Abstract
There is a correlation between teratozoospermia and production of reactive oxygen species leading to poor assisted reproductive techniques outcomes. This study aimed to examine the effect of plasma-rich in growth factors (PRGF) on teratozoospermic samples. Twenty-five teratozoospermic samples were included in this study. After sperm preparation, it was divided into four groups, including 0 (control), 1, 5, and 10% PRGF. Sperm motility, viability (eosin-nigrosin staining), morphology (Papanicolaou staining), DNA fragmentation (sperm chromatin dispersion test), mitochondrial membrane potential (JC-1 staining by flow cytometry), and lipid peroxidation (measurement of malondialdehyde, MDA) were evaluated before and after 1 h of incubation with or without PRGF. Our results showed that after 1 h of incubation, the addition of 1% PRGF improved sperm progressive motility (47.72 ± 13.76%) compared to the control group (17.36 ± 8.50%) (p < 0.001). Also, 1% PRGF preserved the sperm’s total motility (77.50 ± 13.28% vs. 65.63 ± 19.03%, for 1% PRGF and control, respectively) and viability after incubation. The rate of normal sperm morphology was the same between different groups. Higher mitochondrial membrane potential and lower DNA fragmentation were also observed in sperm treated with different concentrations of PRGF compared to the control group, but the differences were non-significant. The MDA levels were significantly decreased in PRGF-treated groups compared to the control group (0.99 ± 0.62, 0.95 ± 0.33, 0.95 ± 0.79, and 1.49 ± 0.27 for 1% PRGF, 5% PRGF, 10% PRGF and control, respectively). Based on our results, it seems that PRGF incubation can improve sperm parameters and especially decrease the level of malondialdehyde as an indicator of oxidative stress, which is one of the main problems of teratozoospermic samples.
Introduction
Teratozoospermia is one of the most important causes of male infertility. Despite the normal average sperm count and motility, morphological disorders limit the fertilization rate (Özer et al. Citation2019). According to WHO, teratozoospermia is defined as a percentage of spermatozoa with normal morphology of less than 4% (World Health Organization Citation2010). One of the main leading causes of the overproduction of seminal reactive oxygen species (ROS) is teratozoospermia (Agarwal et al. Citation2014). Excess cytoplasm in the sperm may be an important source of ROS production in teratozoospermic samples (Venkatesh et al. Citation2009). In addition, leukocytes are another source of free radicals in semen (Saleh and Agarwal Citation2002; Cocuzza et al. Citation2007; Oborna et al. Citation2009). Low and physiological levels of ROS are necessary for many processes such as hyperactivation, acrosome reaction, and fertilization (Agarwal et al. Citation2014). However, high levels of ROS can cause oxidative stress and damage the DNA of spermatozoa (Bui et al. Citation2018). Spermatozoa are susceptible to ROS-induced damage because their plasma membrane contains high levels of unsaturated fatty acids, scanty cytoplasm, and low levels of antioxidants. In addition, the presence of many mitochondria in the middle part of the sperm is another source of ROS production (Walczak-Jedrzejowska et al. Citation2013; Amidi et al. Citation2016). Using various strategies, including in vitro application of antioxidants, could reduce the adverse effects of oxidative stress and improve the quality of teratozoospermia (Keshtgar et al. Citation2012).
Plasma-rich in growth factors (PRGF) is a novel therapeutic supplement that is used in multiple medical fields with beneficial results, for example, in ophthalmology (Anitua et al. Citation2015) and neurobiology (Anitua et al. Citation2013). Its antioxidant potential is due to the presence of various factors such as epidermal growth factor (EGF), platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), transforming growth factor-beta (TGFβ), hepatocyte growth factor (HGF), fibroblast growth factor (FGF), and insulin-like growth factor (IGF-1) (Anitua et al. Citation2008; Anitua and Orive Citation2012). The effects of growth factors in the PRGF were studied solely on the spermatozoa and showed positive results. It was shown that sperm incubation with FGF (Azúa et al. Citation2017) and VEGF (Iyibozkurt et al. Citation2009) could improve human sperm motility. Furthermore, it was reported that human sperm incubation with platelet-rich plasma (PRP) significantly improved sperm motility and viability, and decreased DNA fragmentation, vacuolization, and ROS-positive cells (Bader et al. Citation2020). Recently, it was shown that PRGF has protective effects on human sperm cryopreservation (Mirzaei et al. Citation2022). To the best of our knowledge, the impact of PRGF on sperm incubation after preparation has not been performed. This study aimed to examine the effects of PRGF on human sperm motility, viability, morphology, DNA fragmentation, mitochondrial membrane potential (MMP), and malondialdehyde (MDA) after 1 h of incubation in teratozoospermic samples.
