Abstract
Polycystic ovary syndrome (PCOS) is a disease characterized by metabolic disorders. This study aimed to examine the effects of resveratrol treatment on ovulation in the PCOS rat model. Quantitative real-time PCR and immunohistochemistry were used to determine the mRNA and protein expression levels. TNUEL assay was used to evaluate cell apoptosis in ovary. The metabolites were evaluated by liquid chromatography with tandem mass spectrometry. Resveratrol alleviated disrupted estrous cycle and improved granular cell layers, and reversed the decreased proliferation and increased cell apoptosis of granulosa cells in the ovarian tissues of PCOS rats. Resveratrol restored the changes in the mRNA expression levels in the rate-limiting genes of glycolysis in the PCOS ovary. The expression of lactate dehydrogenase A (LDH-A), pyruvate kinase isozyme M2 (PKM2), and sirtuin 1 (SIRT1) was significantly downregulated in ovarian tissues of the PCOS rats; while the resveratrol treatment significantly increased the expression of LDH-A, PKM2, and SIRT1 in the ovarian tissues of PCOS rats. Collectively, the protective effects of resveratrol in the PCOS rats may be associated with the regulation of glycolysis-related mediators including PKM2, LDH-A, and SIRT1. Resveratrol may represent a good candidate in alleviating the development of PCOS.
Introduction
Polycystic ovary syndrome (PCOS) is a disease characterized by metabolic disorders, often accompanied by clinical symptoms such as acne, obesity, menstrual disorders, and ovulation disorders (Escobar-Morreale Citation2018; Morgante et al. Citation2018; Liu et al. Citation2019). PCOS is a common cause of infertility in women of childbearing age (Zhao et al. Citation2016). Adolescents with PCOS are more likely to have health problems such as diabetes and cardiovascular disease in later life (Zhao et al. Citation2016). However, its mechanism is very complicated, and the molecular mechanisms underlying the pathophysiology of PCOS remains unclear. Studies have shown that glucose metabolism is closely related to follicular development. In the process of normal follicle formation, granulosa cells uptake glucose from the interstitium of ovary through glucose transporters (GLUT) and generate pyruvate and lactic acid for energy supply through glycolysis. Studies have shown that the expression levels of IRS-1 and GLUT4 in the granulosa cells of PCOS rats are reduced, and the cellular glycolysis pathway is disturbed (Zhao et al. Citation2016). Similarly, the expression levels of lactate dehydrogenase, hexokinase, and phosphofructokinase in the ovaries of PCOS mice were significantly lower than those in normal mice (Harris et al. Citation2010). Increasing the levels of lactate and pyruvate in the in vitro cultured atretic follicles from PCOS patients can partially restore the development of follicles (Harris et al. Citation2010). The above findings suggest that the glycolytic pathway may be involved in the regulation of follicular development in PCOS patients.
Resveratrol (3,5,4′-trihydroxy-stilbene) is a natural nonflavonoid polyphenol compound, which is widely present in the roots of dried knotweed, grape skin, peanuts, and other plants (Baur and Sinclair Citation2006). Resveratrol can exert various pharmacological actions including anti-inflammation, anti-oxidation, and anti-apoptosis (Baur and Sinclair Citation2006; Tian and Liu Citation2020). A large number of studies have shown that resveratrol exerts protective effects on ovarian damage induced by metabolic diseases (Morin-Papunen et al. Citation2000). Resveratrol can improve insulin resistance in obese mice, reduce androgen levels, and decrease the number of atretic follicles in the ovaries (Baur et al. Citation2006). Resveratrol can also reduce the apoptosis of granulosa cells in the secondary follicles of PCOS patients, increase the number of secondary follicles, and reduce the number of antral follicles (Ergenoglu et al. Citation2015). In addition, resveratrol can also reduce fasting insulin levels and enhance insulin sensitivity in the PCOS patients (Banaszewska et al. Citation2016). The pharmacological effects of resveratrol are mainly exerted by activating sirtuin 1 (SIRT1) (Price et al. Citation2012). In the ovary, SIRT1 is expressed in the nucleus of granulosa cells and oocytes. SIRT1 is a nicotinamide adenine dinucleotide (NAD)-dependent deacetylase, which requires NAD + as a cofactor to deacetylate intracellular target molecules including transcription factors, signal molecules, and chromatin histones (Chang and Guarente Citation2014). Activation of SIRT1 can reduce reactive oxygen species generated by oxidative stress, protect pancreatic islet β cells, promote insulin secretion, and improve insulin resistance (Pauli et al. Citation2010). In addition, SIRT1 can also prevent histone H3 deacetylation by binding to the PTP-1B promoter to inhibit its expression, reduce the dephosphorylation of insulin receptors, leading to improve insulin sensitivity (Pauli et al. Citation2010). Another study reported that the activation of SIRT1 can up-regulate the ATP level in oocytes and improve the ability of oocytes to develop into the blastocyst stage (Zhang et al. Citation2019). Importantly, our research group previously demonstrate that resveratrol improved follicular development of PCOS rats by regulating the glycolytic pathway (Liang et al. Citation2021). Based on these findings, we speculate that resveratrol may improve glucose metabolism disorders and follicular quality by up-regulating the expression of SIRT1 gene to protect the fertility of PCOS patients.
In this study, we aimed to examine the effects of resveratrol treatment on follicular development of PCOS rat model and to explore the underlying molecular mechanisms. This study could provide a new insight into understanding the mechanistic actions underlying protective effects of resveratrol treatment on PCOS.
