ABSTRACT
The previous study using Sertoli cells cultured in vitro has shown that the protective effects of astragaloside IV (AsIV) on cadmium (Cd)-induced damage to Sertoli cells and its membrane proteins. Yet, it is not known if AsIV has an equivalent effect on Cd-induced damage to the spermatogenesis microenvironment in rats. Using an in vivo model, Cd-induced damage to the spermatogenesis microenvironment and the protective effects of AsIV were studied. Eighteen male Sprague Dawley (SD) rats were randomly divided into three groups (n = 6/group): Cd group, Cd&AsIV group, and control group. Cd was administered to the rats in the Cd group via i.p. at 1 mg/kg body weight once daily, Cd and AsIV was administered to the rats in the Cd&AsIV group via i.p. at 1 mg/kg body weight and 10 mg/kg body weight respectively once daily, and the same volume of saline was administered to the rats in control group via i.p. once daily. The rats in the three groups were injected continuously for 5 days. Vesicular formation in the seminiferous tubules was observed in the Cd treatment group. The average optical density of claudin-11, zonal occludin-1 (ZO-1), and connexin 43 (Cx43) decreased significantly in the Cd treatment group. The ultrastructural damage of the Sertoli cells and tight junctions were also observed by electron microscopy. AsIV treatment rescued the morphologic changes of the seminiferous tubules of the testis and the ultrastructural damage of the Sertoli cells and tight junctions. The average optical density of claudin-11, ZO-1, and Cx43 also increased significantly after AsIV treatment. Cd damages the spermatogenesis microenvironment in rats, which can be rescued by AsIV treatment. These results illustrate that AsIV may also have a protective effect on Cd-induced damage to the spermatogenesis microenvironment in rats.
Abbreviations: AsIV: astragaloside IV; Cd: cadmium; SD: Sprague Dawley; ZO-1: zonal occludin-1; Cx43: connexin 43; BTB: blood-testis barrier; MAPKs: mitogen-activated protein kinases; OSP: oligodendrocyte-specific protein; Cxs: connexins; GJIC: gap junctional intercellular communication; ROS: reactive oxygen species; MDA: malondialdehyde; TGF: tumor growth factor; PBS: phosphate buffer saline; BSA: bovine serum albumin
Introduction
Cd, a primary heavy metal pollutant, has a long half-life of 15–20 years. Cd is widely used in electroplating processes, nickel-Cd batteries, and plastic stabilizer products. It is also found in cigarette smoke, automobile exhaust, pesticides, and phosphate fertilizers. Cd can enter the human body through both diet and inhalation, and its accumulation eventually results in damage to the liver, kidneys, and bones (He et al. Citation2013). Cd can cause many diseases, such as kidney cancer, prostate cancer, and breast cancer. The International Agency for Research on Cancer classified Cd as a human carcinogen in 1993, and the American Toxicology Administration has classified Cd as the seventh most toxic substance threatening human health (Ibiam et al. Citation2013). In recent years, animal experiments have reported that Cd can also affect the reproductive system, especially in males, which can eventually lead to infertility through lipid peroxidation, endocrine disruption, and various signal transduction pathways (Siu et al. Citation2009). It is an endocrine disruptor that regulates sex hormone levels such as testosterone, luteinizing hormone, and follicle stimulating hormone through the hypothalamic-testis-gonadal axis. Cd particularly affects the male reproductive system, however, the process by which it causes reproductive toxicity is complicated and entire mechanism still remains elusive.
The blood-testis barrier (BTB), consisting of tight junctions, gap junctions, adhesive junctions, and other connected complexes, creates a unique microenvironment for meiotic and post meiotic cells, and separates meiotic and post meiotic germ cells from the blood circulation (Chen et al. Citation2018a). These connective complexes can also be used to regulate preleptotene spermatocytes through special ‘open’ and ‘off’ devices on the BTB. Thus, the integrity and function of the BTB is essential for spermatogenesis and maturation (Jiang et al. Citation2014). Sertoli cells are unique cells in the spermatogenic epithelium, as they are in contact with spermatogenic cells and participate in the formation of the BTB to create a stable microenvironment for spermatogenesis. Studies have shown that, rather than the testicular specific vasculature, the BTB is likely the first target of Cd-induced reproductive toxicity (Dong et al. Citation2015).
