ABSTRACT
There are few treatment options, including the use of natural phenolics-based combination therapy for mitigating male infertility conditions associated with chemotherapy. Busulfan is an anti-cancer drug that leads to testicular problems in humans. Here, we studied the effect of co-treatment of rutin and kolaviron against busulfan-induced testis damage. Young adult male Wistar rats were intraperitoneally injected busulfan (4 mg/kg b.w), and then orally administered rutin (30 mg/kg b.w), and kolaviron (50 mg/kg b.w) alone and combined for 60 days. Results revealed that rutin and kolaviron alone or in combination reversed busulfan-induced increase in oxidative stress along with sperm quality of treated animals. However, kolaviron and rutin separately improved the concentrations of MDA and GSH and sperm quality more than when they were combined. Similarly, rutin and kolaviron separately or in combination preserved spermatogenesis and relieved busulfan-induced increase in nitric oxide concentration, myeloperoxidase and 3β-hydroxysteroid dehydrogenase activities. Co-supplementation with kolaviron but not rutin nor when rutin was combined with kolaviron also improved the testicular level of tumor necrosis-alpha. Finally, the histological features in the testes caused by busulfan were reversed by rutin, whereas treatment with kolaviron alone or in combination with rutin partially protected the testis from busulfan-induced injury as demonstrated by the appearance of few germ cells, damaged tubules, loss of round spermatids and defoliation of the seminiferous epithelium. Thus, the combined treatment regimen of rutin and kolaviron sparingly prevented busulfan-induced testicular injuries in rats.
Abbreviations: CAT: Catalase; GSH: Glutathione; 3β-HSD: 3β- hydroxysteroid Dehydrogenase; MDA: Malondialdehyde; TNF-α: Tumor necrosis-alpha; BUS: Busulfan; RUT: Rutin; KV: Kolaviron; TBARS: Thiobarbituric Acid Reactive Substances; MPO: Myeloperoxidase; ELISA: Enzyme-Linked Immunoassay; NAD: Nicotinamide Adenine Dinucleotide (oxidized); ROS: Reactive Oxygen Species.
Introduction
Germ cell formation and development are important targets of some cancer chemotherapeutic drugs (Ezirim et al. Citation2019). The capacities of the germ cells to undergo rapid division make them vulnerable to the toxic insult of cancer treatment drugs like busulfan (Ezirim et al. Citation2019; Abarikwu et al. Citation2020). As an alkyl sulfonate, busulfan or 1,4-butanediol dimethanesulfonate is used in bone marrow transplantation, lymphoma and leukemia treatment and badly affects sperm production (Li et al. Citation2018; Nasimi et al. Citation2018; Ezirim et al. Citation2019). In many cases, these testicular effects could result to oligospermia, azoospernia or infertility in young individuals undergoing chemotherapy treatment (Jung et al. Citation2015; Li et al. Citation2018). Thus, azoospermic experimental animal models are sometimes prepared using busulfan, due to its capacity to destroy differentiated germ cells domiciled in the testes (Abarikwu et al. Citation2020).
Accordingly, the goal of relieving the deleterious impact of busulfan on male fertility health is gaining much traction in the public space and in different laboratories worldwide (Li et al. Citation2018; Nasimi et al. Citation2018; Ezirim et al. Citation2019; Abarikwu et al. Citation2020). Interestingly, phytochemicals are at the epicenter of these therapeutic intervention paradigms due to their plethora of biological activities and tissue-protective potentials (Olayinka et al. Citation2014; Nafees et al. Citation2015; Abarikwu et al. Citation2017). A treatment regimen that involves combining different phytochemicals or their extracts from seeds, nuts or fruits can enhance or reduce the bioavailability and pharmacological effects conferred by the individual phytochemical (Phan et al. Citation2018a). It appears that the combined actions of phytochemicals may elicit heightened or diminished biological properties than the effect of the individual compound (Phan et al. 2018). Therefore, there is the need to evaluate the efficacy of combined phytochemical-based therapies, especially those used as complementary adjuvants, so that their therapeutic potentials are maximized during the treatment of diseases. Synergistic, additive, potentiation, and antagonistic effects are the summative outcomes of these phytochemical mixture interactions that may influence distinct biochemical pathways necessary for cell or tissue survival (Liu Citation2004; Phan et al. 2018; Negrette-Guzmán Citation2019).
The citrus flavonoid, rutin, is made up of disaccharide rutinose and flavonol quercetin with strong antioxidant and potent pharmacological capabilities including, antiviral, anti-angiogenic, antidiarrheal, anti-mutagenic, immunomodulatory and anti-inflammatory attributes (Chen et al. Citation2000; Nakamura et al. Citation2000; Nafees et al. Citation2015; Abarikwu et al. Citation2017). It is commonly available in plums, oranges, tea, buckwheat, cherries, apricots and apples (Abarikwu et al. Citation2020). Kolaviron is a natural medicinal extractive obtained from the Garcinia kola seed, a common nut among most Central and West African populace. The seeds and/or extracts are often utilized to treat certain ailments such as pains, bronchitis, cough, laryngitis, catarrh, liver diseases, dysentery, diarrhea, and hoarseness voice (Iwu and Igboko Citation1982; Iwu et al. Citation1990). Although the nuts contain other bioactive extractives flavonoids (biflavonoid), xanthenes and benzophenones (Iwu and Igboko Citation1982; Iwu et al. Citation1990; Abarikwu et al. Citation2012a), kolaviron is the dominant biflavonoid with several interesting pharmacological properties including, anti-microbial, anti-hepatotoxic, hypocholestrolemic, hypoglycemic, anti-nephrotoxic, neuro-protective, anti-inflammatory, antioxidant and testiculo-protective effects (Olaleye et al. Citation2000, Citation2010; Abarikwu et al. Citation2011, 2012a; Ayepola et al. Citation2014; Olayinka et al. Citation2014; Michel et al. Citation2016). As the interactive effects of bioactive compounds could influence their overall biological properties, the present study was designed to ascertain the outcome of the combination therapy with rutin and kolaviron against busulfan-induced gonadal toxicity in young adult rats.
