ABSTRACT
Endometriosis is a common estrogen-dependent chronic inflammatory disease that leads to infertility in women of reproductive age. Perhaps infertility reflects the reduced expression of integrin αvβ3 and HOXA10 in endometriosis. Previous studies have shown that administration of letrozole, a non-steroidal aromatase inhibitor for cancer treatment, increased the clinical pregnancy rate in women with endometriosis, but the mechanisms remain to be determined. In this communication, a rat model of endometriosis was established. Animals were treated with letrozole at 2ug/kg of body weight, intragastric administration for 15 consecutive days. Letrozole increased the expression of αvβ3 and HOXA10 in the endometriosis model and endometrial receptivity.
Abbreviations: WOI: window of implantation; RGD: Arg-Gly-Asp; HOX: homeobox; E2: estradiol; SPF: specific pathogen-free.
Introduction
Endometriosis is an estrogen-dependent inflammatory disease defined by the presence of endometriotic gland and stroma outside of the uterine cavity (Vallve-Juanico et al. Citation2019). It affects approximately 10% of reproductive age women, with up to 50% experiencing infertility (May et al. Citation2010). Although the underlying mechanisms that lead to endometriosis-induced infertility remain unclear, several causes have been proposed, including pelvic microenvironment, ovarian dysfunction, and altered endometrial receptivity (de Ziegler et al. Citation2010).
Endometrial receptivity is a complex process that provides the embryo with the opportunity to attach, invade, and develop, relying on various mechanisms originating from the endometrium (Lessey and Young Citation2019). Embryo implantation occurs only during the period of endometrial receptivity, also known as the window of implantation (WOI), which occurs between 7 and 10 after ovulation in humans (Wilcox et al. Citation1999), and 5.5 days after mating in rats (Lecce et al. Citation2011). A variety of molecules, including αvβ3 and HOXA10, have been identified as endometrial receptivity markers (Achache and Revel Citation2006).
Integrin αvβ3 is a cell-surface receptor for extracellular matrix proteins that play an important role in embryo implantation (Lessey et al. Citation1992; Lessey, Castelbaum et al. Citation1994). Its expression shows periodic changes, and the peak time is consistent with the implantation window (Tabibzadeh Citation1992; Lessey et al. Citation1994a). The expression of integrin αvβ3 is decreased in women with infertility and endometriosis (Lessey et al. Citation1994a). Estrogen treatment can down-regulate the expression of integrin αvβ3 (Somkuti et al. Citation1997). HOXA10, = upstream gene integrin αvβ3 (Zhu et al. Citation2013), is also highly expressed during the implantation window and is a marker of endometrial receptivity (Gui et al. Citation1999). Estrogen can stimulate the expression of HOXA10, both in the myometrium and the endometrium (Cermik et al. Citation2001). In endometriosis, calpain7 can down-regulate the expression of HOXA10 via the PEST sequence, and the expression of integrin αvβ3 is also reduced (Yan et al. Citation2018). In addition, the expression of HOXA10 is decreased in endometriosis due to promoter methylation (Lu et al. Citation2013).
Estrogen dominance and progesterone resistance caused by inflammation can interfere with endometrial receptivity (Lessey and Kim Citation2017). Letrozole, an aromatase inhibitor, can specifically inactivate aromatase to inhibit estrogen formation. Studies have shown that the combination of letrozole and gonadotropins in IVF leads to higher pregnancy and live birth rates than only using high-dose gonadotropins (Lazer et al. Citation2014). Previous studies have shown that letrozole can treat endometriosis and significantly improve pregnancy rates in women with endometriosis during an IVF cycle (Miller et al. Citation2012; Ferrero et al. Citation2018), but the mechanisms remain to be elucidated.
We tested if letrozole may affect endometrial receptivity in endometriosis by regulating the expression of HOXA10 and integrin αvβ3 using a rat model of endometriosis. We demonstrated that letrozole increases the expression of αvβ3 and HOXA10 and thereby improves endometrial receptivity.