In recent years, the mechanism of Cd-induced toxicity has been extensively studied globally. Some studies have shown that Cd toxicity involves the Cd transporter and metallothionein (Messaoudi et al. Citation2010). Others have demonstrated the effect of Cd on endocrine disruption of the testis, as well as Cd-mediated testicular oxidative stress and disruption of zinc and/or calcium-mediated cellular activity (Jahan et al. Citation2014). Recently, rice produced in many Provinces of China was found to have excess levels of Cd, which had resulted in increased attention to Cd pollution. Rice is the main staple food in China. It is also a major source of nutrients in the Chinese diet, which contributes 40% of the total calorie intake of Chinese people (Huang et al. Citation2013). With the rapid development of industrialization in China, there have been reports of high concentrations of heavy metals, such as Cd, in crops because of soil and water pollution (Wang et al. Citation2019). Cd enter the human body via ingestion of food, as cumulative poisons, consumption of Cd in contaminated rice may cause many toxic effects in humans (He et al. Citation2013). Astragalus membranaceus, whose medical history was over 2000 years, has been routinely used in China for patients with stroke and chronic debilitating diseases and pulmonary fibrosis and myocardial fibrosis. A pilot clinical investigation suggested that astragalus membranaceus was safe and may be beneficial for the treatment of acute cerebral infarction (Li et al. Citation2017). AsIV, a cycloartane-type triterpene glycoside chemical, is one of the major and active components of the root of Astragalus membranaceus. Studies have shown that AsIV has a series of protective effects, such as positive inotropic action, anti-hypertension, anti-viral activity, antinociception, anti-infarction, and anti-inflammation. Besides, there exists a potential protective effect of AsIV on ischemia-reperfusion and cardiovascular diseases (Ren et al. Citation2013; Zhang et al. Citation2020). Mitogen-activated protein kinases (MAPKs) regulate many cellular functions, including cell proliferation, gene expression, apoptosis, immune response, and response to oxidative stress. p38 MAPK is one of the mitogen-activated protein kinases that link extracellular signals to the intracellular machinery to regulate cellular processes (Coulthard et al. Citation2009). The study has demonstrated that AsIV inhibits HSC activation by inhibiting oxidative stress and associated p38 MAPK activation. AsIV acts as an antifibrogenic candidate in the prevention and treatment of liver fibrosis (Li et al. Citation2013). It also has been confirmed that AsIV can reduce blood-brain barrier (BBB) permeability by up-regulating occludin and ZO-1 expression after ischemia-reperfusion injury. AsIV may maintain the integrity of blood-brain barrier by a signal transduction pathway (Qu et al. Citation2009). The current study investigated the effects of AsIV on Cd-induced toxicity in the male reproductive system. The current study investigated the effects of AsIV on Cd-induced toxicity in the male reproductive system.
Results
Morphological changes in the seminiferous tubules
In the control group, testicular parenchyma displayed regular morphology. Seminiferous tubules had regular diameter (250–270 μm) and normal thickness of the lamina propria (80–85 μm). Basement membrane and seminiferous epithelium were well-developed. The epithelium consisted of Sertoli cells and all kinds of spermatogenic cells, such as spermatogonia, spermatocytes, round and elongated spermatids, and spermatozoa. The stages of seminiferous epithelial cycle can be divided into stages I–XII and identified by morphology of spermatogonia, meiosis as well as the relative location of the spermatids. Spermiogenesis can be further subdivided into 16 developmental steps, containing round spermatids (steps 1–8) and differentiating elongating spermatids (steps 9–16). The Sertoli cells were located at the base of the seminiferous tubules. The nuclei were lightly stained and irregular, and the nucleoli were obviously present. The Sertoli cells were inlaid with spermatogenetic cells, and the spermatozoa were abundant in the seminiferous tubules. The interstitial compartment consisted of loose connective tissue and clusters of Leydig cells were noted in this tissue. Leydig cells had conspicuous round or oval nucleus. Sometimes their nucleolus was prominent. Their cytoplasm was abundant, containing few vacuoles. Depending on their location, Leydig cells could be subdivided into two kings: closing to seminiferous tubules and blood vessels in the middle of the interstitial space. ().
Figure 1. Morphology of the seminiferous tubules. HE staining, ×400. in the control group, the Sertoli cells were located at the base of the seminiferous tubules (A). In the Cd group, the seminiferous epithelium in the seminiferous tubules became thinner and disorderly arranged, and the gaps between the cells widened (B). In the Cd&AsIV group, the seminiferous epithelium had more regular arrangement in the seminiferous tubules, vacuolation was reduced in the cytoplasm of sertoli cells, and some spermatogenetic cells were sloughed off (C)
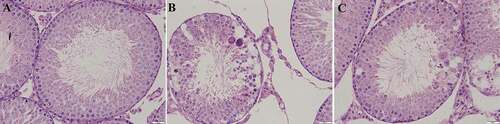
In the Cd group, following continuous treatment with Cd for 5 days, the seminiferous epithelium in the seminiferous tubules became thinner and disorderly arranged, and the gaps between the cells widened. The diameter of the seminiferous tubules and height of the epithelium were significantly reduced. Spermatogenesis went up to the spermatocyte stage. Few number of mature or elongated spermatids together with the spermatozoa in the seminiferous tubules were significantly reduced. Maturation of the seminiferous epithelium was decreased compared to the control group. Giant cells in the seminiferous epithelium were visible, and some spermatids in the lumen of the seminiferous tubules were present, which could be attributed to the effect of premature germ cell sloughing. More vacuoles were also observed in the cytoplasm of the Sertoli cells. The morphology of the loose connective tissue of the interstitial compartment and the types of cells were changed. The number and volume of Leydig cells were decreased. Within the cytoplasm of these cells, numerous small vacuoles were identified. ().
In the Cd&AsIV group, the seminiferous epithelium had a more regular arrangement in the seminiferous tubules and some spermatogenetic cells were sloughed off. The diameter of seminiferous tubules and epithelium height were reduced compared to control group, but significantly higher than the Cd group. Maturation of the seminiferous epithelium was increased compared to the Cd group. Additionally, typical multinucleated giant cells were visible within the seminiferous epithelium. Vacuolation was reduced in the cytoplasm of Sertoli cells. Changes between the loose connective tissue of the interstitial compartment and the number and volume of Leydig cells was inconspicuous. The volume of Leydig cells was lower compared to control group, but significantly higher than the Cd group (). The image analysis results of diameter of seminiferous tubules, epithelium height, volume of Leydig cells, and maturation of the seminiferous epithelium of each group are shown in .
