Abstract
This comparative cross-sectional study aimed to evaluate the relationship between serum leptin and testosterone, FSH, LH, PRL and semen quality in fertile and idiopathic infertile Yemeni men. A total of 30 infertile males with unknown causes and 30 age-matched healthy fertile males were enrolled in this study. The body mass index (BMI) and waist circumference (WC) were measured. Semen samples were analyzed according to the WHO manual for semen analysis. Serum samples were tested for hormones. Subjects were then divided into subgroups and compared based on their main seminal findings. The WC, serum leptin, PRL, FSH and LH levels were significantly higher (p < 0.05) in the infertile subjects than in the fertile group. Serum leptin demonstrated a significant positive correlation (p < 0.01) with body weight, BMI and WC in fertile males and a significant negative correlation (p < 0.01) with testosterone in fertile and infertile males. Similarly, a significant positive correlation was found between serum leptin and FSH (p < 0.01) and LH (p < 0.05) levels in the infertile subjects. The findings showed that non-obstructive azoospermic (NOA) patients have significant (p < 0.05) high levels of serum leptin, FSH, and LH. These findings may support the possibility of a direct peripheral negative effect of leptin on testicular steroidogenesis independent of the suggested indirect effect, and it could directly impact spermatogenesis without inhibiting testosterone production. This effect was accompanied by increasing serum PRL levels. Furthermore, serum leptin and gonadotropins were found to be increased in the idiopathic NOA group. The present study provided valuable insights into the fertile and idiopathic infertile Yemeni males and could establish an important foundation for future andrological-related studies such as investigating the relationship between leptin and other hormones; and infertility-related genetic and epigenetic factors.
Introduction
Leptin, a peptide hormone secreted by adipocytes, has been established to be linked to male fertility in several studies. It is a 167-amino acid protein encoded by the obese ‘ob’ gene. The hormone is involved in regulating food intake, energy balance, and body weight. It is mainly synthesised in the white adipose tissue and secreted in proportion to the mass of adipocytes (Liu et al. Citation2021). However, it is also produced through an exocrine secretion involving salivary glands and the pepsinogen secreting chief epithelial cells of the fundic mucosa (Koepsell Citation2020), placenta, hypothalamus, pituitary, and mammary glands (Manzar and Hussain Citation2011; Silvestris et al. Citation2018).
A significant role of leptin in male reproductive function control has been firmly established (Mintziori et al. Citation2020; Childs et al. Citation2021). Central and peripheral pathways affecting the hypothalamic-pituitary-gonadal (HBG) axis have been hypothesised, considering the wide distribution of leptin receptors throughout the body (Dutta et al. Citation2019; Malik et al. Citation2019). The mechanism by which leptin triggers gonadotropin-releasing hormone (GnRH) neurons to produce gonadotropins is still unclear because these neurons lack leptin receptors. Leptin hormone plays a significant role in male reproduction partially by stimulating the expression of Kisspeptin peptide, premammillary nucleus (PMN), cocaine-and-amphetamine-regulated transcript (CART) and pro-opiomelanocortin (POMC) that stimulate GnRH neurons. Moreover, it could partially via inhibiting the secretion of agouti-related peptide (AgRP) and Neuropeptide Y (NPY), which have an inhibitory effect on GnRH neurons (Malik et al. Citation2019). These mechanisms together can trigger the gonadotropin cascade and, in turn, testicular steroidogenesis and spermatogenesis. Moreover, it is believed that leptin has a direct peripheral role in spermatogenesis and endocrine function of the testes since leptin and its receptors are found in spermatozoa, seminal plasma and testes cells (Egan et al. Citation2017; Dutta et al. Citation2019; Malik et al. Citation2019; Sengupta et al. Citation2019; Khodamoradi et al. Citation2020; Childs et al. Citation2021).
However, high serum leptin levels are associated with obesity and abnormal male reproduction and semen quality (Kasum et al. Citation2016; Dutta et al. Citation2019; Malik et al. Citation2019; Leisegang et al. Citation2021). Leptin could play a direct role on the leptin receptor (Ob-R) of the Leydig cells as a negative regulator of testicular steroidogenesis. It might have a deleterious effect on the proliferation and differentiation of germ cells, independent of gonadotropin regulation (Kasum et al. Citation2016; Dutta et al. Citation2019; Malik et al. Citation2019; Leisegang et al. Citation2021). Moreover, data on the relationship between serum leptin and serum prolactin in infertile males are still in dispute, despite the possible interaction between them in the regulation of reproduction (Guo et al. Citation2014; Kumari et al. Citation2017).