Results
Effect of rutin and kolaviron on testis oxidative stress in busulfan-treated rats
As expected, busulfan injection significantly increased testicular concentration of malondialdehyde (MDA) when compared with the untreated control group. However, rutin and kolaviron alone or in combination diminished the testis MDA concentration compared with the busulfan group (p < 0.05). Kolaviron lowered testis MDA level in busulfan exposed rats more than rutin alone or the combination of kolaviron and rutin (). As noted, busulfan caused a significant decrease in the testis glutathione (GSH) concentration of rats, but co-supplementation with rutin and kolaviron alone or in combination elevated the level of glutathione compared with the busulfan group. The improvement in GSH concentration was more prominent in the testis of rats’ co-administered busulfan and rutin and in animals co-treated with combination of rutin and kolaviron (). Although there was a tendency for CAT activity to increase in all treated groups in comparison with the control, it reaches statistical significant (p < 0.05) level only in the BUS + RUT treated animals ().
Figure 1. Oxidative stress parameters in the studied experimental rat groups. Biochemical markers of oxidative stress were determined in the testes homogenates prepared in ice-cold Tris-KCl buffer as described in the material and methods section. MDA: malondialdehyde (A) GSH: reduced glutathione, (B) CAT: catalase (C). BUS: busulfan; RUT: rutin; KV: kolaviron. Data are mean ± standard deviation (n = 10). *Versus control; **Versus BUS; ***Versus BUS + RUT; ****Versus BUS + KV (p < 0.05)
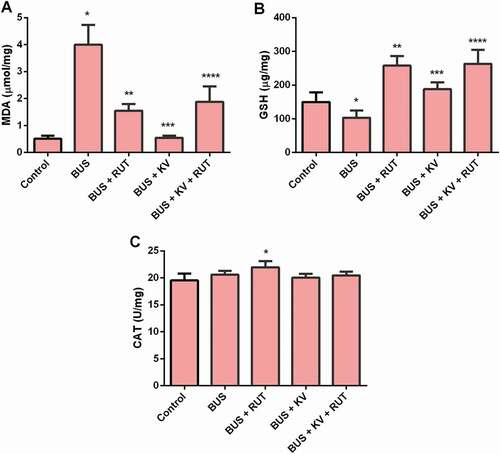
Effects of rutin and kolaviron on testis nitrite and TNF-α concentrations and myeloperoxidase activity in busulfan-treated rats
Animals treated with BUS showed an increased level of nitrite in the testes that decreased after co-treatment with rutin or kolaviron separately or in combination (). Busulfan treatment also caused an increase in testicular myeloperoxidase activity in rats which was significantly (p < 0.05) decreased on co-supplementation with rutin and kolaviron separately or in combination. The testis myeloperoxidase activity was much more decreased after separate co-treatment with rutin than kolaviron. However, the combined treatment regimen of RUT and KV was better than the effect observed after single administration of kolaviron (). Testicular TNF-α concentration was decreased in the BUS and BUS + RUT groups relative to the values seen in the control rats (p < 0.05). The concentration of TNF-α was increased in the testis of BUS + KV relative to the BUS treated animals and was recovered to the values seen in the control while the values of TNF-α in the BUS + KV + RUT animals was reduced compared to the BUS + KV group but toward the values seen in the BUS + RUT – treated animals ().
Figure 2. Nitrite concentrations (A) Myeloperoxidase activity (B) and tumor necrosis factor-alpha (TNF-alpha) level (C) in the studied experimental rat groups. Biochemical parameters of inflammation were determined in the testes homogenates prepared in ice-cold Tris-KCl buffer as described in the material and methods section. BUS: busulfan; RUT: rutin; KV: kolaviron. Data are mean ± standard deviation (n = 10). *Versus control; **Versus BUS; ***Versus BUS + RUT (myeloperoxidase); ***Versus BUS + KV (TNF-alpha) ****Versus BUS + KV (p < 0.05)
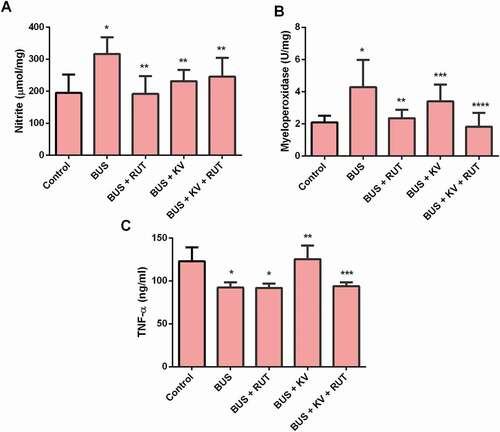
Effect of rutin and kolaviron on the activity 3β-HSD in the testes of busulfan-treated rats
Busulfan treatment increased the activity of 3β-HSD in the testes homogenates of experimental animals, which was reduced toward the control values on co-administration of rutin and kolaviron separately and in combination (p < 0.05). The decrease of 3β-HSD was more effective after a single administration of rutin than after a separate treatment of kolaviron and when kolaviron was administered in combination with rutin ().
Figure 3. Testicular 3β-hydroxysteroid dehydrogenase (3β-HSD) activity in studied experimental rat groups. Enzyme activity level of 3β-HSD was determined in the testes homogenates prepared in ice-cold Tris-KCl buffer as described in the material and methods section. BUS: busulfan; RUT: rutin; KV: kolaviron. Data are mean ± standard deviation (n = 10). *Versus control; **Versus BUS; ***Versus BUS + KV (p < 0.05)
Effect of rutin and kolaviron on the sperm quality and germ cell numbers in the testes of busulfan-treated rats
We also evaluated germ cell counts in the seminiferous tubules and epididymal sperm quality after treatment with BUS separately or combined with RUT, KV and RUT + KV. As expected, BUS treatment significantly decreased the numbers of spermatogonia (Type A and B), spermatocytes (primary and secondary) and round spermatids. Furthermore, BUS treatment also resulted in a significant increase in the percentage of abnormal sperms as well as decreased motility of sperms and the numbers of cauda epididymal sperms (). Co-treatment with RUT or KV separately or in combination significantly increased spermatogonia, spermatocytes (primary and secondary) and round spermatids numbers and also improved the sperm quality parameters of BUS treated animals (p < 0.05). However, their effects on these variables of testicular functions were better in the animals co-treated with RUT and KV separately than in animals treated with their combination (KV + RUT), and had the most effects in the BUS + RUT-treated animals. For instance, it could be noted that spermatogonia, spermatocytes and round spermatids numbers, and sperm quality parameters were statistically lower in the BUS + KV + RUT compared to BUS + RUT-treated animals (; p < 0.05).
Table 1. Germ cell counts in seminiferous tubules and cauda epididymides sperm quality parameters of control and experimental rats at the end of the study.