Results and discussion
Establishing an endometriosis model
The experimental and control groups are outlined in . As shown in , a trimmed segment of endometrial tissue was sutured to the inner side of the abdominal wall. We noted that an ectopic mass in the abdominal wall adhered to the surrounding connective tissues, and neovascularization developed in the surface of the mass (, C), which showed that a model of endometriosis was established. The cyst was 5–10 mm in diameter and contained a clear or purplish-red fluid. shows the vaginal plug, which is an important marker of the endometrium entering the window of implantation (WOI). Hematoxylin-eosin staining analysis, showed the growth of glands in the ectopic mass (, F), modeling endometriosis. The glandular epithelial cells were columnar or cubic, and inflammatory cells and secretions existed in some glandular lumen.
Figure 1. Establishment and results of endometriosis model. (A) The left uterine horn was ligated and excised. The endometrium was exposed along its antimesenteric axis, and 5*5 mm sections were cut. The trimmed endometrial piece was sutured to the inner side of the abdominal wall. (B) Ectopic mass in the abdominal cavity of rats in the endometriosis group. (C) Ectopic mass in the abdominal cavity of rats in the letrozole group. (D) Vaginal plug, a marker of mating between male and female rats. Mating can promote ovulation in female mice, allowing the endometrium to enter the window of implantation. (E) The gland of ectopic mass in the endometriosis group (Hematoxylin-eosin staining, 200×). (F) The gland of ectopic mass in the letrozole group (Hematoxylin-eosin staining, 200×).
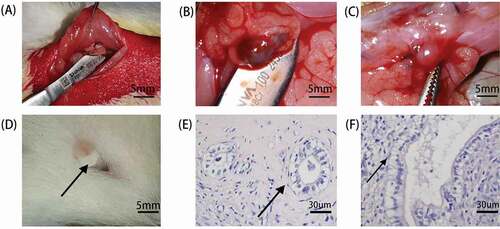
Table 1. Experimental groups.
Integrin αvβ3 and endometrial receptivity
Integrins are cell-surface receptors for extracellular matrix proteins involved in diverse cellular processes, including embryogenesis, wound healing, the immune response, and cancer (Albelda and Buck Citation1990; Hynes Citation1992). Integrin αvβ3, localized in the endometrial luminal and glandular epithelium, is one of the best-characterized endometrial biomarkers related to embryo implantation (Lessey and Castelbaum Citation2002). During blastocyst apposition in embryo implantation, trophoblast cells adhere to the endometrial epithelium. Integrin αvβ3 recognizes and binds to the Arg-Gly-Asp (RGD) amino acid sequence in osteopontin and may participate in cell-cell interactions. Both trophoblast cells and endometrial epithelial cells maintain the same integrin and osteopontin (Vanderpuye et al. Citation1991; Damsky et al. Citation1993), and the expression of integrin αvβ3 persists into pregnancy with expansion to the decidua (Lessey et al. Citation1992, Citation1994a). It is plausible that integrin αvβ3 plays an independent role in the interaction between trophoblastic cells and endometrium. The expression of integrin αvβ3 can be down-regulated by estrogen (Somkuti et al. Citation1997).
The expression of integrin αvβ3 mRNA in endometriosis was lower than its expression in the control group (p = 0.002, ). After applying letrozole, the expression of integrin αvβ3 mRNA was elevated in the endometrium (p = 0.008, ). The expression of αvβ3 protein was also reduced in endometriosis (p = 0.018, , D). After letrozole treatment, the expression of integrin αvβ3 protein in endometrium was elevated (p = 0.002, , D) as summarized in .