Unfortunately, the cause of male infertility remains unknown in about 30 − 40% of the cases and is called idiopathic male infertility (Bracke et al. Citation2018). Leptin was found in high concentrations in unexplained infertile women than in the fertile group (Kumari et al. Citation2017). However, as mentioned by Guo et al. (Citation2014) study, few reports have investigated the correlation between leptin and fertility problems in male patients, particularly in idiopathic asthenozoospermia cases. This observation is also found for other cases of idiopathic infertility because most of the related studies so far neglected many factors that may affect fertility and/or the studied hormones. Despite numerous recent animal studies providing substantial evidence of the direct deleterious effects of leptin on infertility and sperm parameters of adult rats (Haron et al. Citation2010; Abbasihormozi et al. Citation2013), however, more human studies are needed to investigate the role of leptin in human men infertility. Thus, this study was designed to investigate the relationship between serum leptin and sex hormones, prolactin levels and sperm quality in fertile and infertile Yemeni men with unexplained causes for infertility after excluding the most common disorders and factors that may affect fertility.
Results and discussion
Male fertility was reported to be linked to leptin in several studies, but the exact relationship has not been established. Yet, its relationship with idiopathic male infertility has not been studied adequately. In this study, even though fertile and idiopathic infertile males were matched in their age and BMI, the mean WC was significantly higher (p < 0.01) in the infertile subjects (90.43 ± 2.14 cm) than in the fertile ones (81.65 ± 1.84 cm) as shown in . Mean serum leptin concentrations were also significantly higher (p < 0.05) in the infertile subjects (7.07 ± 1.45 ng/mL) than in the fertile ones (3.14 ± 0.72 ng/mL). Moreover, as shown in and , a significant positive correlation was found between serum leptin levels and body weight, BMI and WC (r = 0.483, p = 0.007; r = 0.516, p = 0.004; and r = 0.494, p = 0.005, respectively) in the fertile subjects, and a strong significant negative correlation was found between serum leptin levels and serum testosterone levels in both fertile (r = –0.481, p = 0.007) and in the infertile (r = –0.564, p = 0.001) subjects.
Figure 1. The correlation between serum leptin levels and some parameters in the fertile (A, B, C) and infertile (D, E, F) male subjects. BMI: body mass index; FSH: follicle-stimulating hormone; LH: luteinizing hormone; PRL: prolactin; WC: waist circumference. p-value indicates the significance between leptin and other parameters in the fertile and the infertile groups. r: correlation coefficient.
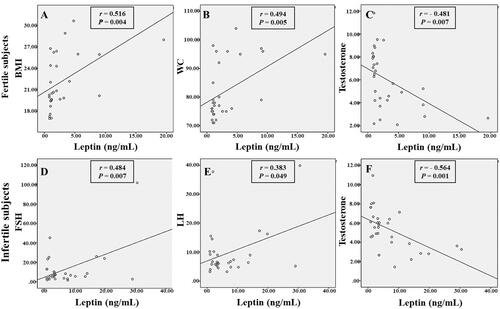
Table 1. Main physical, seminal and serum hormonal parameters of fertile (n = 30) and infertile (n = 30) male subjects (mean ± SEM).
Table 2. The correlation between serum leptin levels and the physical, seminal and hormonal parameters in the fertile (n = 30) and infertile (n = 30) male subjects.
Recently, Lima et al. (2020) assumed that the high levels of leptin, age, and WC were associated with lower values of total testosterone and calculated free testosterone. As well visceral obesity, in particular, was reported to be associated with lower circulating androgen levels in men, which is correlated proportionally to the degree of obesity. Thus, it was found that central obesity is more likely to produce changes in hormone levels than the fat stored in other parts of the body because white adipose tissue is a major endocrine organ which secretes many biologically active peptides and proteins, including adipose-derived hormones and adipokines (Tan and Pu Citation2002; Derby et al. Citation2006; Alves et al. Citation2016; Leisegang et al. Citation2021). The reverse correlation between leptin and testosterone could be explained due to the effect of leptin on LH pulse amplitude and serum LH levels, as well as the possible inhibitory effect of high circulating leptin on testicular steroidogenesis and/or direct action of leptin and other adipocyte-derived hormones on testicular function (Isidori et al. Citation1999; Lima et al. Citation2000; Caprio et al. Citation2003; Caminos et al. Citation2008).