Results
Effects of resveratrol treatment on the body weight and hormone levels in PCOS rats
The study design was illustrated in . Before the induction of PCOS, 6 rats were assigned as control animals and 12 rats were subjected to PCOS induction, and there was not significant difference in the body weight body between control group (178.5 ± 9.27 g) and model group (187.3 ± 10.41 g; P > 0.05, ). After PCOS induction, the body weight of the rats was assessed before resveratrol treatment, and the body weight of the PCOS rats was significantly increased when compared to the control rats (Control group: 253.5 ± 5.54 g; Model group: 297.2 ± 8.52 g; Resveratrol group: 292.8 ± 22.24 g; p < 0.05, ). After resveratrol treatment, the body weight of the PCOS rats was significantly reduced when compared to the PCOS rats without resveratrol treatment (Control group: 272.5 ± 8.56 g; Model group: 349.0 ± 11.31 g; Resveratrol group: 297.8 ± 18.18 g; p < 0.05, ). The analysis of the glucose and insulin levels showed that the HOMA-IR level was significantly elevated in the PCOS group when compared to that in the control group (Supplementary Figure S1A), and resveratrol intervention significantly reduced the HOMA-IR level in the PCOS rats (Supplementary Figure S1A). The testosterone levels in the PCOS group were significantly higher than that in the control group (Supplementary Figure S1B), and resveratrol treatment significantly attenuated the elevated testosterone level in the PCOS rats (Supplementary Figure S1B). In addition, the luteinizing hormone level was significantly higher in the PCOS group than that in the control group (Supplementary Figure S1C), and resveratrol treatment failed to affect the luteinizing hormone level in the PCOS rats (Supplementary Figure S1C).
Figure 1. A diagram of the study design. The animals were fed with high fat diet and intragastric administration with letrozole (1 mg/kg/d) for 21 days followed by oral administration of resveratrol (20 mg/kg/d) in 1% CMC and feeding with high fat diet for another 21 days. Vaginal smear of the animals was performed at 1 week after letrozole initiation, and the establishment of the PCOS model was judged to be successful or not according to the estrous cycle.
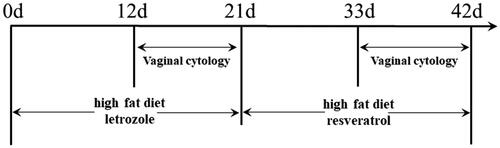
Figure 2. Effects of resveratrol treatment on the body weight in PCOS rats. (A) Body weight of the rats before PCOS modeling. (B) Body weight of the rats after PCOS modeling. (C) Body weight of the rats after resveratrol treatment. *p < 0.05 indicated the significant different between different treatment groups.
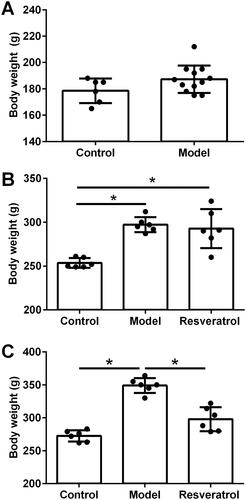
Effects of resveratrol treatment on estrous cycle and ovarian morphology in PCOS rats
The effects of resveratrol treatment on the estrous cycle in the rats were assessed by vaginal cytology. showed the vaginal cytology between day 33 and day 42 (). The rats in the control group showed a complete estrous ; in the model group, the rats showed a disordered estrous cycle on the 10th day after high-fat diet and letrozole intragastric administration . In the resveratrol-treated PCOS rats, the estrous period appeared at 14 days after resveratrol treatment and the complete estrous period was observed at 21 days after resveratrol treatment . The ovarian morphology of the rats was assessed by the H&E staining. As compared to the control group, the rats from the model group showed multiple follicles cystic expansion, and the ovary was vacuolated and had disorganized structure with atretic follicles and corpus luteum . The granulosa cells were sparse and radiating crowns were lost in the model group . After the resveratrol treatment, the PCOS rats showed multiple luteal bodies and follicles at different stages . Moreover, resveratrol treatment improved granular cell layers in the ovary of the PCOS rats .
Figure 3. Effects of resveratrol treatment on estrous cycle and ovarian morphology in PCOS rats. (A) Estrous cycle of the control rats, PCOS rats, and resveratrol-treated PCOS rats was determined by vaginal cytology. (B) Ovary morphology of the control rats, PCOS rats, and resveratrol-treated PCOS rats was evaluated by H&E staining. AT = atretic follicle; CL = corpus luteum; GC = Granular cells.
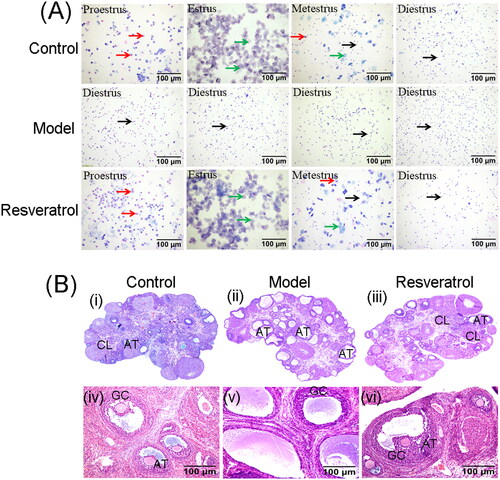
Effects of resveratrol treatment on the PCNA protein distribution and apoptosis-related gene expression in the ovary of PCOS rats
The protein distribution of PCNA in the ovary tissues was determined by IHC. As shown in , a large number of PCNA-positive cells were detected in ovary from the control group; while there were only sparse PCNA-positive cells were detected in the ovary from the model group, which was significantly improved by the treatment with resveratrol . The qRT-PCR assay consistently showed that the mRNA expression of PCNA was significantly decreased in the PCOS rats, which was significantly restored by the treatment with resveratrol . The apoptosis in the rat ovary was assessed using the TUNEL assay, and the apoptosis was significantly increased in the model group when compared to the control group ; while resveratrol treatment significantly attenuated the apoptosis of the ovary in the POCS rats . Furthermore, the mRNA expression levels of apoptosis-related genes were assessed by qRT-PCR. As shown in , the mRNA expression levels of Bax, caspase-3, apaf1, and cytochrome C were significantly increased in the ovary tissues from the PCOS group comparing to that from the control group ; while resveratrol treatment markedly decreased the mRNA expression levels of these genes in the ovary from PCOS rats . Moreover, the Bcl-2 mRNA expression level was significantly increased in the model group, and resveratrol treatment further increased the Bcl-2 mRNA expression level in the PCOS ovary . Importantly, the ratio of Bax/Bcl-2 was significantly increased in the ovary from model group when compared to the control group, and resveratrol treatment markedly reduced the ratio of Bax/Bcl-2 in the PCOS ovary . The protein expression of Bcl-2, Bax, and caspase-3 was further determined by western blot assay, and the results regarding the protein expression of Bcl-2, Bax, and caspase-3 were consistent with that determined by qRT-PCR , Supplementary Figure S2A.