Table 1. Effect of Cd and AsIV on diameter of seminiferous tubules, epithelium height, volume of Leydig cells and maturation of the seminiferous epithelium (mean ± sd, n = 30).
Immunohistochemical staining and image analysis of BTB proteins
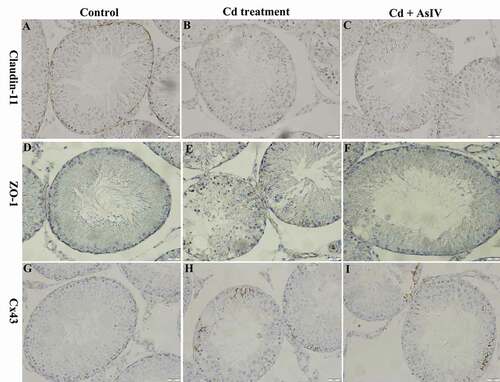
Claudin-11 and ZO-1 staining was brownish yellow and mainly located at the base of the seminiferous tubules, which appeared wavy or dotted. Additionally, there was a small amount of staining in the Leydig cells (). After continuous treatment with Cd for 5 days, the staining was significantly reduced (). After continuous treatment with Cd&AsIV for 5 days, the staining was lower compared to the control group but significantly higher than the Cd group (). Cx43 staining was brownish yellow and mainly found at the base of the seminiferous tubules, which appeared linear or dotted. Additionally, there was a small amount of staining in the spermatogenetic cells, between the spermatogenetic cells and Sertoli cells, and between the testicular Leydig cells (). After continuous treatment with Cd for 5 days, the staining decreased significantly, and we observed a transfer from the basal compartments to the apical compartments. A small amount of staining was found in the cytoplasm of the Sertoli cells (). After continuous treatment with Cd&AsIV for 5 days, the staining was lower than the control group, but significantly higher than the Cd group (). The image analysis results of the average optical density values of claudin-11, ZO-1, and Cx43 protein staining of each group are shown in
Table 2 Effect of Cd and AsIV on expression of claudin-11, ZO-1, Cx43 in the BTB (average optical density, AOD) (mean ± sd, n = 18).
Figure 2. Claudin-11, ZO-1, and Cx43 expression in the seminiferous tubules. immunohistochemistry staining; Streptavidin Peroxidase, ×400. in the control group, claudin-11, ZO-1 and Cx43 staining was brownish yellow and mainly located at the base of the seminiferous tubules (A, D, G). In the Cd group, claudin-11 and ZO-1 staining was significantly reduced (B, E). Cx43 staining significantly decreased, and location was transfer from the basal compartments to the apical compartments (H). In the Cd&AsIV group, the staining was lower compared to control group, but significantly higher than the Cd group (C, F, I)
Ultrastructural changes in the Sertoli cells and tight junctions of the BTB
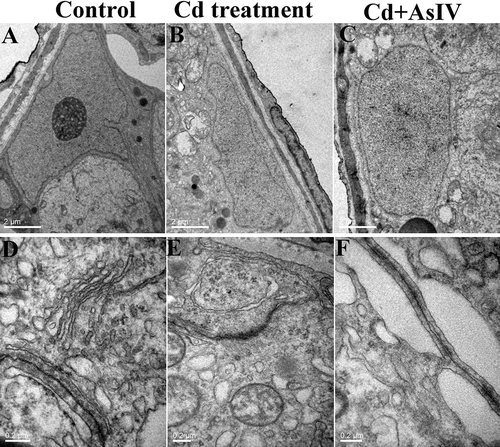
In the control group, the Sertoli cells were adhered to the basement membrane of the seminiferous tubule, and their nuclei were pyramidal, large, and irregular, with nuclear envelopes that were deeply sunken. The nuclei were often rich in chromatin, and the nucleoli were obviously present (). At high magnification, the tight junctions of the BTB remained intact, which appeared as a dense line with high electron density. Near the tight junctions of adjacent Sertoli cells, the endoplasmic reticulum was rich in the cytoplasm, forming a specialized area (). After continuous treatment with Cd for 5 days, the cytoplasmic lysis region of the Sertoli cells was obvious, and vacuoles appeared in the cytoplasm of some Sertoli cells, with swelled mitochondria and myelin-like structures (). Under high magnification, numerous local gaps in the tight junctions in the BTB were enlarged, and the endoplasmic reticulum in the specialization area expanded significantly (). After continuous treatment with Cd&AsIV for 5 days, the ultrastructural damage to the Sertoli cells and the tight junctions of the BTB were weaker compared to the Cd group ().
Figure 3. Transmission electron microscopy images of Sertoli cells and tight junctions of the BTB. In the control group, the Sertoli cells adhered to the basement membrane of the seminiferous tubule, and their nuclei were pyramidal, large, and irregular, with nuclear envelopes that were deeply sunken (A). At high magnification, the tight junctions of the BTB remained intact, which appeared as a dense line with high electron density (D). In the Cd group, the cytoplasmic lysis region of the Sertaaaaoli cells was obvious, and vacuoles appeared in the cytoplasm of some Sertoli cells, with swelled mitochondria and myelin-like structures (B). Under high magnification, numerous local gaps in the tight junctions in the BTB were enlarged, and the endoplasmic reticulum in the specialization area expanded significantly (E). In the Cd&AsIV group, the ultrastructural damage to the sertoli cells and the tight junctions of the BTB were weaker compared to the Cd group (C, F)
Discussion
In the current study, the diameter of the seminiferous tubules, epithelium height, volume of Leydig cells, and maturation of the seminiferous epithelium were reduced and decreased expression of claudin-11, ZO-1, and Cx43 was observed in response to Cd-induced toxicity. AsIV treatment rescued the morphologic changes observed in the seminiferous tubules of the testis, as well as the ultrastructural damage to the Sertoli cells and the tight junctions. In the Cd&AsIV group, the values of diameter of seminiferous tubules, epithelium height, and volume of Leydig cells improved significantly compared to the Cd group (p < 0.05). However, they were still lower as compared to the control group. At the same time, the average optical densities of claudin-11, ZO-1, and Cx43 were increased significantly compared to the Cd group (p < 0.05) but were still lower compared to the control group.