Moreover, the findings showed that mean serum leptin, PRL, FSH and LH concentrations were significantly higher (p < 0.05) in the infertile group than in the fertile ones; and serum leptin displayed a significant positive correlation with FSH and LH (r = 0.484, p = 0.007; r = 0.363, p = 0.049, respectively) in the infertile subjects ( and ). Indeed, some studies have reported no relation between excess body weight or leptin and gonadotropin levels (Guo et al. Citation2014); and it was reported that leptin has a possible direct effect on testicular function and could be independent of FSH and LH regulation (Herrid et al. Citation2008). In another study, leptin levels were negatively correlated with free testosterone and total testosterone levels before and after administration of gonadotropin in males with idiopathic hypogonadotropic hypogonadism (Kilciler et al. Citation2002).
In contrast, some studies reported that serum leptin demonstrated a significant positive correlation with patients’ serum FSH and LH, and there was a significant negative correlation with serum testosterone (Jahan et al. Citation2011; Bracke et al. Citation2018). However, the findings support the possibility of the direct inhibitory effect of leptin on testicular steroidogenesis, independent of gonadotropin regulation, due to the significant increase of serum FSH and LH in the infertile subjects. Leptin receptors were expressed in the seminiferous tubules and seminal plasma on the Leydig and Sertoli cells. This may indicate that leptin has a direct role in spermatogenesis and testicular endocrine function, independent of gonadotropin regulation (Tena-Sempere et al. Citation2001; Glander et al. Citation2002; Zorn et al. Citation2007). The direct effect of leptin on the leptin receptor (Ob-R) of Leydig cells as a negative regulator of testicular steroidogenesis was also reported by Kasum et al. (Citation2016) and in the reviews of Almabhouh et al. (Citation2020) and Malik et al. (Citation2019). Furthermore, leptin could have a negative impact on spermatogenesis independent of inhibiting testicular steroidogenesis. In rats, Haron et al. (Citation2010) and Abbasihormozi et al. (Citation2013) specified the direct deleterious effects of leptin on sperm parameters. These effects could be attributed to the impact of the histone-protamine transition during spermatogenesis, as reported by Almabhouh et al. (Citation2019). Martins et al. (Citation2015) also stated that leptin and its receptors have a modulatory action on the production of acetate and the glycolytic activity of Sertoli cells in the human testes. This glycolytic activity is essential for normal spermatogenesis. This role for leptin and its receptor may clarify the relationship between infertility and obesity in males, as Khosropour et al. (Citation2017) mentioned. Besides, leptin may increase sperm damage by generating reactive oxygen species (ROS) in the seminiferous tubular cells and/or in the epididymis (Malik et al. Citation2019). On the other hand, in primary hypogonadism, the production of inhibin B and testosterone is decreased. This reduces the negative feedback effect on the HPG axis, which leads to an increase in gonadotropins production (Rey Citation2020).
For semen parameters, the results showed no significant differences (p > 0.05) in semen volume between fertile and infertile groups. However, mean sperm concentration, total sperm count, progressive motility, and sperm with abnormal morphology were significantly lower (p < 0.001) in the infertile men than in the fertile group. Besides, serum leptin has no significant correlation (P > 0.05) with semen parameters neither in the fertile nor in the infertile subjects in the current study. This was in parallel with some earlier studies (Guo et al. Citation2014; Amjad et al. Citation2019). Additionally, a meta-analysis by MacDonald et al. (Citation2010) offered evidence of no association between increased BMI and semen parameters. On the contrary, other studies found a negative effect of obesity and serum leptin on some semen parameters (Almabhouh et al. Citation2020; Maghsoumi-Norouzabad et al. Citation2020).
The comparison between the fertile normozoospermic (NZ) group and infertile subgroups showed significant higher differences (p < 0.001) in serum FSH and LH levels and serum leptin levels (p < 0.01) in NOA subgroup compared to the fertile NZ group and the other infertile subgroup. Serum leptin levels were also significantly higher (p < 0.05) in OAZ subgroup than in the fertile NZ group. Serum PRL concentrations were significantly higher (p < 0.05) in NOA and OAT subgroups compared to the fertile NZ group and the infertile OAZ subgroup (). In similar, significant-high concentrations of leptin, FSH and LH were observed in the infertile NOA male patients as reported in the study of Jahan et al. (Citation2011), while another study reported that there was no significant difference in serum leptin levels of obstructive azoospermia (OA), NOA, OAT and NZ men (Zorn et al. Citation2007). However, leptin was suggested to be a diagnostic tool to differentiate between OA and NOA as leptin increases in NOA patients (Gao et al. Citation2011). Additionally, Ellithy et al. (Citation2014) demonstrated that seminal leptin could be an excellent indicator of the accurate prediction for sperm retrieval in NOA men, mainly in combination with FSH. Typically, in patients with NOA primary testicular failure, serum FSH levels are elevated while serum testosterone levels are decreased (Andrade et al. Citation2021).