Figure 4. Effects of resveratrol treatment on the PCNA expression and apoptosis-related gene expression in the ovary of PCOS rats. (A) The PCNA protein expression in control rats, PCOS rats, and resveratrol-treated PCOS rats was determined by IHC. (B) The PCNA mRNA expression in control rats, PCOS rats, and resveratrol-treated PCOS rats was determined by qRT-PCR. (C) The apoptosis in the ovary was assessed using TUNEL assay. (D) The mRNA expression levels of Bax, Bcl-2, caspase-3, apaf1, and cytochrome C in control rats, PCOS rats, and resveratrol-treated PCOS rats were determined by qRT-PCR. (E) The ratio of Bax/Bcl-2 in control rats, PCOS rats and resveratrol-treated PCOS rats was determined by qRT-PCR. (F) The protein expression of Bcl-2, Bax, and caspase-3 in control rats, PCOS rats, and resveratrol-treated PCOS rats was determined by western blot. Red arrows indicated the positively stained cells. N = 6; *p < 0.05 indicated the significant different between different treatment groups.
Effects of resveratrol treatment on the metabolic mediators in ovarian tissues of PCOS rats
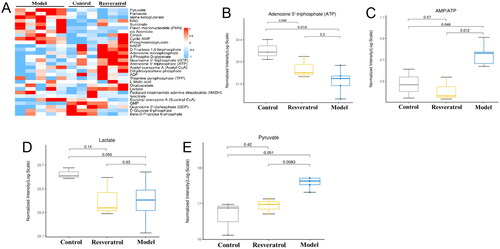
The metabolic mediators in the ovarian tissues were analyzed using the LC-MS/MS analysis. The changes of the 30 metabolic mediators in the ovary from control group, PCOS group, and resveratrol group were presented as the heatmap . Further analysis revealed that the ATP level was elevated and the ratio of AMP/ATP was decreased in the ovary from the PCOS rats, which was significantly restored by the resveratrol treatment . Moreover, the level of lactate was increased and the level of pyruvate was decreased in the ovary from the PCOS group , which was significantly attenuated the resveratrol treatment .
Figure 5. Effects of resveratrol treatment on the metabolic mediators in ovarian tissues of PCOS rats. (A) Effects of DM treatment on the metabolic mediators in the ovarian tissues of PCOS rats by using the targeted metabolomics analysis. (B–E) Effects of resveratrol treatment on the levels of ATP (B), AMP/ATP (C), lactate (D), and pyruvate (E) in the ovarian tissues of PCOS rats after different treatments. N = 6.
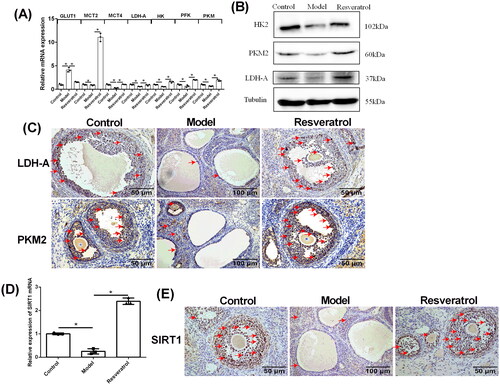
Effects of resveratrol treatment on the PKM2, LDH-A and SRIT1 mRNA and protein expression levels in the ovarian tissues from PCOS rats
The mRNA expression levels of rate-limiting genes of glycolysis in the ovary were assessed by qRT-PCR. As shown in , the mRNA expression level of GLUT1 was significantly increased in the ovary from the PCOS control when compared to the control group, and resveratrol treatment significantly decreased the mRNA expression of GLUT1 in the PCOS ovary . In addition, the mRNA expression levels of MCT2, MCT4, LDH-A, HK, PFK, and PKM were significantly downregulated in the ovary from the model group comparing to the control group ; while resveratrol treatment markedly increased the mRNA expression levels of these genes in the PCOS ovary . The protein expression of HK2, PKM2, and LDH-A was further determined by western blot assay, and the results regarding the protein expression of HK2, PKM2, and LDH-A were consistent with that determined by qRT-PCR , Supplementary Figure S2B. Furthermore, the protein distribution of LDH-A and PKM2 in the rat ovary were assessed by IHC. Consistently, the protein levels of LDH-A and PKM2 were significantly decreased in the ovary of PCOS rats , and resveratrol treatment restored the protein expression levels of LDH-A and PKM2 in the PCOS ovary . Moreover, the mRNA and protein expression levels of SIRT1 were further determined in the ovary tissues. The mRNA and protein expression levels of SIRT1 were significantly decreased in the ovary from the model group comparing to the control group; while resveratrol treatment caused a markedly increase in the mRNA and protein expression levels of SIRT1 in the PCOS ovary .