Results and discussion
Semen characteristics included in this study were reported in . Supplementation with 1, 5, and 10% of PRGF significantly improved the progressive motility compared to the control group (p < 0.0001). 1% PRGF also preserved sperm total motility after incubation (). Statistical analysis also showed that sperm viability after incubation in control, 5, and 10% PRGF groups was significantly lower than 1% PRGF. Motility is one of the essential features of spermatozoa that typically is the first diagnostic marker for evaluating sperm quality. Previous studies have shown a correlation between increased ROS formation and reduction in sperm quality (Sharma et al. Citation1999; Saleh and Agarwal Citation2002). The association between decreased sperm motility and viability and a high level of ROS probably may be due to a cascade of events that inhibit the glycolysis pathway and production of adenosine triphosphate, increased membrane permeability, enzyme dysfunction, decreased axonemal protein phosphorylation, and inactivation of some biochemical pathways (Selley et al. Citation1991; de Lamirande and Gagnon Citation1992; Saleh and Agarwal Citation2002). The results of present study were in line with our previous study that showed sperm cryopreservation with 1% PRGF improved sperm total motility and viability which may be mainly due to the multiple biologically active PRGF ingredients (Mirzaei et al. Citation2022). Increased phosphorylation of the FGF receptor (FGFR) in sperm flagella and activation of kinase signaling pathways upon exposure to FGF2 is regulated by extracellular signaling (ERK) and protein kinase B (PKB or Akt), which increases sperm motility. Interestingly, all responses were eliminated by sperm pre-incubation with a BGJ398 (a potent and selective pan-FGFR antagonist) (Saucedo et al. Citation2015). In addition, Azúa et al. (Citation2017) reported that incubating normozoospermic samples with rFGF2 for 30 min significantly improved sperm motility. VEGF's receptor has been found in spermatids, seminal plasma, Sertoli cells, and Leydig cells (Obermair et al. Citation1999). It was shown that incubation of human spermatozoa, from fertile men, with VEGF has a positive effect on sperm motility in a concentration-dependent manner in vitro (Iyibozkurt et al. Citation2009). Tohidnezhad et al. (Citation2014) showed that VEGF prevents oxidative damage by activating the nuclear factor erythroid 2-related factor 2 (Nrf2) pathway. Recently, Asimakopoulos et al. (Citation2021) reported that incubating human spermatozoa with IGF-I improved sperm motility and viability while the effect on sperm viability was dose-dependent. However, our results were not concentration-dependent and showed that with increasing concentration, the sperm quality decreased which may be explained by impairment in redox balance (Symeonidis et al. Citation2021). Most spermatozoa had multiple defects. Head defects, mid-piece, tail, and excess residual cytoplasm had the highest ratio, respectively (). Normal morphology of spermatozoa after incubation, especially in the control group, showed a significant decrease (p < 0.0001) compared to before incubation. However, the results showed no significant differences in sperm morphology between the control and PRGF groups (). Also, different types of morphological defects were the same among other groups () (). It was shown that density gradient centrifugation (DGC) could improve sperm’s normal morphology. Still, when the rate of abnormal morphology is very high (like in teratozoospermia), this method cannot eliminate all abnormal spermatozoa (Claassens et al. Citation1996). After DGC, there was no change in spermatozoa with cytoplasmic droplets and immature cells in severe teratozoospermia (Hall et al. Citation1995). In line with our results, Bader et al. (Citation2020) also found that sperm incubation with PRP could not improve sperm morphology. Our data showed that sperm morphology may not affect by a short incubation with PRGF and it only can prevent detrimental effects of teratozoospermia.