Table 3. Physical and serum hormonal parameters in the fertile group and infertile subgroups of male subjects (mean ± SEM).
On the other hand, serum leptin and PRL concentrations in our study were significantly higher (p < 0.05) in the infertile group than in the fertile group. This result was consistent with Abd El-Aziz et al. (Citation2008) outcomes, which reported that the PRL and leptin were higher in obese oligozoospermic and azoospermic infertile patients than in obese patients fertile controls. However, no significant correlation was found in the current study between serum leptin and PRL, neither in the fertile nor in infertile subjects. These results were similar to a previous study conducted by Guo et al. (Citation2014). Nevertheless, our findings differed from another study that reported a significant positive correlation between serum leptin and PRL in obese infertile oligozoospermic men (Hofny et al. Citation2010).
Moreover, the current study showed that serum PRL was significantly higher (p < 0.05) in NOA and OAT patients than in the fertile group and OAZ subgroup. This was in agreement with the results of an earlier study (Gao et al. Citation2011). Male infertility was characterised by hyperprolactinaemia (Dabbous and Atkin Citation2018) or other consequences and markers that can disturb semen parameters (El Taieb et al. Citation2020; Ma et al. Citation2020; Elbashir et al. Citation2021). It was found that male and female patients with prolactinoma have higher leptin levels and other adverse metabolic profiles corrected after treatment with cabergoline (Pala et al. Citation2016). With this regard, it was suggested that leptin co-secretion from a prolactinoma might cause elevated serum leptin levels, independently of the peripheral action of prolactin (Balci et al. Citation2009). The absence of the inter-correlation between serum leptin and PRL in our study may rule out the possibility of the interaction between them in the central regulation of fertility that was previously suggested (Guo et al. Citation2014; Kumari et al. Citation2017).
There are some limitations to our study. The current research was carried out in a single centre due to the limited number of specialised professional andrologists and Andrology medical centres in Yemen. Another limitation is the small number of infertile patients involved in the study due to the strict inclusion criteria. Furthermore, our study did not involve Y chromosome microdeletion analysis for patients with severe OZ or NOA, evaluating serum estradiol (E2) and sex hormone-binding globulin (SHBG) levels. Further studies are needed to explore the contribution of these factors.
In conclusion, idiopathic infertile males have high serum leptin levels associated with high levels of serum FSH, LH, and PRL. However, in both fertile and idiopathic infertile men, high serum leptin levels are negatively correlated with serum testosterone levels but not with sperm quality except in infertile men with non-obstructive azoospermia (NOA). Moreover, higher serum leptin levels in idiopathic infertile men are positively correlated with serum gonadotropin levels but not with PRL. These findings may support the possibility of a direct peripheral negative effect of leptin on testicular steroidogenesis independent of the suggested indirect effect and could impact spermatogenesis independent of inhibiting testosterone production.
Materials and methods
Patient characteristics and sample collection
This comparative cross-sectional study was conducted at the consultant medical centre for dermatology, andrology and infertility in Ibb city, Yemen, from March 2015 to August 2015. Samples were collected from men who visited the Andrology clinic for fertility evaluation. The sample size was calculated using OpenEpi software version 2.3. To achieve 80% power and a level of significance of 5%. The sample size was similar to a study conducted by Hanafy et al. (Citation2007). Hence, our study required minimum sample size of 24 patients and 24 control. However, after clinical investigation and interviews, the study involved 30 infertile men of reproductive age (18–50 years old) with abnormal semen parameters and unknown unexplained causes for infertility. The control group involved 30 age-matched fertile volunteers from normozoospermic healthy men. The exclusion criteria were as follows: (a) males aged >18 or <50 years, (b) a history of congenital or acquired disorders of the reproductive organs, (c) clinical varicocele or genital tract infections, (d) patients suspected to have obstructive azoospermia, (e) cases with features suggestive of hypogonadism including repeated low total testosterone and clinical signs and symptoms, (f) history of taking drugs known to compromise male fertility or affecting their sex hormones, (g) exposure to environmental toxicants, excessive heat or irradiation, (h) history of heavy smoking, alcohol intake or drug abuse, (i) cases showing agglutination of their sperms during semen analysis, (j) chronic systemic diseases, and (k) severe obesity (BMI ≥ 40 kg/m2).