Figure 6. Effects of resveratrol treatment on the PKM2, LDH-A, and SRIT1 expression levels in the ovarian tissues from PCOS rats. (A) The mRNA expression levels of GLUT1, MCT2, MCT4, LDH-A, HK, PFK, and PKM in control rats, PCOS rats, and resveratrol-treated PCOS rats were determined by qRT-PCR. (B) The protein expression of HK2, PKM2, and LDH-A in control rats, PCOS rats and resveratrol-treated PCOS rats was determined by western blot assay. (C) The protein expression levels of LDH-A and PKM2 in control rats, PCOS rats, and resveratrol-treated PCOS rats was determined by IHC. (D) The mRNA expression levels of SIRT1 in control rats, PCOS rats, and resveratrol-treated PCOS rats was determined by qRT-PCR. (E) The protein expression levels of SIRT1 in control rats, PCOS rats, and resveratrol-treated PCOS rats was determined by IHC. Red arrows indicated the positively stained cells. N = 6; *p < 0.05 indicated the significant different between different treatment groups.
Discussion
PCOS is a syndrome characterized by metabolic disorders, which can cause hypothalamic-pituitary-ovarian axis dysfunction, increase luteinizing hormone (LH) and androgen levels, accelerate follicular atresia, promote ovaries cystoid changes, impair follicular development and eventually lead to infertility (Charalampakis et al. Citation2016; Lu et al. Citation2018; Zhu et al. Citation2019). Up to date, different rodent models of hormone-induced PCOS have been established. These PCOS models include androgen-induced PCOS model, aromatase inhibitor-induced PCOS model, progesterone receptor antagonist-induced PCOS model, and estrogen-induced PCOS model (Osuka et al. Citation2019). In order to explore the pathogenesis of PCOS, we established a PCOS model by treating rats with high-fat diet and letrozole (Wang et al. Citation2020; Peng et al. Citation2021). Letrozole is a nonsteroidal aromatase inhibitor, which can hinder the conversion of androgens to estrogen, leading to disorders of steroid hormone secretion, increasing androgen levels, which can cause PCOS. Our experimental results showed that the body weight of rats in the model group increased significantly, and the rats were all in the inter-estrous period, and there was no obvious estrus cycle; the rats in the model group showed increased number of vacuolated and cystic follicles, decreased number of granular cells and layers, disordered morphological structure of the ovary, extremely rare corpus luteum, and large number of atretic follicles. The above evidence suggests that the PCOS rat model was successfully established in our study.
Resveratrol is a polyphenol compound extracted from plants and has a wide range of pharmacological effects. Studies have shown that resveratrol has the function of protecting the ovaries (Furat Rencber et al. Citation2018; Brenjian et al. Citation2020). In order to explore the protective effect of resveratrol on the ovaries of PCOS, PCOS rats were treated with resveratrol for consecutive 21 days. Our results showed that resveratrol treatment alleviated disrupted estrous cycle and improved the ovarian morphology in the PCOS ovaries. Consistently, Zhang et al. (Citation2021) showed that resveratrol administration was associated with increased levels of plasma adiponectin and estradiol levels and restoration of normal ovarian morphology in PCOS model animals. In addition, resveratrol improved menstrual cyclicity and hair loss, even though levels of androgens, insulin, and lipids remained unchanged in women with PCOS (Mansour et al. Citation2021). The results suggest that resveratrol can improve the ovary structure and follicular development in PCOS rats.
The proliferation and apoptosis of granulosa cells are closely related to the development of follicles (Zheng et al. Citation2017). Reports have shown that in PCOS patients and animal model of PCOS, a significant increase in the apoptosis of granulosa cells and the expression levels of Bax and caspase-3 from ovaries has been observed (Ding et al. Citation2016). Consistently, our results showed that the expression levels of Bax, Blc-2, caspasse-3, Apaf1, and cytochrome c, and the ratio of Bax/Bcl-2 in the ovaries from the model group were significantly increased, which was prevented by the resveratrol treatment. In order to further study the effect of resveratrol on granulosa cells, the proliferating cell nuclear antigen (PCNA) in rat ovary was detected. The results showed that the expression of PCNA in the ovaries of PCOS rats was significantly downregulated, and PCNA expression was upregulated after resveratrol treatment. This suggests that granulosa cell apoptosis is an important cause of follicular development disorders in PCOS. Resveratrol can reduce granulosa cell apoptosis and play a protective effect on the ovaries.
The occurrence of PCOS is also closely related to abnormal energy metabolism. During normal follicular development, granulosa cells uptake glucose in the surrounding tissues through glucose transporters (GLUT) and generate pyruvate and lactic acid for energy supply through glycolysis. Boland et al., found that developing follicles have high glycolytic activity (Boland et al. Citation1994). The glycolysis rate in goat oocytes is 1.5 times that of oxidative phosphorylation, while the glycolytic activity in COCs is 3 times that of oxidative phosphorylation (Herrick et al. Citation2006). Under hypoxia, the expression of glycolysis-related genes GLUT1, GLUT3, hexokinase 2 (HK2), lactate dehydrogenase (LDH), and M2-type pyruvate kinase (PKM2) in the follicles was significantly upregulated (Makanji et al. Citation2014; Anastácio et al. Citation2017). A large number of studies have shown that pyruvate and lactic acid are important factors to maintain the growth and development of follicles. High concentrations of glucose, pyruvate, and lactic acid can promote the proliferation of granular cells (Peralta et al. Citation2016). In this study, the levels of ATP, lactic acid were decreased, but the ratio of AMP/ATP and the levels of pyruvate were increased in the PCOS rats; in addition, rate-limiting enzyme genes are also downregulated in the PCOS rats, suggesting the dysregulation of glucose metabolism in PCOS rats. After treatment with resveratrol, the level of ATP in the ovaries of PCOS rats was increased, and the ratio of AMP/ATP decreased; the expression levels LDH-A, PKM2, and the rate-limiting enzyme genes in the glycolysis process (MCT2/4, LDH, PFK, PKM, etc.) were increased. These results show that resveratrol can significantly improve the energy metabolism of PCOS rats, to a certain extent, can reverse the disorder of glucose metabolism in PCOS rats, and restore the abnormal glycolysis status.