The BTB is comprised of tight junctions, adhesive junctions, and gap junctions, the tight junctions between the Sertoli cells in the BTB are the most important (Chen et al. Citation2018a). Claudins are the key member of the three classical transmembrane proteins of the tight junction complex, which contains four transmembrane domains with both the amino terminus and the carboxyl terminus on the inside of the cell membrane. The complex interacts with proteins such as ZO-1, ZO-2, and ZO-3, which are of great significance for maintaining the permeability of the tight junction and signal transduction (Yang et al. Citation2014). Among them, claudin-11, also known as oligodendrocyte-specific protein (OSP), is most abundant in the testis and partially expressed in the choroid plexus and renal collecting duct. ZO-1 is a tight junction peripheral protein located in the Sertoli cell membrane. A transmembrane protein attached to one end and an actin microfilament in the membrane attached to its other end, which is essential for tight junction function (Erkanlı Şentürk et al. Citation2012). Connexins (Cxs), gap junction proteins, are widely distributed in various organs and form a direct pathway between adjacent cells. This gap junctional intercellular communication (GJIC) is significant for regulating cell signal transduction, proliferation, differentiation, migration, and apoptosis (Li et al. Citation2012). Risley MS (2000) detected 11 Cx mRNAs in the testis using RT-PCR, of which Cx43 was the most abundant and most important for the developing testis. Cx43 knockout mice showed that Cx43 loss cannot be compensated by other Cx family proteins in Sertoli cells. Cx43 is expressed between Sertoli cells, spermatogenic cells, and interstitial cells. Its distribution also undergoes dynamic changes. As the spermatogenic epithelium of the testis matures, the expression of Cx43 gradually shifts from the apical (adluminal) compartment of the seminiferous epithelium to the basal compartment, becoming an important component of the BTB. Endocrine activity of Leydig cells can also be regulated by Cx43 (Goldenberg et al. Citation2003).
In the current study, we found that the expression of the tight junction proteins claudin-11 and ZO-1 were reduced in the seminiferous tubules after Cd treatment for 5 days. The spermatogenic epithelium was also significantly thinner, and more vacuoles were formed in the cytoplasm. A large number of spermatogenic cells were sloughed off, and the number of sperm was reduced in the lumen of the seminiferous tubules was significantly observed. The ultrastructure of the BTB showed that after continuous Cd treatment for 5 days, vacuoles appeared in the cytoplasm of some Sertoli cells, lysosomes increased, and mitochondria and endoplasmic reticulum were swollen. Numerous local gaps in the tight junctions were enlarged, and endoplasmic reticulum pools in specialized areas were expanded to varying degrees. Several studies have demonstrated that acute high-dose Cd (3 mg/kg body weight) can rapidly lead to altered expression of tight junction and gap junction proteins in the BTB and specialized areas, as well as ultrastructural damage (Medina et al. Citation2017). This is consistent with the findings of the current experimental study. However, the acute dose of Cd (1 mg/kg body weight) used in the current study can better morphologically demonstrate the dynamic changes of Cd-induced destruction of the BTB and the thinning of the spermatogenic epithelium. Here, we also observed that Cx43 decreases with continuous Cd treatment, with a tendency to shift from the basal compartment to the apical (adluminal) compartment. The expression of tight junction proteins claudin-11 and ZO-1 decreased, which may also be due to Cd-induced down-regulation of Cx43 expression and changes in the position resulting in instability of the BTB. Cx43 is closely linked to many proteins, including cytoskeletal proteins (tubulin, actin, and actin-binding protein), adhesive junction proteins (cadherin, and α- and β-catenin), and tight junction proteins (claudin-11, occludin, and ZO-1). Chen reported that Cx43 can specifically bind to the ZO-1 PDZ2 region, and this region can regulate the gap junction composed of Cx43 by phosphorylation and dephosphorylation (Chen et al. Citation2008). The results of the current study showed that expression of Cx43 decreased in the basement membrane of the seminiferous tubules, and was translocated or engulfed in the cytoplasm. At the same time, the tight junctions of the BTB were destroyed to varying degrees, and the expression of tight junction-associated proteins, claudin-11 and ZO-1, was decreased. Therefore, we speculate that Cx43 not only binds closely to the associated proteins of the BTB, but also changes the expression of Cx43, which can affect the expression of tight junction-associated proteins. Thus, this speculation should be further investigated.