Clinical examination and data collection
The clinical evaluation of the subjects included a detailed history, assessment of secondary sexual characters, local examination and measurement of testicular volume, which was performed by one professional andrologist. Body weight and height were measured. BMI (kg/m2) was calculated as weight in kilograms divided by height in meters squared. The WC (cm) was measured using a measuring tape at the point of the minimal waist (Leisegang et al. Citation2021). Furthermore, a face to face interview was carried out to fill a questionnaire designed to match the study needs.
Semen collection and analysis
All semen samples were obtained at the clinic by masturbation after 2–7 days of sexual abstinence into a sterile wide-mouthed plastic specimen container. Semen samples were processed manually according to the guidelines of the WHO (Citation2010). Sperm morphology was evaluated according to the old WHO (Citation1999) criteria due to the lack of the facilities necessary for morphology evaluation using strict criteria defined in the WHO manual, 2010.
Blood sampling and hormonal analysis
A fasting venous blood sample was drawn, allowed to clot, centrifuged at 3000 × g (Centrifuge Humax 4 K, Human, Germany) for 15 minutes and stored at –20 °C until analysis. The concentration of serum leptin was measured using an enzyme-linked immunosorbent assay (ELISA) (Sandwich) technique (DRG International, Inc., Marburg, Germany) as mentioned by the manufacturer. Serum samples for testosterone, follicle-stimulating hormone (FSH), luteinizing hormone (LH) and Prolactin (PRL) were analyzed automatically by a chemiluminescent immunoassay (CLIA) instrument (Cobas E411, Roche Diagnostics, Germany) as mentioned by the manufacturer.
Grouping of the subjects
Subjects were divided into two main groups: fertile normozoospermic (NZ) group (n = 30) and infertile group (n = 30). Infertile subjects were further divided into five subgroups according to their main seminal abnormalities: oligozoospermia (OZ) (n = 5), asthenozoospermia (AZ) (n = 6), oligoasthenozoospermia (OAZ) (n = 9), oligoasthenoteratozoospermia (OAT) (n = 5) and non-obstructive azoospermia (NOA) (n = 5).
Statistical analysis
The IBM SPSS program, version 20.0 (SPSS Inc., Chicago, IL), was used for statistical analysis. Data were expressed as mean ± SEM. An independent sample t-test was applied to evaluate the differences. Pearson correlation test studied relationships between values. One way analysis of variance (ANOVA) followed by a multiple comparison test of Fisher’s least significant difference (LSD) was performed to examine the significant differences in the means of the fertile group and the infertile subgroups. A p < 0.05 was considered significant at 95% confidence levels.
Ethics approval
The study was conducted in accordance with the Declaration of Helsinki (World Medical Association Citation2001), and the approval for the collection of samples was obtained from the Committee of the Faculty of Medicine & Health Sciences at Sana’a University, Sana’a, Yemen (Reference No. 14/4 in 25/3/2013), and from the Committee of the Graduate Studies and Scientific Research at Sana’a University, Sana’a, Yemen (Reference No. 118/4 in 29/12/2014). All participants signed informed consent forms to allow the researcher to take the samples for the experimental procedures.
Authors’ contributions
Performed the experiments, interpreted the data and wrote the first draft of the manuscript: EAA; designed the study and supervised the entire work: FKA; interpreted the results of the manuscript: AAA, MMAN; helped select patients and was involved in the design of the study: RHA; involved in the statistical analysis, interpreted the results, revised and assumed final responsibility for submitting the manuscript: MAA.