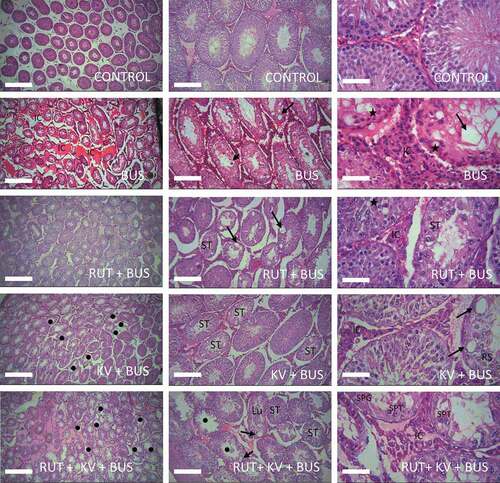
Effect of rutin and kolaviron on the testis histo-cytology of busulfan-treated rats
The cyto-structure of the control rat testis appeared normal with intact lumen and germ cells at different stages of maturation. As expected, the testis cyto-architecture of busulfan treated rats was damaged and has lots of germ cells with abnormal morphology, small-sized tubules with lots of vacuolar spaces and wide interstitial spaces between neighboring tubules. The morphology of the tubules was improved and the epithelium was better organized, although this was accompanied with few degenerate and apoptotic cells in the epithelium after co-treatment with rutin. Furthermore, in rats co-treated with kolaviron, the testis morphology was also improved although the epithelium for some tubules appeared distorted with the presence of few aberrant cells and giant vacuoles. Although few spermatocytes were found in the tubules, the round spermatids were missing in the rat testis co-treated with rutin and kolaviron. Other structural deficits observed were wide lumen for some tubules, wide spaces between neighboring tubules, and tubules with few germ cells. The histology of the testes of these animals appeared not better than BUS + KV or BUS + RUT animals ().
Figure 4. Histopathological examinations of testes isolated from the studied experimental animals. The testes of the control animals showed germ cells that have normal shapes and are seen to be at different stages of maturation in tubules with a centrally located lumen as expected. Many of these cells are seen in layers arising from the basement membrane. As expected, the testes of busulfan (BUS) treated animals have smaller tubules with lots of empty (vacuolar) spaces (long arrows) in the seminiferous epithelium, wide interstitial spaces amongst neighboring tubules and giant apoptotic cells (short arrow) at the luminal border. Note the large populations of interstitial cells between neighboring tubules (IC) along with germ cells (starred) at the basement membrane with abnormal shapes. Spermatids e.g., round spermatids are completely absent in many of the tubules. The tubules had improved morphology similar to the control in the rutin (RUT) + BUS group of animals and better than BUS-treated animals, although there are still few cells within the vacuolated epithelium (arrows) that are somewhat degenerated and with the presence of apoptotic cells (starred). The testes of the kolaviron (KV) + BUS treated animals have few damaged tubules (black dots). Some of the tubular epithelium appeared degenerated (ST) and contain giant vacuoles (arrows) and few round spermatids (RS). The wide intertubular spaces (IC) were seen to contain interstitial cells e.g., Leydig cells. There are more damaged tubules (black dots) with degenerated epithelium (ST), presence of vacuoles (arrows) and defoliated cells in the lumen (Lu) of the KV + RUT + BUS treated animals. The spaces between neighboring tubules are still wide (IC) and few germ cells e.g., spermatocytes (SPT) and spermatogonia (SPG) can still be observed in some tubules. Mag: (4×: Left panel; 10 ×: middle panel; 40 × right panel). Scale bars = 100 µm
Discussion
As expected in the present study, busulfan decreased GSH and increased MDA levels indicative of testicular oxidative stress (Nafees et al. Citation2015; Abarikwu et al. Citation2017, Citation2019, Citation2020; Nasimi et al. Citation2018; Li et al. Citation2018; Njoku et al. Citation2019). Rutin or kolaviron separately or in combination protected against busulfan-induced testicular oxidative stress as demonstrated by the reversed levels of MDA and GSH toward the control values, a finding consistent with the observation that both phenolics are very potent antioxidant compounds (Abarikwu et al. Citation2012b, Citation2020).
Although the rutin and kolaviron combination model effectively reversed the busulfan-induced testicular oxidative damage, this was not better than the individual effects of rutin and kolaviron in the busulfan model. After separate rutin administration, the increase in CAT activity, an important defensive antioxidant enzyme in tissues, recovered to the control values following combined rutin and kolaviron co-treatment. This suggests that the biological effects resulting from the combined treatment was less than the biological outcomes imposed by the individual phyto-compounds. It is plausible that this outcome is due to antagonism between rutin and kolaviron on the inhibition of oxidative stress (Phan et al. Citation2018a, Citation2018b). It is interesting to note that similar antagonistic phytochemical interactions leading to inhibition of lipid peroxidation have been posited. The antioxidant radicals generated during the interactions of both phenolics are not regenerated, leading to reduced antioxidant effects (Song et al. Citation2011). It is thought that where interactions between different antioxidative chemicals results into antioxidant synergistic effects, the synergism is usually the result of the regeneration of a chain-breaking antioxidant by the less efficient antioxidant under the same experimental scenario (Song et al. Citation2011). There is therefore the possibility that unstable radicals are formed in our experimental settings, which exhibit weak scavenging potential (Song et al. Citation2011).