Figure 2. HOXA10 and Integrin αvβ3 expression in endometrium. (A) As compared with control group, the mRNA expression of HOXA10 was reduced in endometriosis (p < 0.0001). After the application of letrozole, the expression of HOXA10 was elevated in endometrium (p = 0.010). (B) The expression of integrin αvβ3 mRNA in endometriosis was lower as compared to its expression in control group ((p = 0.002). After the application of letrozole, the expression of integrin αvβ3 mRNA was elevated in endometrium (p = 0.008). (C and D) The expression of αvβ3 protein was also reduced in endometriosis ((p = 0.018). After letrozole treatment, the expression of integrin αvβ3 protein in endometrium was elevated ((p = 0.002). GAPDH (Glyceraldehyde-3-phosphate dehydrogenase) was used as a loading control. (**p < 0.01, *p < 0.05).
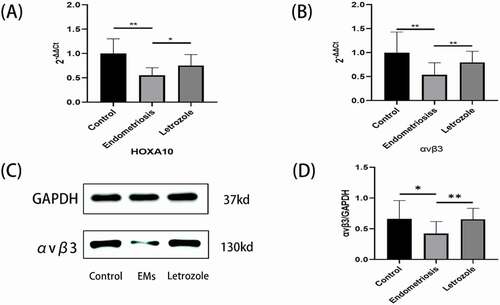
Table 2. The expression of HOXA10 and Integrin αvβ3, and the concentration of serum E2.
HOXA10 and endometrial receptivity
HOXA10, a member of the homeobox (HOX) family, is expressed in the nucleus of endometrial glands and stroma of the endometrium (Gui et al. Citation1999). Its expression depends on the stage of the menstrual cycle, dramatically increasing at the time of implantation (Taylor et al. Citation1998; Gui et al. Citation1999). The expression of HOXA10 is altered in the endometrium of women with infertility, such as patients with endometriosis and polycystic ovary syndrome (Cermik et al. Citation2003). Previous studies have shown that HOXA10 regulates the expression of ITGB3, which encoding integrin αvβ3, affects endometrial receptivity. HOXA10 activates reporter gene expression via a 41-bp β3-Integrin subunit element containing two of the four HOXA10 binding sites (Daftary et al. Citation2002). In addition, HOXA10 can alter embryo viability by regulating tryptophan 2,3-dioxygenase through serotonin signaling (Doherty et al. Citation2011). The elevated expression of HOXA10 promotes the expression of integrin αvβ3 and enhances embryo vitality, promoting embryo implantation.
As shown in , the expression of HOXA10 was reduced in endometriosis but elevated after letrozole application. As compared with control group, the mRNA expression of HOXA10 was reduced in endometriosis (p < 0.0001, ). After the application of letrozole, the expression of HOXA10 was elevated in endometrium (p = 0.010, ).
Endometriosis impairs endometrial receptivity
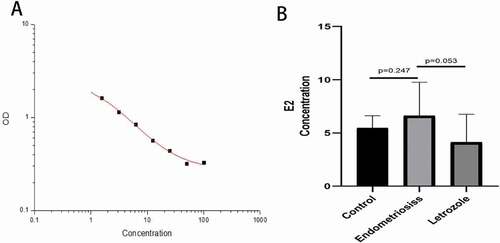
Infertility caused by endometriosis affects reproductive age women and is an estrogen-dependent disease. The upregulation of estrogen in endometriosis may be due to increased aromatase expression and impaired breakdown of estradiol due to decreased 17 beta-hydroxysteroid (Bulun et al. Citation2002). We also found Serum E2 (estradiol) concentration increased in the endometriosis model. As shown in , the standard curve (correlation coefficient was 0.998) was drawn according to the standard substance of gradient concentration and its absorption value. The E2 concentration was 5.48 ± 1.15 pg/ml in the control group, 6.63 ± 3.14 pg/ml in the endometriosis group. E2 concentration in the endometriosis group was slightly higher than that in the control group (P > 0.05), but the difference was not statistically significant, which may be due to small sample size or experimental error.
Figure 3. Serum E2 concentration of rats was detected by ELISA. (A) is the standard curve, the correlation is 0.998. (B) Compared with the control group, estrogen concentration in the endometriosis group was slightly increased (P = 0.247), but the difference was not statistically significant. Estrogen concentration decreased after letrozole administration (P = 0.053), but the difference was not statistically significant.