| Abbreviations | ||
| AZ | = | asthenozoospermia |
| BMI | = | body mass index |
| CLIA | = | chemiluminescent immunoassay |
| ELISA | = | enzyme-linked immunosorbent assay |
| FSH | = | follicle-stimulating hormone |
| GnRH | = | gonadotropin-releasing hormone |
| HBG | = | hypothalamic-pituitary-gonadal |
| LH | = | luteinizing hormone |
| NOA | = | non-obstructive azoospermia |
| NZ | = | fertile normozoospermia |
| OAZ | = | oligoasthenozoospermia |
| OAT | = | oligoasthenoteratozoospermia |
| Ob-R | = | leptin receptor |
| OZ | = | oligozoospermia |
| PRL | = | prolactin |
| SEM | = | standard error of the mean |
| WC | = | waist circumference |
| WHO | = | World Health Organization. |
Acknowledgments
The authors express sincere appreciation to the Faculty of Medicine and Health Sciences at the University of Sana’a, and to the consultant medical centre for Dermatology, Andrology and Infertility at Ibb governorate for their support and research facilities.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Abbasihormozi S, Shahverdi A, Kouhkan A, Cheraghi J, Akhlaghi AA, Kheimeh A. 2013. Relationship of leptin administration with production of reactive oxygen species, sperm DNA fragmentation, sperm parameters and hormone profile in the adult rat. Arch Gynecol Obstet. 287(6):1241–1249.
- Abd El-Aziz D, Mahran AM, Hofny ER, Abdel-Hafez HZ, Mahran A, Abd El-Azeem HG, Tag LM, Sherif TM. 2008. Semen parameters, gonadotrophic, sex hormones, and serum leptin in obese infertile males. Al-Azhar Assiut Med J. 6(1):1687–1693.
- Almabhouh FA, Ahmad Muhammad FI, Ibrahim H, Singh H. 2019. Leptin: a pleitropic factor in physiology. JCHS. 4(2):31–57.
- Almabhouh FA, Md Mokhtar AH, Malik IA, Aziz N, Durairajanayagam D, Singh HJ. 2020. Leptin and reproductive dysfunction in obese men. Andrologia. 52(1):e13433.
- Alves GM, Jesus TT, Sousa M, Goldberg E, Silva MB, Oliveira FP. 2016. Male fertility and obesity: are ghrelin, leptin and glucagon-like peptide-1 pharmacologically relevant? Curr Pharm Des. 22(7):783–791.
- Amjad S, Baig M, Zahid N, Tariq S, Rehman R. 2019. Association between leptin, obesity, hormonal interplay and male infertility. Andrologia. 51(1):e13147.
- Andrade DL, Viana MC, Esteves SC. 2021. Differential diagnosis of Azoospermia in men with infertility. J Clin Med. 10(14):3144.
- Balci H, Akgun-Dar K, Gazioglu N, Kapucu A, Bolayirli M, Oz B. 2009. The relationship between prolactin (PRL), leptin, nitric oxide (NO), and cytokines in patients with hyperprolactinemia. Pituitary. 12(3):170–176.
- Bracke A, Peeters K, Punjabi U, Hoogewijs D, Dewilde S. 2018. A search for molecular mechanisms underlying male idiopathic infertility. Reprod Biomed Online. 36(3):327–339.
- Caminos JE, Nogueiras R, Gaytán F, Pineda R, González CR, Barreiro ML, Castaño JP, Malagón MM, Pinilla L, Toppari J, et al. 2008. Novel expression and direct effects of adiponectin in the rat testis. Endocrinology. 149(7):3390–3402.
- Caprio M, Fabbrini E, Ricci G, Basciani S, Gnessi L, Arizzi M, Carta AR, De Martino MU, Isidori AM, Frajese GV, et al. 2003. Ontogenesis of leptin receptor in rat leydig cells. Biol Reprod. 68(4):1199–1207.
- Childs GV, Odle AK, MacNicol MC, MacNicol AM. 2021. The importance of leptin to reproduction. Endocrinology. 162(2):bqaa204.
- Dabbous Z, Atkin SL. 2018. Hyperprolactinaemia in male infertility: clinical case scenarios. Arab J Urol. 16(1):44–52.
- Derby CA, Zilber S, Brambilla D, Morales KH, McKinlay JB. 2006. Body mass index, waist circumference and waist to hip ratio and change in sex steroid hormones: the Massachusetts male ageing study. Clin Endocrinol. 65(1):125–131.
- Dutta S, Biswas A, Sengupta P. 2019. Obesity, endocrine disruption and male infertility. Asian Pac J Reprod. 8(5):195–202.
- Egan OK, Inglis MA, Anderson GM. 2017. Leptin signaling in AgRP neurons modulates puberty onset and adult fertility in mice. J Neurosci. 37(14):3875–3886.