SIRT1 is a deacetylase, expressed in the nucleus of oocytes and granulosa cells (Han et al. Citation2017; González-Fernández et al. Citation2019). With the increase of age, the damage of oocytes by oxidative stress gradually intensifies (Han et al. Citation2017; González-Fernández et al. Citation2019). SIRT1 can avoid this damage, thereby delaying the decline of ovarian function (Han et al. Citation2017; González-Fernández et al. Citation2019). Activated SIRT1 can also significantly inhibit the production of reactive oxygen species and nitric oxide, and improve insulin resistance related to oxidative stress (Kitada et al. Citation2019). In addition, SIRT1 can also inhibit the deacetylation of histone H3 from binding to the PTP-1B promoter to inhibit its expression, thereby reducing the dephosphorylation of the insulin receptor and improving insulin sensitivity (Wójcik et al. Citation2018). In this study, our results showed that the expression of SITR1 in the model group was significantly decreased compared with the control group; and the expression of SITR1 in the resveratrol group was significantly increased compared with the model group. These results may indicate that resveratrol may improve the glycolysis disorder of PCOS rats by upregulating the expression of SIRT1. In the comparison of our previous work (Liang et al. Citation2021), first, our work further showed that resveratrol could attenuate apoptosis in the ovary of PCOS rats; second, the effects of resveratrol on glycolytic pathway was profiled with RNA sequencing in the previous work (Liang et al. Citation2021), and our study performed the metabolomics analysis in the ovarian tissues of PCOS rats to reveal the changes in glycolytic pathway; third, previous work emphasized the role of SIRT2 in the resveratrol-mediated protective effects on PCOS ovary (Liang et al. Citation2021), while our work further deciphered that the protective actions of resveratrol in PCOS may be related to SIRT1.
Several limitations in this study should be considered. First, the effects of resveratrol on the PCOS rats were limited to in vivo studies, and future in vitro studies should be considered to decipher the mechanistic action of resveratrol. Second, the expression levels of GLUT1, MCT2, MCT4, PFK, Apaf1, and cytochrome C were only determined by qRT-PCR, and future studies should perform western blot assay to determine the protein expression levels of these mediators when primary antibodies are available. Third, we should be cautious when interpreting hormonal results in this study, as the anesthesia could influence the hormonal levels in the rats. Fourth, this study used the 5-week-old rats for experiments, which did not reflect the total population; further studies should use rats with different ages to confirm the current findings.
In conclusion, resveratrol treatment improved the pathological changes in the PCOS rats. Resveratrol can improve the energy metabolism of the ovary via regulating the glycolysis pathway. Further evidence indicated that the protective effects of resveratrol in the PCOS rats may be associated with the regulation of glycolysis-related mediators including PKM2, LDH-A, and SIRT1. Resveratrol may represent a good candidate in alleviating the development of PCOS, and further mechanistic studies and clinical investigation may be performed to confirm the therapeutic potential of resveratrol in PCOS.
Materials and methods
Animals and treatments
The Sprague Dawley (SD) rats (130–140 g, 5 weeks old) were obtained from The Affiliated Hospital of Guilin Medical University. For the establishment of the PCOS, the animals were fed with high fat diet and intragastric administration with letrozole (1 mg/kg/d; Heng Rui Pharmaceutical Factory, Lianyungang, China) dissolved in 1% (w/w) carboxymethyl cellulose (CMC) for consecutive 21 days. Vaginal smear of the animals was performed at 1 week after letrozole initiation, and the establishment of the PCOS model was judged to be successful or not according to the estrous cycle (Yarmolinskaya et al. Citation2021; Dubey et al. Citation2022). In this study, the rats were randomly divided into the three groups: control group (n = 6), model group (n = 6), and resveratrol group (n = 6). For the control group, the animals were orally administered 1% CMC and fed with normal diet for 42 days; for the model group, the animals were fed with high fat diet (consisting of 61.5% standard food, 12% lard, 5% sucrose, 5% milk powder, 5% peanut, 10% egg, 1% sesame oil, 0.5% salt) and intragastric administration with letrozole (an aromatase inhibitor; 1 mg/kg/d) for 21 days followed by oral administration of 1% CMC and feeding with high fat diet for another 21 days (Wang et al. Citation2020; Peng et al. Citation2021); for the resveratrol group, the animals were fed with high fat diet and intragastric administration with letrozole (1 mg/kg/d) for 21 days followed by oral administration of resveratrol (20 mg/kg/d, Solarbio, Beijing, China) in 1% CMC and feeding with high fat diet for another 21 days (Yazir et al. Citation2015; Liang et al. Citation2021). At the end of the experiments, the animals were sacrificed by overdose of pentobarbitone (80 mg/kg, intraperitoneal).
Measurement of blood glucose, insulin, testosterone, and luteinizing hormone levels
At the end of the experiment, the rats were anesthetized by intraperitoneal injection of 10% chloral hydrate (0.2 mL/100 g). Blood was collected by cardiac puncture, and the serum was isolated by centrifuging at 2500 rpm for 20 min at 4 °C. The serum insulin levels were detected by a commercial insulin ELISA kit (Abcam, Cambridge, USA). Glucose levels in the serum were detected by Glucose Meter (Contournext, Parsippany, USA). The HOMA-IR was calculated based on the following formula: (fasting blood glucose levels x fasting blood insulin levels)/22.5 (Matthews et al. Citation1985). The serum levels of testosterone, luteinizing hormone were determined by corresponding commercial ELISA kits (Abcam, Cambridge, USA).
Vaginal cytology
Consecutively vaginal smears were performed for 10 days which carried out, respectively, before the ending of modeling and resveratrol treatment according to our previous study (Zhang et al. Citation2020).
Morphological analysis of ovary
The ovarian tissues collected from the rats were fixed in 4% paraformaldehyde overnight at 4 °C, embedded in paraffin, sectioned into 5-μm thick slices, deparaffinized, and stained with hematoxylin and eosin (Servicebio, Wuhan, China) according to the manufacturer’s protocol. A light microscope (OLYMPUS cellSens Standard, OLYMPUS, PA, USA) was used to evaluate the morphology of the ovarian tissues.