Estrogen dominance and progesterone resistance can impair endometrial receptivity (Lessey and Kim Citation2017), this may reflect that estrogen can inhibit the expression of integrin αvβ3. This reduced endometrial receptivity may be due to a shift away from normal progesterone action and excessive estrogen activity (Aghajanova et al. Citation2010). Previous studies have shown that the expression of integrin αvβ3 was reduced in endometriosis (Lessey et al. Citation1994a), and HOXA10 was also downregulated (Wu et al. Citation2005). Our results also confirm these conclusions.
The effects of letrozole
The effect of letrozole on the fertility of women varies in current reports. Some investigators have shown that letrozole can boost fertility by increasing the number of follicles (Kilic-Okman et al. Citation2003). Others have shown that letrozole can increase pregnancy rate by elevating the expression of αvβ3 (Miller et al. Citation2012). Possible explanations are that the research methods and the dosage regimen in studies may be different. Unlike previous studies, we established a model of endometriosis to begin to investigate the effect of low-dose letrozole on endometrial receptivity and endometriosis. We found that letrozole could promote the expression of integrin αvβ3 and HOXA10 in endometrium of endometriosis and may improve endometrial receptivity.
Letrozole is an aromatase inhibitor, which can competitively bind to the heme of the cytochrome P450 subunit, thereby reducing estrogen levels. Aromatase is a rate-limiting enzyme in the biological synthesis of androgen to estrogen, and elevated aromatase levels are associated with IVF implantation failure (Brosens et al. Citation2004). As shown in , we found that the serum E2 concentration was 4.13 ± 2.64 pg/ml in letrozole group. As compared with endometriosis group, E2 the concentration decreased after letrozole administration (P = 0.053), and the difference was not statistically significant, which may reflect the small sample size and/or associated error. As we showed, low-dose letrozole improved the expression of integrin αvβ3 perhaps by lowering the estrogen level. In addition, low dose letrozole increased the expression of HOXA10. In the past few years, many studies have demonstrated favorable results of letrozole in assisted reproductive technology, with no short – or long-term side effects (Palomba Citation2015; Tatsumi et al. Citation2017). In summary, letrozole can promote the expression of integrin αvβ3 and HOXA10 in the endometrium during endometriosis. It should be noted that the specific regulatory mechanisms will require a larger randomized set of in vitro and in vivo experiments.
Materials and methods
Animals
Forty-three Sprague-Dawley rats at 8 weeks old, weighing 240 ± 20 g, were used for the study. The study was conducted in Animal Laboratory Center of Shandong Provincial Hospital. Animals were raised in a specific pathogen-free (SPF) facility with a temperature-controlled (22°C-26°C) and humidity-controlled (40%-60%) environment, under a 12-h light/dark cycle, and ad libitum access to food and water. The experimental groups are shown in .
Modeling and tissue collection
Step 1: Establishment of the endometriosis model
All animals were anesthetized through intraperitoneal administration of 2% pentobarbital sodium (Sigma, USA) at a dose of 70 mg/kg. Endometriosis was surgically induced using the method described in the previous literature with a minor modification according to our previous experience (Yildirim et al. Citation2010). Using aseptic techniques, the left uterine horn was ligated and excised, placed in sterile saline. The endometrium was exposed along its antimesenteric axis, and 5*5 mm sections were cut. The trimmed endometrial piece was sutured to the inner side of the abdominal wall. Abdominal layers were closed anatomically. The control group also received laparotomy, only ligation of the left uterine horn, without implantation of abdominal wall ectopic endometrium.
Step 2: Drug intervention
After the surgery, the rats were raised for 28 days and during this time they did not receive medication. After 28 days, they were randomly divided into two groups, the endometriosis group and the letrozole group. Letrozole group was administered intragastrically letrozole (MCE, USA), 2ug/kg of body weight daily in 2 mL of normal saline for 15 days. Referring to the research on reproductive toxicity in letrozole’s drug label, we selected this dose which can inhibit estrogen dominance in endometriosis and maintain a certain estrogen concentration. The control group and endometriosis group were administered normal saline.