Figure 1. Evaluation of sperm morphology using Papanicolaou staining method. (A) Morphology of spermatozoa after incubation in the control group; a) small head, b) thick mid-piece, c) mid-piece and acrosome abnormality, (B) Morphology of spermatozoa after incubation in the 1% PRGF group; a) doubled tail, b) thick mid-piece, c) excess residual cytoplasm, (C) Morphology of spermatozoa after incubation in the 5% PRGF group; a) thick mid-piece, b) excess residual cytoplasm, c) amorphous head, (D) Morphology of spermatozoa after incubation in the 10% PRGF group; a) low acrosome area and coiled tail, b) coiled tail, c) thick mid-piece, (E) Morphology of spermatozoa in raw semen, a) excess residual cytoplasm, b) coiled tail, c) small head (×1,000).
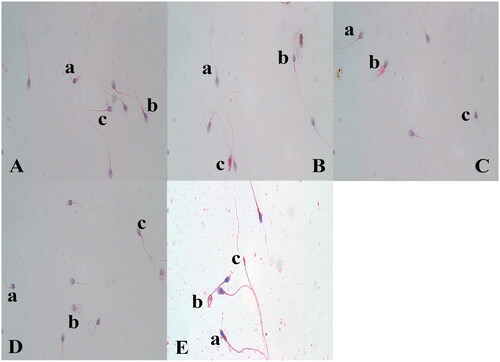
Table 1. Semen characteristics included in this study.
Table 2. Comparison of sperm parameters (mean ± SD) before and after incubation between different groups.
Table 3. Different types of morphological abnormalities between different groups.
The stability of DNA is a crucial factor in ensuring embryo development, and an increase in DNA fragmentation rate is related to adverse reproductive outcomes such as miscarriage (Coughlan et al. Citation2015). DNA fragmentation in teratozoospermia was higher than in normozoospermia. The samples can be linked to excessive ROS generation by teratozoospermia (Said et al. Citation2005a; Citation2005b). Our results indicated that DNA fragmentation increased dramatically in the control group and PRGF could protect DNA integrity, but the difference was not significant () (). In line with our findings, Yan et al. (Citation2021) reported that a slight reduction of DNA fragmentation was observed in post-thawed sperm supplemented with PRP, but the difference was not significant. Recently, Mirzaei et al. (Citation2022) reported that supplementing freezing medium with 1% PRGF significantly improved DNA integrity. It has been shown that sperm incubation with IGF-1, a factor included in PRGF, leads to a significant decrease in DNA fragmentation (Susilowati et al. Citation2015). The molecular mechanism of IGF-1 occurs through the regulation of the mitochondrial cytochrome c/caspase pathway (Li et al. Citation2003). In addition, Bader et al. (Citation2020) showed that in both non-stressed and stressed conditions (caused by hydrogen peroxide (H2O2)), incubation with 2% PRP significantly improved human sperm motility and viability, decreased DNA fragmentation, vacuolization, and ROS-positive cells. They found that in stress conditions, the results had a stronger significance. Perhaps one of the reasons that PRGF failed to improve DNA integrity, in our study, is that the extent of DNA damage in teratozoospermia caused by ROS is more significant than in normozoospermia due to abnormal morphology, and PRGF could not affect the double-stranded structure of DNA.
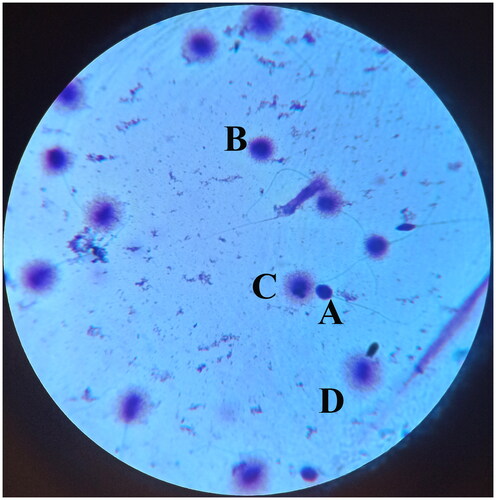
Figure 2. Sperm DNA fragmentation was evaluated with sperm chromatin dispersion test. In this test, spermatozoa without halos and with small halos are considered abnormal with fragmented DNA. Spermatozoa with medium and large halos are considered normal with intact DNA. (A) Spermatozoon without halo, (B) Spermatozoon with small halo, (C) Spermatozoon with medium halo, (D) Spermatozoon with large halo (×1,000).