Immunohistochemical (IHC) localization of proliferating cell nuclear antigen (PCNA), lactate dehydrogenase A (LDH-A), pyruvate kinase isozyme M2 (PKM2), and SIRT1
The sections in 5-μm thick slices were permeabilized with 1% Triton X-100 in phosphate buffered saline (PBS) for 30 min at room temperature, boiled in 100 mM sodium citrate (pH 6.0) three times for 6 min each at 5-min intervals for antigen retrieval, and then incubated with 3% hydrogen peroxide for 30 min to remove endogenous peroxidase followed by blocking in 5% bovine serum albumin at room temperature for 1 h. The sections were then incubated overnight at 4 °C with primary rabbit polyclonal antibodies including PCNA (1:100 dilution, 60097-1-Ig, Protein Tech Group Inc., USA), LDH-A (1:100 dilution, ab52488, Abcam, USA), PKM2 (1:100 dilution, ab137791, Abcam) and SIRT1 (1:100 dilution, 60303-1-Ig, Protein Tech Group Inc.), and normal IgG was served as a negative control. After that, the sections were incubated with rabbit anti-goat biotin-SP-conjugated antibody (1:100; SA00004-4, Protein Tech Group Inc.) at room temperature for 1 h. The stained PCNA, LDH-A, PKM2, and SIRT1 proteins were visualized using the 3, 3-diaminobenzidine chromogen.
Terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) assay
TUNEL assay kit (Roche Diagnostic Systems, Branchburg, USA) was used to detect the apoptotic cells in the ovary according to our previous study (Zhang et al. Citation2020).
Quantitative real‑time PCR (qPCR)
RNA extraction from the tissues was performed using TRIzol reagent (Takara, Dalian, China). Synthesis of cDNA was performed using the TransScript II One-Step gDNA Removal and cDNA Synthesis SuperMix kit (Transgen Biotech, Beijing, China). Real-time PCR analyses for the gene expression level were performing on the Applied Biosystems 7500 Real-time PCR System (Applied Biosystems, Foster City, CA, USA). The primer sequences were shown in . GAPDH was used as the reference control, and gene expression levels were calculated using comparative Ct method.
Table 1. Primer sequences of the qRT-PCR analysis.
Liquid chromatography with tandem mass spectrometry (LC-MS/MS) analysis of ovarian metabolites
A total of 40 mg rat ovarian tissue for each sample was homogenized in 200 μL pre-cooled ultrapure water. The homogenized samples were incubated with 800 μL pre-cooled methanol/acetonitrile (1:1, v/v) by vortex mixing with ultrasound in ice bath for 20 min, followed by incubation for 1 h at –20 °C and centrifuge at 14,000 rpm for 4 min at 4 °C. The supernatant was collected and dried in the vacuo. For mass spectrometry, 100 μL of acetonitrile-water solution (1:1, v/v) was reconstituted, centrifuging at 14,000 rpm for 4 min at 4 °C, and the supernatant was taken for analysis. Samples were separated by using an Agilent 1290 Infinity LC Ultra Performance LC System. Mobile phase: liquid A was 10 mM aqueous ammonium acetate solution, and liquid B was acetonitrile. The sample was placed in a 4 °C autosampler at a column temperature of 45 °C with a flow rate of 300 μL/min and an injection volume of 2 μL. The relevant liquid-phase gradient was as follows: 0–18 min, B liquid linearly changes from 90% to 40%; 18–18.1 min, B liquid linearly changes from 40% to 90%; 18.1–23 min, B liquid is maintained at 90%. A quality control sample is set up for each experimental sample in the sample queue for the detection and evaluation of the stability and repeatability of the system; a standard mixture of energy metabolites is set in the sample queue for the correction of chromatographic retention time. Mass spectrometry was performed in negative ion mode using a 5500 QTRAP mass spectrometer (AB SCIEX). The 5500 QTRAP ESI source conditions were as follows: source temperature 450 °C, ion Source Gas 1: 45, Ion Source Gas 2: 45, Curtain gas: 30, ion Spray Voltage Floating 4500 V; multi-response monitoring mode detects the pair of ions to be tested. The peak area and retention time were extracted using Multiquant software. Standardization of energy metabolites was used to correct retention time for metabolite identification.
Western blot analysis
The proteins from the ovarian tissues were extracted using the RIPA buffer supplemented with the protease inhibitors. The protein concentrations were determined by the BCA assay kit (Thermo Fisher Scientific). Equal amounts of proteins were resolved on a 10% SDS-PAGE gel followed by the transferred to the PVDF membranes. After blocking with 1.5% skimmed milk in PBST at room temperature for 1 h, the PVDF membranes were incubated with primary antibodies including Bcl-2 (1:500 dilution, sc-23960, Santa Cruz Biotechnology Inc., USA), Bax (1:500 dilution, sc-20067, Santa Cruz Biotechnology Inc., USA), caspase-3 (1:1000 dilution, #9662, Cell Signaling Technology, USA), HK2 (1:1000 dilution, A0994, ABclonal, China), PKM2 (1:1000 dilution, #4053, Cell Signaling Technology, USA), LDH-A (1:1000 dilution, #3558, Cell Signaling Technology, USA), and tubulin (1:3000 dilution, 66031-1-Ig, Protein Tech Group Inc., USA) overnight at 4 °C. After washing with PBST for 5 min x 3 times, the PVDF membranes were incubated with horseradish peroxidase-conjugated secondary antibodies at room temperature for 2 h. The bands on the PDVF membranes were visualized using ECL kit (Thermo Fisher Scientific). Tubulin was used as the reference control for protein expression.
Statistical analysis
The experimental data are presented as the mean ± standard deviation. Data were analyzed using SPSS 18.0 software (SPSS, Inc., Chicago, IL, USA). The unpaired Student’s t-test was used to analyze the comparison between the two groups. One-way ANOVA followed by Bonferroni’s multiple comparison tests was used for comparison among multiple groups. A P value less than 0.05 was considered to indicate a statistically significant result.