Step 3: Sample collection
Female rats were caged with male rats and vaginal plug were checked every morning and evening. All the male rats had undergone vasectomy. The mating behavior can stimulate ovulation in female rats and make the endometrium enter the window of implantation. The day of vaginal plug discovery (confirming insemination) was designated as Day 1. The rats were anesthetized on Day 5.5, when the endometrium was in the implantation window (Lecce et al. Citation2011). The ectopic mass and the right endometrium of the rats were removed. Blood was collected from the inferior vena cava and serum was collected.
Hematoxylin-eosin staining
The ectopic tissue was washed and fixed with 4% paraformaldehyde. After dehydration and paraffin-embedding, samples were cut into 4um thick sections and placed on glass slides. After 5 mins of dewaxing, hydration, hematoxylin staining, the sample were stained with eosin stained for 2 minutes. The stained sections were scanned by Digital Tissue Section Scanner (TissueGnostics, magnification, ×200).
Total RNA extraction and real-time PCR analysis
Total RNA was extracted from fresh endometrium using TRIzol reagents (Accurate, Hunan, China). Extracted RNA was reverse-transcribed by Evo M-MLV RT Premix (Accurate, Hunan, China) to obtain cDNA. RT-PCR of mRNA were performed on the Light Cycler 480 System (Roche, Basel, Switzerland) with SYBR® Green Pro Taq HS Premix (Accurate, Hunan, China). The qPCR amplification conditions were as follows: 40 cycles of 95°C for 5 sec, 60°C for 30 sec. Quantification of the target gene expression in the samples was calculated and adjusted to the quantitative expression of β‑actin. Relative gene expression was calculated using the 2‑∆∆Ct method. The primer for HOXA10 was 5ʹ-AGTGCTGGGCTGTGTTTAATCA-3ʹ and 5ʹ-CACGCTTCCATACTCTGCTCTT-3ʹ. The primer for integrin αvβ3 was 5ʹ-TAGAAGAGCCTGAGTGTCCTAAG-3ʹ and 5ʹ-TTCCAGATGAGCAGAGTAGCA-3ʹ. The primer for β-Actin was 5ʹ- GGAGATTACTGCCCTGGCTCCTA-3ʹ and 5ʹ- GACTCATCGTACTCCTGCTTGCTG-3ʹ.
Tissue protein extraction and Western blot analysis
Fresh endometrium samples were lysed in RIPA lysis buffer (Beyotime, Shanghai, China) with protease inhibitors (MCE, USA) to extract whole‑cell proteins. Protein levels were measured using the BCA Protein Concentration Determination kit (Solarbio, Beijing, China). Total proteins (20ug) were run on a 12.5% polyacrylamide gel and separated through electrophoresis, then transferred onto polyvinylidene fluoride membranes (Millipore Corporation, Billerica, USA). Membranes were blocked with 5% fat‑free milk followed by incubation with 0.5% integrin β3 (NB600-1342; Novus, Colorado, USA) and 0.1% GAPDH (Glyceraldehyde-3-phosphate dehydrogenase) (10,494; Proteintech, Wuhan, China) overnight at 4°C. Membranes were then incubated with 0.5‰ Anti-mouse IgG Antibody (7076; CST, USA) or 0.2‰ Goat Anti‑rabbit IgG Antibody (ZB‑2301; Zhongshan Golden Bridge Biotechnology, Beijing, China) in blocking buffer, respectively. Protein bands were detected by using an ECL detection kit (BD Biosciences, Franklin Lakes, NJ).
Enzyme linked immunosorbent assay (ELISA)
Rat E2 ELISA Kit (E-EL-0152 c, Elabscience, Shanghai, China) was used in the experiment. Standard and sample were first added, followed by rapid addition of biotinylated antibody. After incubation and washing, the enzyme conjugate was added. Incubated and washed again, added substrate reagent and stop solution respectively. The optical density of each hole was measured immediately with the enzyme plate instrument (450 nm).