Astragalus is the root of Mongolian Astragalus or the membrane folder astragaloside of leguminous plants and contain chemical components such as glycosides, polysaccharides, flavonoids, amino acids, and trace elements. AsIV, the main active ingredient in Astragalus preparations, has various pharmacological effects, such as preventing ischemia-reperfusion injury, protecting dopaminergic neurons, inhibiting oxidative stress and apoptosis, and regulating gene expression, among others. Qu et al. (Citation2009) confirmed that AsIV can up-regulate occludin and ZO-1 in the BBB after ischemia-reperfusion, thereby reducing the permeability of the BBB (Qu et al. Citation2009). In the current study, we observed that AsIV can partially antagonize the Cd-induced decreased expression of claudin-11, ZO-1, and Cx43 of the BTB. We also showed that AsIV can protect against Cd-induced morphological damage to the seminiferous tubules and ultrastructure of tight junctions of the BTB and Sertoli cells. Moreover, Cd increased the generation of intracellular reactive oxygen species (ROS), enhanced production of malondialdehyde (MDA), and induced mitochondrial dysfunction. Various studies have demonstrated the protective effect of antioxidants in cases of male infertility. For example, onions are a natural antioxidant that protects DNA and important molecules from oxidative stress and improves sperm quality, leading to decreased infertility rates (Khaki et al. Citation2017). A recent study suggested that the antioxidant quercetin decreased MDA and ROS levels caused by Cd in goat sperm, which facilitated increased motility, survival, membrane integrity, and mitochondria activity during storage in vitro and subsequent embryo development. Chen et al. (Citation2018c) reported that ascorbic acid protects against BTB destruction by inhibiting oxidative stress and the tumor growth factor (TGF)-β3/p38 MAPK signaling pathway in the testis of Cd-exposed rats (Chen et al. Citation2018c). It has been reported in the literature that the Sertoli cells are capable of producing a variety of proteases (such as MAPK) and their corresponding protease inhibitors. These enzymes and their inhibitors maintain the normal dynamics of the BTB, ensuring the successful progress of spermatogenesis. Therefore, we speculate that Cd interferes with the homeostasis of intracellular proteases and their corresponding inhibitors of the Sertoli cells, leading to overexpression of certain proteases (MAPK), which in turn destroy the BTB-associated proteins. Thus, the mechanism by which AsIV antagonizes Cd-induced male reproductive toxicity may be related to inhibition of the MAPK signaling pathway. However, Zhu JB et al indicated that AsIV was maternally toxic at 1.0 mg/kg body weight in rats and fetotoxic at a dose higher than 0.5 mg/kg body weight, but devoid of teratogenic effects in rabbits. In light of these findings, it is perhaps prudent to advise caution for women who might use AsIV to combat cardiovascular disease during pregnancy (Jiangbo et al. Citation2009; De Barros et al. Citation2018). In addition, its poor bioavailability after oral administration is mainly caused by its very low solubility in biologic fluids, resulting in disappointing clinical application (Qing et al. Citation2019). Thus, we need look for new drug development of AsIV that might provide better clinical efficacy and be a potential candidate for treatment of infertility. In future studies, we will explore the mechanism of Cd-induced BTB destruction in the male reproductive system and develop corresponding targeted drugs, which could be clinically used to improve fertility rates.
In conclusion, we used a Cd-induced male rat model to observe the effects of Cd on the expression of related connective proteins in the BTB and the destruction of the BTB ultrastructure, as well as the protective effect of AsIV. The results showed that Cd can lead to decreased expression of tight junction proteins, claudin-11 and ZO-1, as well as gap junction protein, Cx43, of the BTB, causing ultrastructural damage. AsIV can antagonize Cd’s effect on the expression of the above-mentioned related connective proteins and reduce the degree of ultrastructural damage of the BTB.
Materials and methods
Animals and experimental material
This study was approved by the Institutional Animal Research Ethics Committee at the Army Medical University, Chongqing, China (approval no. SYXK20070002). A total of 18, 7 week-old SPF male SD male rats (average body weight: 200–220 g) were purchased from the experimental animal center at the Army Medical University, Chongqing, China [Certification number: SCXK (Yu) 2012–0001]. Cd chloride (batch number: 20,100,601; purity of 99.0% or higher) was purchased from the Kelon Chemical Reagent Factory, Chengdu. AsIV (batch number 120,220; purity of 98.0% or higher) was purchased from Fang Sheng Biological Development Limited Company (Baoji, China). Dimethyl sulfoxide (DMSO) was purchased from Sigma (United States). Rabbit anti-claudin-11 (36–4500) and rabbit anti-ZO-1 (61–7300) antibodies were purchased from Invitrogen (United States). Rabbit anti-Cx43 antibody (ab11370) was purchased from Abcam (United States). Horseradish peroxidase labeling goat anti-rabbit IgG (H + L) was purchased from Blue Skies Biotechnology Institute (Jiangsu, China). Two-step immunohistochemical detection kits and the DAB chromogenic reagent kit were purchased from Boster Biological Engineering Limited (Wuhan, China). Both Pyramitome and Ultratome were purchased from Reichert-Jung (Austria). The Ultratome Hitachi-7500 was purchased from Hitachi Limited (Japan).
Concentration of Cd and AsIV choosing
The pre-experiment was designed to test the doses of Cd in current investigation following the instruction of published guideline (Chen et al. Citation2018b). Male SD rats were exposed to Cd with dose of 1 mg/kg body weight and 3 mg/kg body weight, respectively. We found that Cd-induced damages to the morphology of Seminiferous tubules was distinctly observed at the dose of 1 mg/kg body weight. However, The Seminiferous tubules was changed significantly with dose of 3 mg/kg body weight, and the testis was completely destroyed on the fifth day, even some of rats have died. Therefore, we used the dose of 1 mg/kg body weight for injection. In our previous study, we processed MTT cell activity test, we added AsIV to the Sertol cell culture medium containing Cd with doses of 5 mg/L, 10 mg/L and 20 mg/L, respectively. We observed that Sertol cells had the preferable activity in the 10 mg/L dose group. Besides, Cd was administered to the rats at 1 mg/kg body weight day by day, Some of rats died after 5 days. Thus 5 day-administration period is a proper span for selection.