- El Taieb MA, Hegazy EM, Ibrahim HM, Ibrahim AK. 2020. Seminal and serum leptin levels in male patients with varicocele and isolated asthenozoospermia before and after repair. Aging Male. 23(5):579–584.
- Elbashir S, Magdi Y, Rashed A, Henkel R, Agarwal A. 2021. Epididymal contribution to male infertility: an overlooked problem. Andrologia. 53(1):e13721.
- Ellithy MMS, Shaeer OKZ, Gaafar KM. 2014. Correlation between leptin content and sperm retrieval in cases of functional azoospermia. J Basic Appl Zool. 67(5):164–172.
- Gao L, Chen B, Lu YN, Hu K, Wang HX, Han YF, Wang YX, Huang YR. 2011. Leptin level in azoospermic patients and its clinical value. Zhonghua Nan Ke Xue = Natl J Androl. 17(6):492–497. [
- Glander HJ, Lammert A, Paasch U, Glasow A, Kratzsch J. 2002. Leptin exists in tubuli seminiferi and in seminal plasma. Andrologia. 34(4):227–233.
- Guo J, Zhao Y, Huang W, Hu W, Gu J, Chen C, Zhou J, Peng Y, Gong M, Wang Z. 2014. Sperm motility inversely correlates with seminal leptin levels in idiopathic asthenozoospermia. Int J Clin Exp Med. 7(10):3550–3555.
- Hanafy S, Halawa FA, Mostafa T, Mikhael NW, Khalil KT. 2007. Serum leptin correlates in infertile oligozoospermic males. Andrologia. 39(5):177–180.
- Haron MN, D'Souza UJA, Jaafar H, Zakaria R, Singh HJ. 2010. Exogenous leptin administration decreases sperm count and increases the fraction of abnormal sperm in adult rats. Fertil Steril. 93(1):322–324.
- Herrid M, Xia Y, O'Shea T, McFarlane JR. 2008. Leptin inhibits basal but not gonadotrophin-stimulated testosterone production in the immature mouse and sheep testis. Reprod Fertil Dev. 20(4):519–528.
- Hofny ERM, Ali ME, Abdel-Hafez HZ, Kamal EE-D, Mohamed EE, Abd El-Azeem HG, Mostafa T. 2010. Semen parameters and hormonal profile in obese fertile and infertile males. Fertil Steril. 94(2):581–584.
- Isidori AM, Caprio M, Strollo F, Moretti C, Frajese G, Isidori A, Fabbri A. 1999. Leptin and androgens in male obesity: evidence for leptin contribution to reduced androgen levels. J Clin Endocrinol Metab. 84(10):3673–3680.
- Jahan S, Bibi R, Ahmed S, Kafeel S. 2011. Leptin levels in infertile males. J Coll Physicians Surg Pak. 21(7):393–397.
- Kasum M, Anić-Jurica S, Čehić E, Klepac-Pulanić T, Juras J, Žužul K. 2016. Influence of male obesity on fertility. Acta Clin Croat. 55(2):301–308.
- Khodamoradi K, Parmar M, Khosravizadeh Z, Kuchakulla M, Manoharan M, Arora H. 2020. The role of leptin and obesity on male infertility. Curr Opin Urol. 30(3):334–339.
- Khosropour S, Hamidi M, Fattahi A, Khodadadi I, Karami M, Fazilati M, Vaisi-Raygani A, Tavilani H. 2017. Leptin and leptin-receptor polymorphisms in fertile and infertile men. Syst Biol Reprod Med. 63(1):7–14.
- Kilciler G, Ozata M, Oktenli C, Sanisoglu SY, Bolu E, Bingol N, Kilciler M, Ozdemir IC, Kutlu M. 2002. Diurnal leptin secretion is intact in male hypogonadotropic hypogonadism and is not influenced by exogenous gonadotropins. J Clin Endocrinol Metab. 87(11):5023–5029.
- Koepsell H. 2020. Glucose transporters in the small intestine in health and disease. Pflugers Arch – Eur J Physiol. 472(9):1207–1248.
- Kumari P, Jaiswar SP, Shankhwar P, Deo S, Ahmad K, Iqbal B, Mahdi AA. 2017. Leptin as a predictive marker in unexplained infertility in north Indian population. J Clin Diagn Res. 11(3):Qc28–Qc31.
- Leisegang K, Sengupta P, Agarwal A, Henkel R. 2021. Obesity and male infertility: mechanisms and management. Andrologia. 53(1):e13617.