Statistical analysis
Data were analyzed using SPSS 22.0. The statistical significance of the difference between groups were determined by independent T-test. The value of p < 0.05 was considered statistically significant.
Ethics approval
The experimental procedures were approved by the Committee for Animal Research. All animal studies strictly conformed to the animal experiment guidelines of the Committee for Humane Care.
Authors’ contributions
Designed the study, performed experiments, and wrote major parts of the manuscript, Figures and Tables: JZ. Helped with study design, supervised all the experiments and the progress of the study, and wrote parts of the manuscript: ML. Guided manuscript writing and wrote parts of the manuscript: CL, HZ. Performed some of the experiments: LW, RL. All authors read and approved the final manuscript.
Acknowledgments
The authors are grateful to all study participants, gynecologists, pathologists, animal laboratory researchers and central laboratory researchers at Shandong Provincial Hospital affiliated to Shandong University.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Achache H, Revel A. 2006. Endometrial receptivity markers, the journey to successful embryo implantation. Hum Reprod Update. 12(6):731–746. doi:https://doi.org/10.1093/humupd/dml004.
- Aghajanova L, Velarde MC, Giudice LC. 2010. Altered gene expression profiling in endometrium: evidence for progesterone resistance. Semin Reprod Med. 28(1):51–58. doi:https://doi.org/10.1055/s-0029-1242994.
- Albelda SM, Buck CA. 1990. Integrins and other cell adhesion molecules. FASEB J. 4(11):2868–2880. doi:https://doi.org/10.1096/fasebj.4.11.2199285.
- Brosens J, Verhoeven H, Campo R, Gianaroli L, Gordts S, Hazekamp J, Hagglund L, Mardesic T, Varila E, Zech J, et al. 2004. High endometrial aromatase P450 mRNA expression is associated with poor IVF outcome. Hum Reprod. 19(2):352–356. doi:https://doi.org/10.1093/humrep/deh075.
- Bulun SE, Gurates B, Fang Z, Tamura M, Sebastian S, Zhou J, Amin S, Yang S. 2002. Mechanisms of excessive estrogen formation in endometriosis. J Reprod Immunol. 55(1–2):21–33. doi:https://doi.org/10.1016/S0165-0378(01)00132-2.
- Cermik D, Karaca M, Taylor HS. 2001. HOXA10 expression is repressed by progesterone in the myometrium: differential tissue-specific regulation of HOX gene expression in the reproductive tract. J Clin Endocrinol Metab. 86(7):3387–3392. doi:https://doi.org/10.1210/jcem.86.7.7675.
- Cermik D, Selam B, Taylor HS. 2003. Regulation of HOXA-10 expression by testosterone in vitro and in the endometrium of patients with polycystic ovary syndrome. J Clin Endocrinol Metab. 88(1):238–243. doi:https://doi.org/10.1210/jc.2002-021072.
- Daftary GS, Troy PJ, Bagot CN, Young SL, Taylor HS. 2002. Direct regulation of beta3-integrin subunit gene expression by HOXA10 in endometrial cells. Mol Endocrinol. 16(3):571–579. doi:https://doi.org/10.1210/mend.16.3.0792.
- Damsky C, Sutherland A, Fisher S. 1993. Extracellular matrix 5: adhesive interactions in early mammalian embryogenesis, implantation, and placentation. FASEB J. 7(14):1320–1329. doi:https://doi.org/10.1096/fasebj.7.14.8224605.
- de Ziegler D, Borghese B, Chapron C. 2010. Endometriosis and infertility: pathophysiology and management. Lancet. 376(9742):730–738. doi:https://doi.org/10.1016/S0140-6736(10)60490-4.
- Doherty LF, Kwon HE, Taylor HS. 2011. Regulation of tryptophan 2,3-dioxygenase by HOXA10 enhances embryo viability through serotonin signaling. Am J Physiol Endocrinol Metab. 300(1):E86–93. doi:https://doi.org/10.1152/ajpendo.00439.2010.