Animal grouping and processing
Modeling with disruption of the BTB by Cd (Siu et al. Citation2009; Qu et al. Citation2009; Chen et al. Citation2018a), 18 SPF-class, 7-week-old male SD rats, with an average body weight (200–220) g were randomly divided into three groups (n = 6/group) as control, Cd, and Cd & AsIV. 0.1% Cd chloride was administered to the rats in Cd group via i.p. at 1 mg/kg body weight once daily, 0.1% Cd chloride and AsIV was administered to the rats in Cd&AsIV group via i.p. at 1 mg/kg body weight and 10 mg/kg body weight respectively once daily, and the same volume of saline was administered to the rats in control group via i.p. once daily. The rats in three groups were injected continuously for 5 days. On the sixth day, the rats were anesthetized with isoflurane. The left testis was surgically removed, immediately dissected with a cleaning blade, and quickly placed in a vial containing Bouin’s fixative. After being fixed for approximately 20 min, the testis was cut with a blade into approximately 3 mm thick pieces and placed in fixing solution to continue the fixation. The right testis was injected with 4% glutaraldehyde for 20 min in vivo, removed, immediately cut into 1 mm3 tissue blocks with a clean double-sided blade, and placed in 4% glutaraldehyde for fixation.
Hematoxylin and eosin (HE) and immunohistochemical staining
After fixation in Bouin’s fixative solution, the testicular tissue was routinely dehydrated with alcohol, made transparent with xylene, embedded in paraffin, sliced at a thickness of 5 μm, and observed after HE staining. Further tissue preparation was performed according to the instructions of the two-step immunohistochemistry test kit. The sections were deparaffinized in water, washed thrice with distilled water (2 min each time), incubated in 3% H2O2 for 10 min at room temperature to eliminate endogenous peroxidase activity, and washed thrice with distilled water (2 min each time). Then, the tissues were immersed in 0.01 mol/L citrate buffer (pH 6.0), boiled in a microwave oven for 5 min, cooled for 20 min at room temperature, and washed thrice with phosphate buffer saline (PBS) (2 min each time). The sections were incubated in 5% bovine serum albumin (BSA) at room temperature for 10 min, followed by incubation with rabbit anti-rat claudin-11 primary antibody (1:100), rabbit anti-rat ZO-1 primary antibody (1:100), or rabbit anti-rat Cx43 primary antibody (1:1,000), overnight at 4°C (~14–16 h). After rewarming for 1 h in a 37°C water bath, the sections were washed thrice with PBS (2 min each time), incubated in an HRP-labeled anti-rabbit IgG secondary antibody in a 37°C water bath for 30 min, and washed thrice with PBS (2 min each time). The sections were then stained with DAB, dehydrated, sealed, and observed under a light microscope. The negative control group was incubated with PBS instead of the primary antibodies.
Transmission electron microscopy
The 1 mm3 testicular tissue sections were fixed with 4% glutaraldehyde pre-fixation and 1% osmium tetroxide (prepared in PBS) post fixation (pH 7.2–7.4) (2 h each time). The conventional gradient dehydration was done using ethanol and acetone. Epoxy resin 618# soaking, embedding, polymerization, and ultratome were used to prepare 70 nm ultrathin sections. The uranyl acetate and lead citrate were used for contrasting and staining. A Hitachi-7500 transmission electron microscope was used to observe and image the sections at 120 KV.
Image analysis
Light microscopy (400x) was used to image the immunohistochemistry staining for claudin-11, ZO-1, and Cx43 in the control group, Cd group, and Cd&AsIV group, respectively. A total of 18 images per group (3 fields per animal) were obtained, resulting in a total of 162 images in 9 groups. The BM2000 Biomedical Image Analysis System at Beijing University of Aeronautics and Astronautics was used to average the optical density analysis of the images. Light microscopy (400x) was used to evaluate diameter of seminiferous tubules and epithelium height. Five fields of seminiferous tubules were evaluated from per animal in the control group, Cd group and Cd & AsIV group by the software Image-Pro Plus, Version 6.0 conducting selected measurement manually. Measuring method: diameter of semiferous tubules is the distance between the outer-ring of the two lamina propria through the center, epithelium height is the distance between edge of basement membrane and apical seminiferous epithelium. The stages of seminiferous epithelial cycle were used to assess dynamics of spermatogenesis. The stages of seminiferous epithelial cycle can be divided into stages I–XII and identified by morphology of spermatogonia, meiosis as well as relative location of the spermatids. According to the stages of seminiferous epithelial cycle, seminiferous epithelium can be assigned a value on the basis of higher frequency of mature cell population, which was divided into two grades: grade one refers to primary or secondary spermatocytes, grade two refers to spermatids with rounded nuclei, which were in the I to VIII stages of seminiferous epithelial cycle. Therefore, the degree of maturation of the seminiferous epithelium can be further used to evaluate dynamics of spermatogenesis. Five fields of seminiferous tubules were evaluated from per animal in the control group, Cd group and Cd & AsIV group at random. The average degree of maturation of the seminiferous epithelium in the control group, Cd group and Cd & AsIV group was that the number of seminiferous tubules in each of the degrees was multiplied by the value of grade and the obtained values were summed and divided by thirty. The volume of Leydig cells has been regarded as a sensitive indicator to evaluate testosterone production. Five regular circular or oval nuclei were chosen randomly from per animal in the control group, Cd group and Cd & AsIV group. The diameter of the nucleus of the Leydig cell was measured by using the software Image-Pro Plus, Version 6.0. Leydig cell nuclear volume was calculated by the mathematical formula, which is Diameter3 × π × 1/6. The percent of Leydig cells occupied by nuclei was evaluated by using a grid containing 441 points the same as a 40X objective lens. Leydig cell cytoplasmic volume was calculated by the mathematical formula, which is cytoplasm%×Leydig cell nuclear volume/nucleus%. The volume of Leydig cells was summed by Leydig cell nuclear volume and cytoplasmic volume.