Testicular inflammatory toxicity of busulfan was demonstrated in the present study by the increased nitrite concentration and myeloperoxidase activity in the testes of busulfan-treated rats. Concurrent increase of testicular nitrite concentration and myeloperoxidase activity is linked to gonadal inflammation that promotes impairment of testicular function (Imamoğlu et al. Citation2012; Maiocchi et al. Citation2017; Abarikwu et al. Citation2020; Qu et al. Citation2020). Our studies demonstrated that rutin and kolaviron when administered alone or in combination, protected against the busulfan-induced increase in the level of testicular nitrite and myeloperoxidase activity, suggesting that these phenolics exhibited anti-inflammatory potencies against inflammatory toxicities induced by busulfan (Ayepola et al. Citation2014; Abarikwu Citation2015; Nafees et al. Citation2015; Al‐Roujeaie et al. Citation2017; Kandemir et al. Citation2020). However, the capacity to recover to the control level of nitrite was lower after combined treatment of rutin and kolaviron, further suggesting the antagonism of both phenolics in our experimental model. This was also confirmed in the myeloperoxidase and TNF-α results because the increase in myeloperoxidase activity and TNF-α concentration found after separate administration of kolaviron was significantly inhibited after combined treatment of rutin and kolaviron. This phenomenon could reduce the therapeutic benefits of the drug combination as therapeutic treatment regimen against busulfan-induced testicular toxicity. In many experimental models, TNF-α has been associated with the immune suppressive effects of busulfan ((Nilsson et al. Citation2005; Vos Citation2007; Hirayanagi et al. Citation2015; Uchida et al. Citation2019) which was also confirmed in the present study. The decrease of TNF-α by busulfan was not inhibited when rutin was co-administered separately (BUS + RUT) nor when kolaviron and rutin were co-administered (BUS + KV + RUT) together. Testicular concentrations of TNF- α were only found to be increased only when kolaviron was co-administered separately (BUS + KV), suggesting that kolaviron inhibited the immunosuppressive effects of busulfan in the rat’s testes. The fact that kolaviron co-treatment normalized TNF-α value close to control values but not when kolaviron was combined with rutin further suggests an antagonistic effect between both phenolics in a rat model of busulfan-induced testicular injury (Abu-soud and Hazen Citation2000; Hadad and Levy Citation2012; Phan et al. Citation2018a). Therefore, rutin and kolaviron co-treatment at the tested dose combination might not be suitable therapy against the immuno-suppressive effects of busulfan in the rat’s testes.
3β-hydroxysteroid dehydrogenase is a key enzyme in the biosynthesis of all active steroid hormones, and it exerts a regulatory control on the testicular steroid hormone cascade system (Abarikwu et al. Citation2014; Alamdar et al. Citation2017). It is believed that the activity of 3β-HSD in the testis is essential for normal steroidogenesis and subsequently for the reproductive capacity of most mammalian animal species (Rasmussen et al. Citation2013). The finding that 3β-HSD activity was increased in the BUS-treated animals in the present study is consistent with our previous study, and thought to be a compensatory attempt by the testes to drive androgen synthesis (Abarikwu et al. Citation2020). Interestingly, rutin and kolaviron co-treatment alone or in combination decreased 3β-HSD activity. However, rutin co-treatment (BUS + RUT) had better protective effects on 3β-HSD activity than when kolaviron and rutin were combined (BUS + KV + RUT) or when kolaviron was administered separately (BUS + KV). It may appear that kolaviron and rutin did not exhibit a strong protective effect on 3β-HSD activity in the rat’s testes as a result of the antagonism of both phenolics (Phan et al. Citation2018b). Considering that these phenolics occur in nature and prevalently in combinations in seeds and fruits, giving them together might not amplify their efficacy against busulfan-induced disturbances in androgen production in animals and clinical models.
The histological features seen in the testes of animals treated with busulfan which have also been confirmed in previous studies, include germ cell losses, vacuolar spaces and wide interstitial spaces, damaged seminiferous tubules and spermatogenesis arrest (Ezirim et al. Citation2019; Qu et al. Citation2020). The testes of animals co-treated with rutin alone, where few tubules were seen to be damaged, protected the testis better than co-treatment with kolaviron alone. The protective effects of rutin and kolaviron on the morphology of the testes have also been reported in previous studies and support the present findings (Olayinka et al. Citation2014; Nafees et al. Citation2015; Abarikwu et al. Citation2020). Similarly, rutin co-treatment was also better than kolaviron in improving tubular germ cell (spermatogonia, spermatocytes and spermatids) counts as well as sperm quality parameters including forward sperm motility, normal sperms and cauda epididymal sperm numbers. When rutin and kolaviron were co-administered together, spermatogonia, spermatocytes and round spermatids numbers, and the sperm quality parameters were lower than the effects seen when kolaviron (BUS + KV) and rutin (BUS + RUT) were co-administered separately. Therefore, one can assume that rutin preserved the morphology of the testis and spermatogenesis better than kolaviron in a rat model of busulfan-induced testicular injury. However, their combination did not protect against busulfan-induced testicular deficits better than their separate treatments. In fact, there were more damaged tubules with defoliated epithelium and giant cells in the testes of busulfan-treated rats co-treated with rutin and kolaviron, further confirming that the effects of kolaviron and rutin co-treatment is less than their individual effects. It is therefore believed that rutin and kolaviron co-treatment following busulfan treatment afford little protection to the testis. Kolaviron and rutin structures contain catechol moieties that are effective inhibitors of lipid peroxidation, and additional molecular features, including unsaturated bonds in conjugation with oxo-bonds. This further makes them effective scavengers of free radicals (Farombi and Nwaokeafor Citation2005; Farombi et al. Citation2007; Kumar and Pandey Citation2013; Omole et al. Citation2018). However, their physico-chemical properties and/or locations in the membranes could influence their combined capacity to inhibit oxidative damage of tissues. For example, rutin interacts with the polar head of phospholipids at water lipid interface, protecting the membranes from oxidative damage (Erlejman et al. Citation2004; Kumar and Pandey Citation2013). The bulky size of the kolaviron biflavonoid complex and the 3ʹ,8” linkage between flavone-flavone dimers could sterically hinder their interaction with membrane molecules, thereby preventing the biflavonoids from gaining a deeper penetration into lipid bilayer membranes or on the bilayer–water interface (Ogunwa Citation2018; Ogunwa et al. Citation2019). These differences may restrict better and cooperative antioxidant effects resulting in antagonistic effects (Hadad and Levy Citation2012; Ogunwa Citation2018). Furthermore, the fact that multimodal therapeutic intervention (rutin-kolaviron combination) failed to provide better therapeutic outcomes than monotherapy (rutin or kolaviron alone) in a model of busulfan-induced testicular injury could also indicate that multimodal drug interventions should be tested with combine effective doses that are different from those used during separate administration. These assumptions seem reasonable to suggest but need to be tested in future experimental trials. Additionally, to better understand the mechanism of action of the treatment regimen on the testes, it would be interesting to examine if the combined kolaviron-rutin therapy could directly influence the expression of spermatogenesis genes.
In conclusion, rutin and kolaviron when administered separately reversed busulfan-induced testicular toxicity, but their combined regimen exhibited reduced cytoprotective and anti-lipoperoxidative effects. The antagonistic effects that often interfere with expected biological outcomes of a combined treatment regimen might be responsible for the minor protective effects of the phenolic chemicals on testicular functions. Overall, combination treatment regimens provide a promising approach for the prevention of drug-induced testicular toxicities, but antagonistic interactions between bioactive phyto-constituents may also occur to lower intervention efficacy. Therefore, the present study indicates that these natural phenolic compounds, when used in combination at the tested doses, might not be suitable cytoprotectants against busulfan-induced testicular damage in young adult rats.