Ethics approval
The animal experiments were approved by the Animal Ethics Committee of The Affiliated Hospital of Guilin Medical University (No. 2019-0009).
Authors’ contributions
Data collection, data analysis, and manuscript writing: PH; Data collection, data analysis, and manuscript writing: ML; Data collection and data analysis: JL; Protocol development: CZ; Protocol development and manuscript writing: JY, SZ.
| Abbreviations | ||
| IHC | = | immunohistochemistry |
| LC-MS/MS | = | liquid chromatography with tandem mass spectrometry |
| LDH-A | = | lactate dehydrogenase A |
| NAD | = | nicotinamide adenine dinucleotide |
| PBS | = | phosphate buffered saline |
| PCNA | = | proliferating cell nuclear antigen |
| PCOS | = | polycystic ovary syndrome |
| PKM2 | = | pyruvate kinase isozyme M2 |
| qPCR | = | quantitative real-time PCR |
| SD | = | Sprague Dawley |
| SIRT1 | = | sirtuin 1 |
| TUNEL | = | terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling |
Supplemental Material
Download PDF (127.3 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Anastácio A, Rodriguez-Wallberg KA, Chardonnet S, Pionneau C, Fédérici C, Almeida Santos T, Poirot C. 2017. Protein profile of mouse ovarian follicles grown in vitro. Mol Hum Reprod. 23(12):827–841.
- Banaszewska B, Wrotyńska-Barczyńska J, Spaczynski RZ, Pawelczyk L, Duleba AJ. 2016. Effects of resveratrol on polycystic ovary syndrome: A double-blind, randomized, placebo-controlled trial. J Clin Endocrinol Metab. 101(11):4322–4328.
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, et al. 2006. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 444(7117):337–342.
- Baur JA, Sinclair DA. 2006. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 5(6):493–506.
- Boland NI, Humpherson PG, Leese HJ, Gosden RG. 1994. The effect of glucose metabolism on murine follicle development and steroidogenesis in vitro. Hum Reprod. 9(4):617–623.
- Brenjian S, Moini A, Yamini N, Kashani L, Faridmojtahedi M, Bahramrezaie M, Khodarahmian M, Amidi F. 2020. Resveratrol treatment in patients with polycystic ovary syndrome decreased pro-inflammatory and endoplasmic reticulum stress markers. Am J Reprod Immunol. 83(1):e13186.
- Chang HC, Guarente L. 2014. SIRT1 and other sirtuins in metabolism. Trends Endocrinol Metab. 25(3):138–145.
- Charalampakis V, Tahrani AA, Helmy A, Gupta JK, Singhal R. 2016. Polycystic ovary syndrome and endometrial hyperplasia: an overview of the role of bariatric surgery in female fertility. Eur J Obstet Gynecol Reprod Biol. 207:220–226.
- Ding L, Gao F, Zhang M, Yan W, Tang R, Zhang C, Chen ZJ. 2016. Higher PDCD4 expression is associated with obesity, insulin resistance, lipid metabolism disorders, and granulosa cell apoptosis in polycystic ovary syndrome. Fertil Steril. 105(5):1330–1337.e1333.
- Dubey P, Shi T, Coltharp M, Reddy S. 2022. Effects of resveratrol on metabolic, biochemical, and endocrine manifestations in polycystic ovary syndrome. Dietetics. 1(2):66–77.
- Ergenoglu M, Yildirim N, Yildirim AG, Yeniel O, Erbas O, Yavasoglu A, Taskiran D, Karadadas N. 2015. Effects of resveratrol on ovarian morphology, plasma anti-mullerian hormone, IGF-1 levels, and oxidative stress parameters in a rat model of polycystic ovary syndrome. Reprod Sci. 22(8):942–947.
- Escobar-Morreale HF. 2018. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol. 14(5):270–284.
- Furat Rencber S, Kurnaz Ozbek S, Eraldemır C, Sezer Z, Kum T, Ceylan S, Guzel E. 2018. Effect of resveratrol and metformin on ovarian reserve and ultrastructure in PCOS: an experimental study. J Ovarian Res. 11(1):55.
- González-Fernández R, Martín-Ramírez R, Rotoli D, Hernández J, Naftolin F, Martín-Vasallo P, Palumbo A, Ávila J. 2019. Granulosa-lutein cell sirtuin gene expression profiles differ between normal donors and infertile women. IJMS. 21(1):295.
- Han Y, Luo H, Wang H, Cai J, Zhang Y. 2017. SIRT1 induces resistance to apoptosis in human granulosa cells by activating the ERK pathway and inhibiting NF-κB signaling with anti-inflammatory functions. Apoptosis. 22(10):1260–1272.
- Harris SE, Maruthini D, Tang T, Balen AH, Picton HM. 2010. Metabolism and karyotype analysis of oocytes from patients with polycystic ovary syndrome. Hum Reprod. 25(9):2305–2315.
- Herrick JR, Lane M, Gardner DK, Behboodi E, Memili E, Blash S, Echelard Y, Krisher RL. 2006. Metabolism, protein content, and in vitro embryonic development of goat cumulus-oocyte complexes matured with physiological concentrations of glucose and L-lactate. Mol Reprod Dev. 73(2):256–266.
- Kitada M, Ogura Y, Monno I, Koya D. 2019. Sirtuins and type 2 diabetes: role in inflammation, oxidative stress, and mitochondrial function. Front Endocrinol (Lausanne). 10:187.
- Liang A, Huang L, Liu H, He W, Lei X, Li M, Li S, Liang H, Chen G, Tang J, et al. 2021. Resveratrol improves follicular development of PCOS rats by regulating the glycolytic pathway. Mol Nutr Food Res. 65(24):e2100457.
- Liu Q, Xie YJ, Qu LH, Zhang MX, Mo ZC. 2019. Dyslipidemia involvement in the development of polycystic ovary syndrome. Taiwan J Obstet Gynecol. 58(4):447–453.