- Ferrero S, Evangelisti G, Barra F. 2018. Current and emerging treatment options for endometriosis. Expert Opin Pharmacother. 19(10):1109–1125. doi:https://doi.org/10.1080/14656566.2018.1494154.
- Gui Y, Zhang J, Yuan L, Lessey BA. 1999. Regulation of HOXA-10 and its expression in normal and abnormal endometrium. Mol Hum Reprod. 5(9):866–873. doi:https://doi.org/10.1093/molehr/5.9.866.
- Hynes RO. 1992. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 69(1):11–25. doi:https://doi.org/10.1016/0092-8674(92)90115-S.
- Kilic-Okman T, Kucuk M, Altaner S. 2003. Comparison of the effects of letrozole and clomiphene citrate on ovarian follicles, endometrium, and hormone levels in the rat. Fertil Steril. 80(6):1330–1332. doi:https://doi.org/10.1016/j.fertnstert.2003.05.002.
- Lazer T, Dar S, Shlush E, Al Kudmani BS, Quach K, Sojecki A, Glass K, Sharma P, Baratz A, Librach CL. 2014. Comparison of IVF outcomes between minimal stimulation and high-dose stimulation for patients with poor ovarian reserve. Int J Reprod Med. 2014:581451. doi:https://doi.org/10.1155/2014/581451.
- Lecce L, Kaneko Y, Madawala RJ, Murphy CR. 2011. ICAM1 and fibrinogen-gamma are increased in uterine epithelial cells at the time of implantation in rats. Mol Reprod Dev. 78(5):318–327. doi:https://doi.org/10.1002/mrd.21307.
- Lessey BA, Castelbaum AJ. 2002. Integrins and implantation in the human. Rev Endocr Metab Disord. 3(2):107–117. doi:https://doi.org/10.1023/A:1015450727580.
- Lessey BA, Castelbaum AJ, Buck CA, Lei Y, Yowell CW, Sun J. 1994a. Further characterization of endometrial integrins during the menstrual cycle and in pregnancy**Supported by the National Institutes of Health grants HD-29449 and HD-30476–1 (B.A.L.), Bethesda, Maryland.††Presented at the 5th leukocyte differentiation antigen conference, Boston, November 3 to 7, 1993. Fertil Steril. 62(3):497–506.
- Lessey BA, Castelbaum AJ, Sawin SW, Buck CA, Schinnar R, Bilker W, Strom BL. 1994b. Aberrant integrin expression in the endometrium of women with endometriosis. J Clin Endocrinol Metab. 79(2):643–649. doi:https://doi.org/10.1210/jcem.79.2.7519194.
- Lessey BA, Damjanovich L, Coutifaris C, Castelbaum A, Albelda SM, Buck CA. 1992. Integrin adhesion molecules in the human endometrium. Correlation with the normal and abnormal menstrual cycle. J Clin Invest. 90(1):188–195. doi:https://doi.org/10.1172/JCI115835.
- Lessey BA, Kim JJ. 2017. Endometrial receptivity in the eutopic endometrium of women with endometriosis: it is affected, and let me show you why. Fertil Steril. 108(1):19–27. doi:https://doi.org/10.1016/j.fertnstert.2017.05.031.
- Lessey BA, Young SL. 2019. What exactly is endometrial receptivity? Fertil Steril. 111(4):611–617. doi:https://doi.org/10.1016/j.fertnstert.2019.02.009.
- Lu H, Yang X, Zhang Y, Lu R, Wang X. 2013. Epigenetic disorder may cause downregulation of HOXA10 in the eutopic endometrium of fertile women with endometriosis. Reprod Sci. 20(1):78–84. doi:https://doi.org/10.1177/1933719112451146.
- May KE, Conduit-Hulbert SA, Villar J, Kirtley S, Kennedy SH, Becker CM. 2010. Peripheral biomarkers of endometriosis: a systematic review. Hum Reprod Update. 16(6):651–674. doi:https://doi.org/10.1093/humupd/dmq009.