Statistical analysis
Statistical analyses were performed using SPSS Statistics for Windows, Version 19.0 (IBM Corp, Armonk, NY, USA). Data are expressed as mean ± standard deviation (sd)., and one-way analysis of variance (ANOVA) was used for comparison between groups. Statistically significant differences were indicated by P values of < 0.05.
Ethics approval
All experimental procedures were approved by the Research Council and Animal Care and Use Committee of Army Medical University, Chongqing, China (approval no. SYXK20070002) and were performed in accordance with the Guide for the Care and Use of Laboratory Animals published by National Institutes of Health.
Authors’ contributions
Conceived and designed the experiments: ZXY, SHY; performed the experiments and analyzed the data: WN, XYD, YCW, XLY; drafted the manuscript: WN; contributed reagents, materials and laboratory environment: JX; completes electron microscopic detection: XGL. All authors read and approved the final manuscript.
Data availability statement
All the data supporting your findings is contained within the manuscript. The datasets used and/or analyzed in the current study is available from the corresponding author on reasonable request.
Disclosure statement
The authors declare that they have no competing interests.
Additional information
Funding
References
- Chen H, Lui WY, Mruk DD, Xiao X, Ge R, Lian Q, Lee WM, Silvestrini B, Cheng CY. 2018a. Monitoring the Integrity of the Blood-Testis Barrier (BTB): an in vivo assay. Methods Mol Biol (Clifton, NJ). 1748:245–252. eng. doi: https://doi.org/10.1007/978-1-4939-7698-0_17.
- Chen H, Lui W-Y, Mruk DD, Xiao X, Ge R, Lian Q, Wm L, Silvestrini B, Cheng CY. 2018b. Monitoring the Integrity of the Blood-Testis Barrier (BTB): An In Vivo Assay. In: Alves M., Oliveira P. (eds) Sertoli Cells. Methods in Molecular Biology. 1748. Humana Press, New York, NY. doi:https://doi.org/10.1007/978-1-4939-7698-0_17.
- Chen J, Pan L, Wei Z, Zhao Y, Zhang M. 2008. Domain-swapped dimerization of ZO-1 PDZ2 generates specific and regulatory connexin43-binding sites. EMBO J. 27(15):2113–2123. eng. doi:https://doi.org/10.1038/emboj.2008.138.
- Chen N, Su P, Wang M, Li YM. 2018c. Ascorbic acid inhibits cadmium-induced disruption of the blood-testis barrier by regulating oxidative stress-mediated p38 MAPK pathways. Environ Sci Pollut Res Int. 25(22):21713–21720. eng. doi:https://doi.org/10.1007/s11356-018-2138-4.
- Coulthard LR, White DE, Jones DL, McDermott MF, Burchill SA. 2009. p38(MAPK): stress responses from molecular mechanisms to therapeutics. Trends Mol Med. 15(8):369–379. eng. doi:https://doi.org/10.1016/j.molmed.2009.06.005.
- De Barros JWF, Borges CDS, Missassi G, Pacheco TL, De Grava Kempinas W. 2018. Impact of intrauterine exposure to betamethasone on the testes and epididymides of prepubertal rats. Chem Biol Interact. 291:202–211. eng. doi:https://doi.org/10.1016/j.cbi.2018.06.030.
- Dong H, Chen Z, Wang C, Xiong Z, Zhao W, Jia C, Lin J, Lin Y, Yuan W, Zhao AZ, et al. 2015. Rictor regulates spermatogenesis by controlling sertoli cell cytoskeletal organization and cell polarity in the mouse testis. Endocrinology. 156(11):4244–4256. eng. doi:https://doi.org/10.1210/en.2015-1217.
- Erkanlı Şentürk G, Ersoy Canillioĝlu Y, Umay C, Demiralp-Eksioglu E, Ercan F. 2012. Distribution of Zonula Occludens-1 and Occludin and alterations of testicular morphology after in utero radiation and postnatal hyperthermia in rats. Int J Exp Pathol. 93(6):438–449. eng. doi:https://doi.org/10.1111/j.1365-2613.2012.00844.x.
- Goldenberg RC, Fortes FS, Cristancho JM, Morales MM, Franci CR, Varanda WA, Campos de Carvalho AC. 2003. Modulation of gap junction mediated intercellular communication in TM3 Leydig cells. J Endocrinol. 177(2):327–335. eng. doi:https://doi.org/10.1677/joe.0.1770327.
- He P, Lu Y, Liang Y, Chen B, Wu M, Li S, He G, Jin T. 2013. Exposure assessment of dietary cadmium: findings from Shanghainese over 40 years, China. BMC Public Health. 13(1):590. doi:https://doi.org/10.1186/1471-2458-13-590.