- Lu C, Hutchens EG, Farhy LS, Bonner HG, Suratt PM, McCartney CR. 2018. Influence of sleep stage on LH pulse initiation in the normal late follicular phase and in polycystic ovary syndrome. Neuroendocrinology. 107(1):60–72.
- Makanji Y, Tagler D, Pahnke J, Shea LD, Woodruff TK. 2014. Hypoxia-mediated carbohydrate metabolism and transport promote early-stage murine follicle growth and survival. Am J Physiol Endocrinol Metab. 306(8):E893–E903.
- Mansour A, Samadi M, Sanginabadi M, Gerami H, Karimi S, Hosseini S, Shirzad N, Hekmatdoost A, Mahdavi-Gorabi A, Mohajeri-Tehrani MR, et al. 2021. Effect of resveratrol on menstrual cyclicity, hyperandrogenism and metabolic profile in women with PCOS. Clin Nutr (Edinburgh, Scotland). 40(6):4106–4112.
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. 1985. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 28(7):412–419.
- Morgante G, Massaro MG, Di Sabatino A, Cappelli V, De Leo V. 2018. Therapeutic approach for metabolic disorders and infertility in women with PCOS. Gynecol Endocrinol. 34(1):4–9.
- Morin-Papunen LC, Vauhkonen I, Koivunen RM, Ruokonen A, Tapanainen JS. 2000. Insulin sensitivity, insulin secretion, and metabolic and hormonal parameters in healthy women and women with polycystic ovarian syndrome. Hum Reprod. 15(6):1266–1274.
- Osuka S, Nakanishi N, Murase T, Nakamura T, Goto M, Iwase A, Kikkawa F. 2019. Animal models of polycystic ovary syndrome: a review of hormone-induced rodent models focused on hypothalamus-pituitary-ovary axis and neuropeptides. Reprod Med Biol. 18(2):151–160.
- Pauli JR, Ropelle ER, Cintra DE, De Souza CT, da Silva AS, Moraes JC, Prada PO, de Almeida Leme JA, Luciano E, Velloso LA, et al. 2010. Acute exercise reverses aged-induced impairments in insulin signaling in rodent skeletal muscle. Mech Ageing Dev. 131(5):323–329.
- Peng MF, Tian S, Song YG, Li CX, Miao MS, Ren Z, Li M. 2021. Effects of total flavonoids from Eucommia ulmoides Oliv. leaves on polycystic ovary syndrome with insulin resistance model rats induced by letrozole combined with a high-fat diet. J Ethnopharmacol. 273:113947.
- Peralta OA, Bucher D, Angulo C, Castro MA, Ratto MH, Concha I. 2016. Tissue localization of GM-CSF receptor in bovine ovarian follicles and its role on glucose uptake by mural granulosa cells. Anim Reprod Sci. 170:157–169.
- Price NL, Gomes AP, Ling AJ, Duarte FV, Martin-Montalvo A, North BJ, Agarwal B, Ye L, Ramadori G, Teodoro JS, et al. 2012. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 15(5):675–690.
- Tian B, Liu J. 2020. Resveratrol: a review of plant sources, synthesis, stability, modification and food application. J Sci Food Agric. 100(4):1392–1404.
- Wang MX, Yin Q, Xu X. 2020. A rat model of polycystic ovary syndrome with insulin resistance induced by letrozole combined with high fat diet. Med Sci Monit. 26:e922136.
- Wójcik M, Krawczyńska A, Antushevich H, Herman AP. 2018. Post-receptor inhibitors of the GHR-JAK2-STAT pathway in the growth hormone signal transduction. IJMS. 19(7):1843.
- Yarmolinskaya M, Bulgakova O, Abashova E, Borodina V, Tral T. 2021. The effectiveness of resveratrol in treatment of PCOS on the basis of experimental model in rats. Gynecol Endocrinol. 37(sup1):54–57.
- Yazir Y, Utkan T, Gacar N, Aricioglu F. 2015. Resveratrol exerts anti-inflammatory and neuroprotective effects to prevent memory deficits in rats exposed to chronic unpredictable mild stress. Physiol Behav. 138:297–304.
- Zhang N, Zhuang L, Gai S, Shan Y, Wang S, Li F, Chen L, Zhao D, Liu X. 2021. Beneficial phytoestrogenic effects of resveratrol on polycystic ovary syndromein rat model. Gynecol Endocrinol. 37(4):337–341.
- Zhang T, Du X, Zhao L, He M, Lin L, Guo C, Zhang X, Han J, Yan H, Huang K, et al. 2019. SIRT1 facilitates primordial follicle recruitment independent of deacetylase activity through directly modulating Akt1 and mTOR transcription. FASEB J. 33(12):14703–14716.
- Zhang S, Tu H, Yao J, Le J, Jiang Z, Tang Q, Zhang R, Huo P, Lei X. 2020. Combined use of Diane-35 and metformin improves the ovulation in the PCOS rat model possibly via regulating glycolysis pathway. Reprod Biol Endocrinol. 18(1):58.
- Zhao S, Xu H, Cui Y, Wang W, Qin Y, You L, Chan WY, Sun Y, Chen ZJ. 2016. Metabolic actions of insulin in ovarian granulosa cells were unaffected by hyperandrogenism. Endocrine. 53(3):823–830.
- Zheng Q, Li Y, Zhang D, Cui X, Dai K, Yang Y, Liu S, Tan J, Yan Q. 2017. ANP promotes proliferation and inhibits apoptosis of ovarian granulosa cells by NPRA/PGRMC1/EGFR complex and improves ovary functions of PCOS rats. Cell Death Dis. 8(10):e3145.
- Zhu JL, Chen Z, Feng WJ, Long SL, Mo ZC. 2019. Sex hormone-binding globulin and polycystic ovary syndrome. Clin Chim Acta. 499:142–148.