- Miller PB, Parnell BA, Bushnell G, Tallman N, Forstein DA, Higdon HL, Kitawaki J, Lessey BA. 2012. Endometrial receptivity defects during IVF cycles with and without letrozole. Human Reproduction. 27(3):881–888. doi:https://doi.org/10.1093/humrep/der452.
- Palomba S. 2015. Aromatase inhibitors for ovulation induction. J Clin Endocrinol Metab. 100(5):1742–1747. doi:https://doi.org/10.1210/jc.2014-4235.
- Somkuti SG, Yuan L, Fritz MA, Lessey BA. 1997. Epidermal growth factor and sex steroids dynamically regulate a marker of endometrial receptivity in Ishikawa cells. J Clin Endocrinol Metab. 82(7):2192–2197. doi:https://doi.org/10.1210/jcem.82.7.4102.
- Tabibzadeh S. 1992. Patterns of expression of integrin molecules in human endometrium throughout the menstrual cycle. Hum Reprod. 7(6):876–882. doi:https://doi.org/10.1093/oxfordjournals.humrep.a137753.
- Tatsumi T, Jwa SC, Kuwahara A, Irahara M, Kubota T, Saito H. 2017. No increased risk of major congenital anomalies or adverse pregnancy or neonatal outcomes following letrozole use in assisted reproductive technology. Hum Reprod. 32(1):125–132. doi:https://doi.org/10.1093/humrep/dew280.
- Taylor HS, Arici A, Olive D, Igarashi P. 1998. HOXA10 is expressed in response to sex steroids at the time of implantation in the human endometrium. J Clin Invest. 101(7):1379–1384. doi:https://doi.org/10.1172/JCI1597.
- Vallve-Juanico J, Houshdaran S, Giudice LC. 2019. The endometrial immune environment of women with endometriosis. Hum Reprod Update. 25(5):564–591. doi:https://doi.org/10.1093/humupd/dmz018.
- Vanderpuye OA, Labarrere CA, McIntyre JA. 1991. A vitronectin-receptor-related molecule in human placental brush border membranes. Biochem J. 280(1):9–17. doi:https://doi.org/10.1042/bj2800009.
- Wilcox AJ, Baird DD, Weinberg CR. 1999. Time of implantation of the conceptus and loss of pregnancy. N Engl J Med. 340(23):1796–1799. doi:https://doi.org/10.1056/NEJM199906103402304.
- Wu Y, Halverson G, Basir Z, Strawn E, Yan P, Guo SW. 2005. Aberrant methylation at HOXA10 may be responsible for its aberrant expression in the endometrium of patients with endometriosis. Am J Obstet Gynecol. 193(2):371–380. doi:https://doi.org/10.1016/j.ajog.2005.01.034.
- Yan Q, Huang C, Jiang Y, Shan H, Jiang R, Wang J, Liu J, Ding L, Yan G, Sun H. 2018. Calpain7 impairs embryo implantation by downregulating beta3-integrin expression via degradation of HOXA10. Cell Death Dis. 9(3):291. doi:https://doi.org/10.1038/s41419-018-0317-3.
- Yildirim G, Attar R, Ozkan F, Kumbak B, Ficicioglu C, Yesildaglar N. 2010. The effects of letrozole and melatonin on surgically induced endometriosis in a rat model: a preliminary study. Fertil Steril. 93(6):1787–1792. doi:https://doi.org/10.1016/j.fertnstert.2009.09.021.
- Zhu L-H, Sun L-H, Hu Y-L, Jiang Y, Liu H-Y, Shen X-Y, Jin X-Y, Zhen X, Sun H-X, Yan G-J. 2013. PCAF impairs endometrial receptivity and embryo implantation by down-regulating β3-Integrin expression via HOXA10 acetylation. J Clin Endocrinol Metab. 98(11):4417–4428. doi:https://doi.org/10.1210/jc.2013-1429.