Materials and methods
Chemicals and reagents
Rutin and busulfan were purchased from Sigma-Aldrich (St. Louis, MO, USA). Kolaviron was isolated from fresh Garcinia kola seeds as previously described by Iwu (Citation1985), and as modified by Farombi et al. (Citation2000). All tested chemicals were prepared in corn oil at the beginning of study, and used throughout the experiment. The other chemicals utilized in this study were of the highest commercial grade available.
Animals and treatment
Fifty adult male Wistar rats 8-week old were used for the study. The animals weighing 153 g were purchased from the Department of Biochemistry animal house and were allowed to acclimatize for a week before the start of experiment. The rats were randomly divided into five (5) groups of ten (10) animals per each group. During the whole time of the experiment, the rats were kept in standard rat’s cages with free access to drinking water and standard feeds for laboratory animals, with a diurnal light-to-dark cycle (12 hours light and 12 hours dark) and at room temperature. The following treatment procedure was adopted for the study. Group I: Control received corn oil by gavage (2 mL/kg body weight) for 60 days. Group II: BUS treated; received one dose of busulfan (4 mg/kg b.w) intraperitoneally (i.p.) once a week for 2 weeks during the 60 days period of the experiment (Abarikwu et al. Citation2020). Group III: BUS + RUT (rutin) treated; received BUS at 4 mg/kg b.w., i.p. once a week for 14 days and RUT at 30 mg/kg b.w., by oral gavage thrice weekly for 60 days (Abarikwu et al. Citation2020). Group IV: BUS + KV (kolaviron) treated; received BUS at 4 mg/kg b.w., i.p. once a week for 14 days and KV at 50 mg/kg b.w. (Michel et al. Citation2016), by oral gavage thrice weekly for 60 days. Group V: BUS + KV + RUT treated; received BUS at 4 mg/kg b.w., i.p. once a week for 14 days, and KV at 50 mg/kg b.w., along with RUT at 30 mg/kg b.w., by oral gavage thrice weekly for 60 days. The dose of RUT was previously reported to have antioxidant protective and anti-inflammatory effects (Abarikwu et al. Citation2013, Citation2020) in rat’s testes, while that of busulfan could have a direct toxic effect on the morphology of the testes of rats with no significant mortality rate when administered via the i.p. route (Abarikwu et al. Citation2020). The treatment regimen for BUS that required a minimum of two doses in 2 weeks during the 60 days treatment period was also reported to be sufficient to cause depopulation of germ cells in the seminiferous tubules (Ogawa et al. Citation1999; Ezirim et al. Citation2019). The anti-inflammatory effects of kolaviron were previously reported by Michel et al. (Citation2016) at 50 mg/kg b.w. in Wistar rats. After 60 days treatment period, rats were fasted overnight and then anesthetized for tissue collection. The paired testes were quickly removed and weighed. Next, the right testes were rinsed in ice-cold 1.15% potassium chloride and homogenized in ice-cold 50 mM Tris-HCl buffer (pH 7.4) containing 1.15% potassium chloride. The resulting homogenate was centrifuged at 10,000 × g for 30 minutes at 4°C, and the collected supernatant was kept in the freezer until it was used for the biochemical assays. The left testes were immediately fixed in Bouin’s solution overnight at room temperature, sectioned and stained routinely with hematoxylin and eosin for microscopy.
Determination of concentration of thiobarbituric acid reactive substances (TBARS) – malondialdehyde (MDA) level in testes homogenates
Lipid peroxidation in the sample homogenate was evaluated by quantifying the concentration of malondialdehyde (MDA) at 532 nm as previously described by Ohkawa et al. (Citation1979). The concentration of MDA in was expressed as micromole per milligram protein. The results were calculated using the extinction coefficient for MDA of 1.56 × 105/M/cm.
Determination of reduced glutathione (GSH) level and catalase activity in the testes homogenates
Reduced glutathione (GSH) concentration in the sample homogenates was measured as described by Moron et al. (Citation1979) using Ellman’s reagent (5, 5ʹ – dithiobis-(2-nitrobenzoic acid)) as the substrate for color development. The absorbance was read at 412 nm and the results were expressed as microgram per milligram protein. Catalase (CAT) was determined spectrophotometrically at 240 nm in testes tissue homogenate as previously described by Chance and Maehly (Citation1955). The activity was expressed as U/milligram of protein.
Determination of myeloperoxidase activity, tumor necrosis factor- alpha (TNF-α) and nitrite concentrations in the testes homogenates
To assay for myeloperoxidase (MPO), the sample homogenates were mixed with buffered sodium acetate (80 mM, pH 5.4) in test-tubes containing 18.4 mM tetramethylbenzidine (TMB) and 15% H2O2. The mixture was incubated for 5 min at 37°C. At the end of the incubation period, the reaction was stopped with H2SO4 (2M) and the absorbance was read at 450 nm in a spectrophotometer. The results were expressed as U/mg protein (Ezirim et al. Citation2019). Nitrite concentrations in the tissue samples were measured spectrophotometrically at 540 nm using the Griess method as previously described by Singh et al. (Citation2015). The result was extrapolated from sodium nitrate standard curve (0–500 µmol/mL). Testicular concentrations of TNF-α were assayed in the sample homogenates by enzyme-linked immunoassay (ELISA) as described in the manufacturer’s protocols (Biolegend ELISA kits, San Diego, USA). The absorbance was read at 450 nm in a microplate reader (BioTek, Vermont, USA) within 15 min of addition of the stop solution (0.175 M H2SO4). The concentration of TNF-α was extrapolated from a calibration curve (16–1000 pg/ml) and expressed as ng/ml. Protein concentrations in the samples were determined as described by Lowry et al. (Citation1951).
Determination of 3β-hydroxy steroid dehydrogenase (HSD) activity in the testes homogenates
To assay for 3β–HSD enzyme activity in the sample homogenate, 0.2 mL tissue sample was mixed with 100 mM sodium pyrophosphate buffer (pH 9.0) containing 0.2 ml of NAD (0.5 mM) and 0.1 mM pregnenolone. Then, 2 mL of distilled water was added to the reaction mixture and mixed by inversion. The change in absorbance was measured at 340 nm for 3–5 min at 30 s intervals. The activity of the enzyme was expressed as U/mg protein (Abarikwu et al. Citation2014).