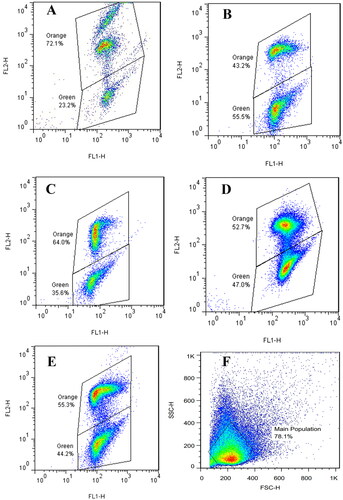
As shown in , sperm MMP decreased significantly after incubation only in the control group compared to before incubation (p < 0.01) and PRGF addition tended to restore MMP compared to the control group () due to its antioxidant activity. According to previous studies, mitochondrial activity is closely related to sperm motility (Martinez-Pastor et al. Citation2004; Espinoza et al. Citation2009). The positive effect of IGF-1 on the membrane integrity of the sperm has been shown previously (Padilha et al. Citation2012). Also, adding PRP had no beneficial effects on sperm MMP during cryopreservation which Yan et al. (Citation2021) reported may be due to fewer antioxidant properties. Due to the maintenance of MMP during 1 h of incubation, the rate of improvement may be increased with increasing incubation time. Our study showed that MDA levels increased in the control group after incubation compared to before incubation. Also, a significant difference was observed between the control group with 1, 5, and 10% PRGF (). MDA, a specific marker of lipid peroxidation, is applied as an indicator of oxidative stress. Previous studies have reported an increased level of ROS production in teratozoospermia (Aziz et al. Citation2004; Keshtgar et al. Citation2012). ROS production is associated with lipid peroxidation. ROS is a weaker predictor of spermatozoa function than lipid peroxidation. Lipid peroxidation is a clinical test of spermatozoa quality and considered as an indirect technique to measure oxidative stress (Williams and Ford Citation2005; Symeonidis et al. Citation2021). Similar to our results, Fanaei et al. (Citation2014) showed that in the prepared teratozoospermic samples, after 1 h of incubation, the MDA level increased, and the addition of ascorbic acid to the incubation medium prevented the further increase of MDA concentration. Several studies have shown that MDA levels negatively correlate with spermatozoa quality and are significantly higher in the seminal plasma of infertile men (Collodel et al. Citation2015; Dorostghoal et al. Citation2017). Recently, Palani and Alahmar (Citation2020) reported no significant difference in MDA levels between infertile and fertile groups. One explanation for the difference in the results of the previous studies is the dissimilarity in patient selection, methods used to assess oxidative stress, and genetic factors. Further studies are estimating additional sperm parameters such as ROS level and molecular mechanisms of PRGF effects on sperm quality and function to help the clinicians to improve in vitro fertilization (IVF) results. One of the challenges in infertility treatment is facing to teratozoospermia. PRGF as a main source of growth factors could open a new era of treatment in different aspects of assisted reproductive technology. Our results indicated that PRGF could significantly maintain sperm motility, viability, DNA integrity, and MMP in teratozoospermic samples. Further studies are needed to evaluate the clinical application of PRGF to improve the fertilizing ability of teratozoospermic samples.
Figure 3. Dot plots from JC-1 staining of spermatozoa in different groups. Effects of incubation of different PRGF dosages (1%, 5%, and 10%) on mitochondrial membrane potential. After incubation with spermatozoa, JC-1 staining method was used and the samples were read by flowcytometry. The fluorescence response for orange spermatozoa (high mitochondrial membrane potential); and green spermatozoa (low mitochondrial membrane potential). (A) After density gradient centrifugation, (B) control, (C) 1% PRGF, (D) 5% PRGF, (E) 10% PRGF, (F) Forward scatter (FSC) intensity and side scatter (SSC) intensity plot.
