv. Apr15 6(16):eaaz7602. PMID: 32494621; PMCID: PMC7159919 doi:https://doi.org/10.1126/sciadv.aaz7602.
- Oyeyipo IP, van der Linde M, Du Plessis SS. 2017. Environmental exposure of sperm sex-chromosomes: a gender selection technique. Toxicol Res. 33(4):315–333. doi:https://doi.org/10.5487/TR.2017.33.4.315.
- Rahman MS, Pang MG. 2020. New biological insights on X and Y chromosome-bearing spermatozoa. Front Cell Dev Biol. 7:388. Published 2020 Jan 21. doi:https://doi.org/10.3389/fcell.2019.00388.
- Rorie RW, Delgado P, Lester T. 2014. Variation among beef bulls in the ratio of X- to Y-chromosome bearing spermatozoa. Adv Reprod Sci. 2:69–75. doi:https://doi.org/10.4236/arsci.2014.24008.
- Sharifi-Rad M, Anil Kumar NV, Zucca P, Varoni EM, Dini L, Panzarini E, Rajkovic J, Tsouh Fokou PV, Azzini E, Peluso I, et al. 2020. Lifestyle, oxidative stress, and antioxidants: back and forth in the pathophysiology of chronic diseases. Front Physiol. 11:694. Published 2020 Jul 2. doi:https://doi.org/10.3389/fphys.2020.00694.
- Umehara T, Tsujita N, Shimada M, Yamashita YM. 2019. Activation of Toll-like receptor 7/8 encoded by the X chromosome alters sperm motility and provides a novel simple technology for sexing sperm. PLoS Biol. 17(8):e3000398. Published 2019 Aug 13. doi:https://doi.org/10.1371/journal.pbio.3000398.
- Van Larebeke NA, Sasco AJ, Brophy JT, Keith MM, Gilbertson M, Watterson A. 2008 Apr-Jun. Sex ratio changes as sentinel health events of endocrine disruption. Int J Occup Environ Health. 14(2):138–143. PMID: 18507291. doi:https://doi.org/10.1179/oeh.2008.14.2.138.
- You YA, Kwon WS, Saidur Rahman M, Park YJ, Kim YJ, Pang MG. 2017. Sex chromosome-dependent differential viability of human spermatozoa during prolonged incubation. Hum Reprod. Jun1 32(6):1183–1191. PMID: 28430968 doi:https://doi.org/10.1093/humrep/dex080.