Determination of sperm quality parameters and morphometrical analysis of the testes
The cauda epididymides were placed in normal saline and minced into small pieces to obtain the sperm samples that were used for sperm quality assessment. Abnormal sperms were assessed by evaluating the percentage of sperms stained with 1% eosin and 5% nigrosine. The forward motility of epididymal sperm was evaluated immediately after their isolation from the cauda. The mean was used as the final percentage scores. Finally, sperm numbers were calculated with a hemocytometer using the improved Neubauer count chamber. Spermatozoa were counted for five hemocytometer chambers and were averaged to obtain the sperm numbers (Abarikwu et al. Citation2014; Anjamrooz et al. Citation2007). Both abnormal sperms and motility estimations were performed from five different fields in each sample by counting 400 sperms under a light microscope. To count the germ cells (spermatogonia: Type A and B, spermatocytes: primary and secondary and round spermatids), a 240-intersection grid was overlaid on the tissue section being evaluated, and each structure touched by the grid point was counted using the ‘cell counter’ tool of Image J. Twenty randomly chosen fields in five sections per testis at × 400 magnification were evaluated for germ cell numbers. The location and morphology of the cells within the seminiferous tubules were used to identify them (Anjamrooz et al. 2007; Abarikwu et al. Citation2019).
Statistical analysis
Statistical analyses of the acquired data were done using GraphPad Prism (version 6.0). (GraphPad Software, Inc., San Diego, CA, USA). Data were presented as the mean ± standard deviation (SD). The results were analyzed using one-way analysis of variance (ANOVA) and Tukey’s post hoc multiple comparison test and values were considered statistically different at p < 0.05.
Ethical approval for animal study
Animal use and handling followed internationally recognized guidelines for the protection of animal welfare.
Authors’ contributions
Investigation of study and statistical analysis of data: IGJ; conceptualization, investigation, supervision, writing – review & editing of draft manuscript and data curation: SOA; Writing – draft manuscript: RCCN; participated in supervision of the study: BAA; participated in performing the experiment: CLO, CJMO
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Abarikwu SO. 2014. Kolaviron, a natural flavonoid from the seeds of Garcinia kola, reduces LPS-induced inflammation in macrophages by combined inhibition of IL-6 secretion, and inflammatory transcription factors, ERK1/2, NF-κB, p38, Akt, p-c-JUN and JNK. Biochim Biophys Acta. 1840(7):2373–2381. doi:https://doi.org/10.1016/j.bbagen.2014.03.006.
- Abarikwu SO. 2015. Anti-inflammatory effects of kolaviron modulate the expressions of inflammatory marker genes, inhibit transcription factors ERK1/2, p-JNK, NF-κB, and activate Akt expressions in the 93RS2 Sertoli cell lines. Mol Cell Biochem. 401(1–2):197–208. doi:https://doi.org/10.1007/s11010-014-2307-9.
- Abarikwu SO, Akiri OF, Durojaiye MA, Alabi AF. 2014. Combined administration of curcumin and gallic acid inhibits gallic acid-induced suppression of steroidogenesis, sperm output, antioxidant defenses and inflammatory responsive genes. J Steroid Biochem Mol Biol. 143:49–60. doi:https://doi.org/10.1016/j.jsbmb.2014.02.008.
- Abarikwu SO, Farombi EO, Kashyap MP, Pant AB. 2011. Kolaviron protects apoptotic cell death in PC12 cells exposed to atrazine. Free Radic Res. 45(9):1061–1073. doi:https://doi.org/10.3109/10715762.2011.593177.
- Abarikwu SO, Farombi EO, Pant AB. 2012a. Kolaviron bioflavonoids of Garcinia kola seeds protect atrazine-induced cytotoxicity in primary cultures of rat Leydig cells. Int J Toxicol. 31(4):407–415. doi:https://doi.org/10.1177/1091581812445476.
- Abarikwu SO, Iserhienrhien BO, Badejo TA. 2013. Rutin- and selenium-attenuated cadmium-induced testicular pathophysiology in rats. Hum Exp Toxicol. 32(4):395–406. doi:https://doi.org/10.1177/0960327112472995.
- Abarikwu SO, Mgbudom-Okah CJ, Onuah CJ. 2020. The protective effect of rutin against busulfan-induced testicular damage in adult rats. Drug Chem Toxicol. 1–9. doi:https://doi.org/10.1080/01480545.2020.1803905
- Abarikwu SO, Mgbudom-Okah CJ, Onuah CL, Ogunlaja A. 2019. Fluted pumpkin seeds protect against busulfan-induced oxidative stress and testicular injuries in adult mice. Drug Chem Toxicol. 1–11. doi:https://doi.org/10.1080/01480545.2019.1657885
- Abarikwu SO, Njoku RC, Lawrence CJ, Charles IA, Ikewuchi JC. 2017. Rutin ameliorates oxidative stress and preserves hepatic and renal functions following exposure to cadmium and ethanol. Pharma Biol. 55(1):2161–2169. doi:https://doi.org/10.1080/13880209.2017.1387575.
- Abarikwu SO, Otuechere CA, Ekor M, Monwuba K, Osobu D. 2012b. Rutin ameliorates cyclophosphamide-induced reproductive toxicity in male rats. Toxicol Int. 19:207–214. doi:https://doi.org/10.4103/0971-6580.97224.
- Abu-soud H, Hazen SL. 2000. Nitric oxide modulates the catalytic activity of myeloperoxidase. J Biol Chem. 275(8):5425–5430. doi:https://doi.org/10.1074/jbc.275.8.5425.
- Alamdar A, Xi G, Huang Q, Tian M, Eqani SAMAS, Shen H. 2017. Arsenic activates the expression of 3β-HSD in mouse Leydig cells through repression of histone H3K9 methylation. Toxicol Appl Pharmacol. 326:7–14. doi:https://doi.org/10.1016/j.taap.2017.04.012.
- Al‐Roujeaie AS, Abuohashish HM, Ahmed MM, Alkhamees OA. 2017. Effect of rutin on diabetic‐induced erectile dysfunction: possible involvement of testicular biomarkers in male rats. Andrologia. 49(8):e12737. doi:https://doi.org/10.1111/and.12737.
- Anjamrooz SH, Movahedin M, Mowla SJ, Bairanvand SP. 2007. Assessment of morphological and functional changes in the mouse testis and epididymal sperms following busulfan treatment. Iran Biomed J. 11(1):15–22.