- Huang Z, Pan XD, Wu PG, Han JL, Chen Q. 2013. Health risk assessment of heavy metals in rice to the population in Zhejiang, China. PloS One. 8(9):e75007. eng. doi:https://doi.org/10.1371/journal.pone.0075007.
- Ibiam AU, Ugwuja EI, Ejeogo C, Ugwu O. 2013. Cadmium-induced toxicity and the hepatoprotective potentials of aqueous extract of jessiaea nervosa leaf. Adv Pharm Bull. 3(2):309–313. eng.
- Jahan S, Zahra A, Irum U, Iftikhar N, Ullah H. 2014. Protective effects of different antioxidants against cadmium induced oxidative damage in rat testis and prostate tissues. Syst Biol Reprod Med. 60(4):199–205. eng. doi:https://doi.org/10.3109/19396368.2014.912363.
- Jiang XH, Bukhari I, Zheng W, Yin S, Wang Z, Cooke HJ, Shi QH. 2014. Blood-testis barrier and spermatogenesis: lessons from genetically-modified mice. Asian J Androl. 16(4):572–580. eng. doi:https://doi.org/10.4103/1008-682X.125401.
- Jiangbo Z, Xuying W, Yuping Z, Xili M, Yiwen Z, Tianbao Z. 2009. Effect of astragaloside IV on the embryo-fetal development of Sprague-Dawley rats and New Zealand White rabbits. J Appl Toxicol. 29(5):381–385. eng. doi:https://doi.org/10.1002/jat.1422.
- Khaki A, Rajabzadeh A, Khaki AA. 2017. Side effects of pyrethroid and supporting role of onion in the male rat’s spermatogenesis. Chin Med J. 130(24):3015–3016. eng. DOI:https://doi.org/10.4103/0366-6999.220297.
- Li L, Hou X, Xu R, Liu C, Tu M. 2017. Research review on the pharmacological effects of astragaloside IV. Fundam Clin Pharmacol. 31(1):17–36. eng. DOI:https://doi.org/10.1111/fcp.12232.
- Li MW, Mruk DD, Cheng CY. 2012. Gap junctions and blood-tissue barriers. Adv Exp Med Biol. 763:260–280. eng.
- Li X, Wang X, Han C, Wang X, Xing G, Zhou L, Li G, Niu Y. 2013. Astragaloside IV suppresses collagen production of activated hepatic stellate cells via oxidative stress-mediated p38 MAPK pathway. Free Radic Biol Med. 60:168–176. eng. DOI:https://doi.org/10.1016/j.freeradbiomed.2013.02.027.
- Medina MF, Arrieta MC, Villafañe MN, Klyver SMR, Odstrcil IMA, González ME. 2017. Early signs of toxicity in testes and sperm of rats exposed to low cadmium doses. Toxicol Ind Health. 33(7):576–587. eng. DOI:https://doi.org/10.1177/0748233716689524.
- Messaoudi I, Banni M, Saïd L, Saïd K, Kerkeni A. 2010. Evaluation of involvement of testicular metallothionein gene expression in the protective effect of zinc against cadmium-induced testicular pathophysiology in rat. Reprod Toxicol (Elmsford, Ny). 29(3):339–345. eng. DOI:https://doi.org/10.1016/j.reprotox.2010.01.004.
- Qing LS, Chen TB, Sun WX, Chen L, Luo P, Zhang ZF, Ding LS. 2019. Pharmacokinetics comparison, intestinal absorption and acute toxicity assessment of a novel water-soluble astragaloside IV derivative (Astragalosidic Acid, LS-102). Eur J Drug Metab Pharmacokinet. 44(2):251–259. eng. DOI:https://doi.org/10.1007/s13318-018-0515-5.
- Qu YZ, Li M, Zhao YL, Zhao ZW, Wei XY, Liu JP, Gao L, Gao GD. 2009. Astragaloside IV attenuates cerebral ischemia-reperfusion-induced increase in permeability of the blood-brain barrier in rats. Eur J Pharmacol. 606(1–3):137–141. eng. DOI:https://doi.org/10.1016/j.ejphar.2009.01.022.
- Ren S, Zhang H, Mu Y, Sun M, Liu P. 2013. Pharmacological effects of Astragaloside IV: a literature review. J Tradi Chine Med. 33(3):413–416. eng.
- Siu ER, Mruk DD, Porto CS, Cheng CY. 2009. Cadmium-induced testicular injury. Toxicol Appl Pharmacol. 238(3):240–249. eng. DOI:https://doi.org/10.1016/j.taap.2009.01.028.
- Wang P, Chen H, Kopittke PM, Zhao FJ. 2019. Cadmium contamination in agricultural soils of China and the impact on food safety. Environ Pollu (Barking, Essex: 1987). 249:1038–1048. eng. DOI:https://doi.org/10.1016/j.envpol.2019.03.063.
- Yang Q, Hao J, Chen M, Li G. 2014. Dermatopontin is a novel regulator of the CdCl2-induced decrease in claudin-11 expression. Toxicol in Vitro Int J Pub Assoc BIBRA. 28(6):1158–1164. eng. DOI:https://doi.org/10.1016/j.tiv.2014.05.013.
- Zhang J, Wu C, Gao L, Du G, Qin X. 2020. Astragaloside IV derived from Astragalus membranaceus: a research review on the pharmacological effects. Adv Pharma (San Diego, Calif). 87:89–112. eng.