- Lima N, Cavaliere H, Knobel M, Halpern A, Medeiros-Neto G. 2000. Decreased androgen levels in massively obese men may be associated with impaired function of the gonadostat. Int J Obes. 24(11):1433–1437.
- Lima TFN, Nackeeran S, Rakitina E, Lima GFN, Arora H, Kargi AY, Ramasamy R. 2020. Association of leptin with total and free testosterone: results from the national health and nutrition examination surveys. Androg Clin Res Ther. 1(1):94.
- Liu H, Du T, Li C, Yang G. 2021. STAT3 phosphorylation in central leptin resistance. Nutr Metab. 18(1):39– 13.
- Ma J-X, Wang B, Li H-S, Jiang X-J, Yu J, Ding C-F, Chen W-Q. 2020. Association between obesity-associated markers and semen quality parameters and serum reproductive hormones in Chinese infertile men. Reprod Biol Endocrinol. 18(1):95.
- MacDonald AA, Herbison GP, Showell M, Farquhar CM. 2010. The impact of body mass index on semen parameters and reproductive hormones in human males: a systematic review with meta-analysis. Hum Reprod Update. 16(3):293–311.
- Maghsoumi-Norouzabad L, Zare Javid A, Aiiashi S, Hosseini SA, Dadfar M, Bazyar H, Dastoorpur M. 2020. The impact of obesity on various semen parameters and sex hormones in iranian men with infertility: a cross-sectional study. Res Rep Urol. 12:357–365.
- Malik IA, Durairajanayagam D, Singh HJ. 2019. Leptin and its actions on reproduction in males. Asian J Androl. 21(3):296–299.
- Manzar D, Hussain ME. 2011. Leptin rhythmicity and its relationship with other rhythm markers. Biol Rhythm Res. 42(2):163–180.
- Martins AD, Moreira AC, Sá R, Monteiro MP, Sousa M, Carvalho RA, Silva BM, Oliveira PF, Alves MG. 2015. Leptin modulates human Sertoli cells acetate production and glycolytic profile: a novel mechanism of obesity-induced male infertility? Biochim Biophys Acta – Mol Basis Dis. 1852(9):1824–1832.
- Mintziori G, Nigdelis MP, Mathew H, Mousiolis A, Goulis DG, Mantzoros CS. 2020. The effect of excess body fat on female and male reproduction. Metabolism. 107:154193.
- Pala NA, Laway BA, Misgar RA, Shah ZA, Gojwari TA, Dar TA. 2016. Profile of leptin, adiponectin, and body fat in patients with hyperprolactinemia: response to treatment with cabergoline. Indian J Endocr Metab. 20(2):177–181.
- Rey RA. 2020. Biomarkers of male hypogonadism in childhood and adolescence. Adv Lab Med. 1(2), 24.
- Sengupta P, Bhattacharya K, Dutta S. 2019. Leptin and male reproduction. Asian Pac J Reprod. 8(5):220–226.
- Silvestris E, de Pergola G, Rosania R, Loverro G. 2018. Obesity as disruptor of the female fertility. Reprod Biol Endocrinol. 16(1):22.
- Tan RS, Pu SJ. 2002. Impact of obesity on hypogonadism in the andropause. Int J Androl. 25(4):195–201.
- Tena-Sempere M, Pinilla L, Zhang F-P, González LC, Huhtaniemi I, Casanueva FF, Dieguez C, Aguilar E. 2001. Developmental and hormonal regulation of leptin receptor (Ob-R) messengerribonucleic acid expression in rat testis. Biol Reprod. 64(2):634–643.
- WHO. 1999. World Health Organisation, laboratory manual for the examination of human semen and sperm-cervical mucus interaction. Cambridge: Cambridge University Press. https://www.aab.org/images/WHO%204th%20manual.pdf.
- WHO. 2010. World Health Organisation, laboratory manual for the examination and processing of human semen. 5th ed. Geneva: World Health Organization; [accessed 2010]. https://apps.who.int/iris/bitstream/handle/10665/44261/9789241547789_eng.pdf?sequence=1&isAllowed=y.
- World Medical Association. 2001. World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. Bull World Health Organ [373–374 p.]. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2566407/pdf/11357217.pdf.
- Zorn B, Osredkar J, Meden-Vrtovec H, Majdic G. 2007. Leptin levels in infertile male patients are correlated with inhibin B, testosterone and SHBG but not with sperm characteristics. Int J Androl. 30(5):439–944.