- Ayepola OR, Cerf ME, Brooks NL, Oguntibeju OO. 2014. Kolaviron, a biflavonoid complex of Garcinia kola seeds modulates apoptosis by suppressing oxidative stress and inflammation in diabetes-induced nephrotoxic rats. Phytomedicine. 21(14):1785–1793. doi:https://doi.org/10.1016/j.phymed.2014.09.006.
- Chance B, Maehly AC. 1955. Assay of catalase and peroxidases. Methods Enzymol. 2:764–775.
- Chen SS, Gong J, Liu FT, Mohammed U. 2000. Naturally occurring polyphenolic antioxidants modulate IgE‐mediated mast cell activation. Immunology. 100(4):471–480. doi:https://doi.org/10.1046/j.1365-2567.2000.00045.x.
- Erlejman AG, Verstraeten SV, Fraga CG, Oteiza PI. 2004. The interaction of flavonoids with membranes: potential determinant of flavonoid antioxidant effects. Free Radic Res. 38(12):1311–1320. doi:https://doi.org/10.1080/10715760400016105.
- Ezirim CY, Abarikwu SO, Uwakwe AA, Mgbudom-Okah CJ. 2019. Protective effects of Anthocleista djalonensis A. Chev root extracts against induced testicular inflammation and impaired spermatogenesis in adult rats. Mol Biol Rep. 46(6):5983–5994. doi:https://doi.org/10.1007/s11033-019-05033-w.
- Farombi EO, Abarikwu SO, Adedara IA, Oyeyemi MO. 2007. Curcumin and kolaviron ameliorate di-n-butylphthalate-induced testicular damage in rats. Basic Clin Pharmacol Toxicol. 100:43–48. doi:https://doi.org/10.1111/j.1742-7843.2007.00005.x.
- Farombi EO, Nwaokeafor IA. 2005. Antioxidant mechanisms of kolaviron: studies on serum lipoprotein oxidation, metal chelation and oxidative membrane damage in rats. Clin Exp Pharmacol Physiol. 32:667–674. doi:https://doi.org/10.1111/j.0305-1870.2005.04248.x.
- Farombi EO, Tahntenyg DG, Agboola AO, Nwankwo JO, Emerole GO. 2000. Analgesic and Anti-inflammatory effects of kolaviron (a Garcia kola seed extract). Food Chem Toxicol. 38(6):535–541. doi:https://doi.org/10.1016/S0278-6915(00)00039-9.
- Hadad N, Levy R. 2012. The synergistic anti-inflammatory effects of lycopene, lutein, β-carotene, and carnosic acid combinations via redox-based inhibition of NF-κB signalling. Free Radic Biol Med. 53:1381–1391. doi:https://doi.org/10.1016/j.freeradbiomed.2012.07.078.
- Hirayanagi Y, Qu N, Hirai S, Naito M, Terayama H, Hayashi S, Hatayama N, Kuramasu M, Ogawa Y, Itoh M. 2015. Busulfan pretreatment for transplantation of rat spermatogonial differentially affects immune and reproductive systems in male recipient mice. Anat Sci Int. 90:264–274. doi:https://doi.org/10.1007/s12565-014-0261-y.
- Imamoğlu M, Bülbül SS, Kaklikkaya N, Sarihan H. 2012. Oxidative, inflammatory and immunologic status in children with undescended testes. Pediatr Int. 54(6):816–819. doi:https://doi.org/10.1111/j.1442-200X.2012.03695.x.
- Iwu M, Igboko O. 1982. Flavonoids of Garcinia kola seeds. J Nat Prod. 45(5):650–651. doi:https://doi.org/10.1021/np50023a026.
- Iwu MM. 1985. Antihepatotoxic constituents of Garcinia kola seeds. Experientia. 41(5):670–699. doi:https://doi.org/10.1007/BF02007729.
- Iwu MM, Igboko OA, Tempesta MS. 1990. Biflavonoid constituents of Garcinia kola roots. Fitoterapia. 61(2):178–181.
- Jung SW, Kim HJ, Lee BH, Choi SH, Kim HS, Choi YK, Kim JY, Kim E-S, Hwang S-H, Lim KY, et al. 2015. Effects of Korean Red Ginseng extract on busulfan-induced dysfunction of the male reproductive system. J Ginseng Res. 39(3):243–249. doi:https://doi.org/10.1016/j.jgr.2015.01.002.
- Kandemir FM, Caglayan C, Aksu EH, Yildirim S, Kucukler S, Gur C, Eser G. 2020. Protective effect of rutin on mercuric chloride‐induced reproductive damage in male rats. Andrologia. 52(3):e13524. doi:https://doi.org/10.1111/and.13524.
- Kumar S, Pandey AK. 2013. Chemistry and biological activities of flavonoids: an overview. Scientific World J. 2013:162750. doi:https://doi.org/10.1155/2013/162750.
- Li B, He X, Zhuang M, Niu B, Wu C, Mu H, Tang F, Cui Y, Liu W, Zhao B, et al. 2018. Melatonin ameliorates busulfan-induced spermatogonial stem cell oxidative apoptosis in mouse testes. Antioxid Redox Signal. 28(5):385–400. doi:https://doi.org/10.1089/ars.2016.6792.
- Liu RH. 2004. Potential synergy of phytochemicals in cancer prevention: mechanism of action. J Nutr. 134(12):3479S–3485S. doi:https://doi.org/10.1093/jn/134.12.3479S.
- Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. 1951. Protein measurement with folin phenol reagent. J Biol Chem. 193:265–275. doi:https://doi.org/10.1016/S0021-9258(19)52451-6.
- Maiocchi SL, Morris JC, Rees MD, Thomas SR. 2017. Regulation of the nitric oxide oxidase activity of myeloperoxidase by pharmacological agents. Biochem Pharmacol. 135:90–115. doi:https://doi.org/10.1016/j.bcp.2017.03.016.
- Michel TK, Ottoh AA, Chukwunonye UCE, Obodoike EC, Christopher O, Mmaduakolam IM. 2016. Bio-flavonoids and Garcinoic acid from Garcinia kola seeds with promising anti-inflammatory potentials. Pharmacog J. 8:56–58. doi:https://doi.org/10.5530/pj.2016.1.12.
- Moron MS, Depierre JW, Mannervik B. 1979. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta. 582:67–78. doi:https://doi.org/10.1016/0304-4165(79)90289-7.
- Nafees S, Rashid S, Ali N, Hasan SK, Sultana S. 2015. Rutin ameliorates cyclophosphamide induced oxidative stress and inflammation in Wistar rats: role of NFκB/MAPK pathway. Chem Biol Interact. 231:98–107. doi:https://doi.org/10.1016/j.cbi.2015.02.021.
- Nakamura Y, Ishimitsu S, Tonogai Y. 2000. Effects of quercetin and rutin on serum and hepatic lipid concentrations, fecal steroid excretion and serum antioxidant properties. J Health Sci. 46(4):229–240. doi:https://doi.org/10.1248/jhs.46.229.
- Nasimi P, Tabandeh MR, Roohi S. 2018. Busulfan-mediated oxidative stress and genotoxicity decrease in sperm of Satureja Khuzestanica essential oil-administered mice. Sys Biol Reprod Med. 64(5):348–357. doi:https://doi.org/10.1080/19396368.2018.1449915.
- Negrette-Guzmán M. 2019. Combinations of the antioxidants sulforaphane or curcumin and the conventional antineoplastics cisplatin or doxorubicin as prospects for anticancer chemotherapy. Eur J Pharmacol. 859:172513. doi:https://doi.org/10.1016/j.ejphar.2019.172513.
- Nilsson C, Forsman J, Hassan Z, Abedi-Valugerdi M, O’Connor C, Concha H, Jansson M, Hassan M. 2005. Effect of altering administration order of busulphan and cyclophosphamide on the myeloablative and immunosuppressive properties of the conditioning regimen in mice. Exp Hemal. 33:380–387. doi:https://doi.org/10.1016/j.exphem.2004.12.003.
- Njoku R-C-C, Abarikwu SO, Uwakwe AA, Mgbudom-Okah CJ, Ezirim CY. 2019. Dietary fluted pumpkin seeds induce reversible oligospermia and androgen insufficiency in adult rats. Sys Biol Reprod Med. 65(6):437–450. doi:https://doi.org/10.1080/19396368.2019.1612482.
- Ogawa T, Dobrinski I, Brinster RL. 1999. Recipient preparation is critical for spermatogonial transplantation in the rat. Tissue Cell. 31(5):461–472. doi:https://doi.org/10.1054/tice.1999.0060.
- Ogunwa TH. 2018. Computer-aided modeling of interaction between aldehyde dehydrogenase and Garcinia biflavonoids. Int J Comput Appl. 179:18–25.
- Ogunwa TH, Fasimoye RY, Adeyelu TT. 2019. Studies on the interaction mechanisms of Garcinia kolaviron constituents with selected diabetes and neurodegenerative disease targets. J Proteins Proteom. 10:221–234. doi:https://doi.org/10.1007/s42485-019-00021-x.
- Ohkawa H, Ohishi N, Yagi K. 1979. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 95:351‑358. doi:https://doi.org/10.1016/0003-2697(79)90738-3.
- Olaleye SB, Farombi EO, Adewoye EA, Onasanwo SA. 2000. Analgesic and anti-inflammatory effects of kolaviron (a Garcinia kola seed extract). Afr J Biomed Res. 3:171–174.
- Olaleye SB, Onasanwo SA, Ige AO, Wu KK, Cho CH. 2010. Anti-inflammatory activities of a kolaviron-inhibition of nitric oxide, prostaglandin E2 and tumor necrosis factor alpha production in activated macrophage-like cell line. Afr J Med Med Sci. 39:41–46.
- Olayinka ET, Ore A, Fashiku KA. 2014. Kolaviron and L-ascorbic acid ameliorates chlorambucil-induced hepatic and renal toxicity in rat. Int J Toxicol Appl Pharmacol. 4(1):23–32.
- Omole JG, Ayoka OA, Alabi QK, Adefisayo MA, Asafa MA, Olubunmi BO, Fadeyi BA. 2018. Protective effect of kolaviron on cyphosphamide-induced cardiac toxicity in rats. J Evid Based Integr Med. 23:1–11. doi:https://doi.org/10.1177/2156587218757649.
- Phan MAT, Bucknall M, Arcot J. 2018b. Effect of different anthocyanidin glucosides on lutein uptake by caco-2 cells, and their combined activities on anti-oxidation and anti-inflammation in vitro and ex vivo. Molecules. 23:2035–2036. doi:https://doi.org/10.3390/molecules23082035.
- Phan MAT, Paterson J, Bucknall M, Arcot J. 2018a. Interactions between phytochemicals from fruits and vegetables: effects on bioactivities and bioavailability. Crit Rev Food Sci Nutr. 58(8):1310–1329. doi:https://doi.org/10.1080/10408398.2016.1254595.
- Qu N, Kuramasu M, Nagahori K, Ogawa Y, Hayashi S, Hirayanagi Y, Terayama H, Suyama K, Sakabe K, Itoh M. 2020. Co-Administration of the traditional medicines Hachimi-Jio-Gan and Hochu-Ekki-To can reverse busulfan-induced aspermatogenesis. Int J Mol Sci. 21(5):1716–1730. doi:https://doi.org/10.3390/ijms21051716.
- Rasmussen MK, Ekstrand B, Zamaratskaia G. 2013. Regulation of 3β-hydroysteroid dehydrogenaseΔ5-Δ4 isomerase: a review. Int J Mol Sci. 14(9):17926–17942. doi:https://doi.org/10.3390/ijms140917926.
- Singh MP, Jakhar R, Kang SC. 2015. Morin hydrate attenuates the acrylamide-induced imbalance in antioxidant enzymes in a murine model. Int J Mol Med. 36(4):992–1000. doi:https://doi.org/10.3892/ijmm.2015.2306.
- Song LL, Liang R, Li DD, Xing YD, Han RM, Zhang JP, Skibsted LH. 2011. β-Carotene radical cation addition to green tea polyphenols. Mechanism of antioxidant antagonism in peroxidizing liposomes. J Agric Food Chem. 59(23):12643–12651. doi:https://doi.org/10.1021/jf2030456.
- Uchida N, Nassehi T, Drysdale CM, Gamer J, Yapundich M, Bonifacino AC, Krouse AE, Linde N, Hsieh MM, Donahue RE, et al. 2019. Busulfan combined with immunosuppression allows efficient engraftment of gene-modified cells in a rhesus macaque model. Mol Ther. 27:1586–1596. doi:https://doi.org/10.1016/j.ymthe.2019.05.022.
- Vos JG. 2007. Immune suppression as related to toxicology. J Immunotoxicol. 4:175–200. doi:https://doi.org/10.1080/15476910701508262.