Materials and methods
Study population
Twenty-five human teratozoospermic semen samples from patients (20–45 years, mean ± SD: 34.75 ± 6.8) referred to the IVF clinics of Gandhi and Akbarabadi hospitals were assessed in this study. All samples had less than 4% normal sperm morphology. All patients with varicocele and a history of using drugs, tobacco, alcohol, or vitamins were excluded from this study. Unless noted, all chemicals were purchased from Sigma-Aldrich (Steinheim, Germany).
Preparation of semen samples
Semen samples were collected into sterile plastic containers after 3–5 days of abstinence and were incubated at 37 °C for 30 min to allow liquefaction. Semen samples were prepared using the density gradient technique according to WHO guidelines (World Health Organization Citation2010). Briefly, the density gradient medium (SpermGrad, Vitrolife, Göteborg, Sweden) was prepared by layering 1 mL of 40% (v/v) over the 1 mL of 80% (v/v) medium, and 1 mL of semen sample was added over the layers mentioned above. Then, the centrifugation was performed at 300–400 g for 15 min. After centrifugation, most of the supernatant from the sperm pellet was gently removed and the pellet was resuspended in 5 mL of the supplemented medium by gentle pipetting. Two centrifugations were performed (200 g for 5 min) and the final pellet was resuspended in sperm medium by gentle pipetting. Spermatozoa were incubated in the presence or absence of PRGF (concentrations of 1, 5, and 10%) to determine the effect of PRGF on sperm parameters (Mirzaei et al. Citation2022). After 1 h of incubation at 37 °C, sperm parameters were assessed.
PRGF preparation
Briefly, blood samples were collected from 5 healthy male donors (27–40 years, mean ± SD: 34. 5 ± 4.4) with no history of viral infection during the last six months and screened for blood-borne viruses like hepatitis B, hepatitis C, human immunodeficiency virus, cytomegalovirus, and Epstein–Barr. The samples were mixed with 3.8% sodium citrate in a tube. At 580 g for 15 min at room temperature, the entire plasma layer was separated after initial centrifugation. 10% calcium chloride was added to the plasma and incubated for 30 min at 37 °C. Then, the released supernatants were collected and incubated at 56 °C for 60 min. Finally, the plasma was filtered, aliquoted, and stored at −80 °C (Mirzaei et al. Citation2022).
Sperm motility, viability, and morphology
Sperm motility was evaluated via the CASA (Version: 2.6.2) (ETC CASA, Emerging Technology of Century, Tehran, Iran) (World Health Organization Citation2010). The criterion for considering a spermatozoon as progressive was linearity ≥0.45 µm. The camera (Mshot MD30, Guangzhou, China) speed was 30 frames recorded per second. Eosin-nigrosin staining was used to evaluate sperm viability. At least 200 spermatozoa were examined under a light microscope (Labomed (CxL), CA, USA), magnification: ×1,000). Sperm morphology was evaluated using the Papanicolaou staining method (World Health Organization Citation2010). The prepared slides were reviewed by bright-field light microscopy (Labomed (CxL), CA, USA), magnification: ×1,000). The spermatozoa were checked for head, neck, mid-piece, and tail abnormalities. Two expert operators blindly evaluated at least 200 spermatozoa, and the rate of normal morphology was presented as a percentage.
Sperm DNA fragmentation
SCD test was used to evaluate spermatozoa DNA fragmentation. About 50–60 µL of the sperm/agarose mixture was placed on a slide covered with agarose (0.65%). The coverslip was removed after 5 min, and the drops were kept in 0.08% HCl solution for 7 min. The slides were immersed in the lysing solution (distilled water, sodium dodecyl sulfate, NaCl, Tris-HCl, EDTA, and mercaptoethanol) for 25 min and washed in distilled water for 5 min. The slides were then dehydrated with 70, 90, and 99.7% ethanol, and finally, they were stained with a Wright solution. At least 200 spermatozoa were evaluated with light microscopy (Labomed (CxL), CA, USA), magnification: ×1,000). In this test, the spermatozoa with medium and large haloes are normal, and spermatozoa with a slight halo and no halo are considered abnormal (Najafi et al. Citation2019).
Sperm mitochondrial membrane potential
JC-1 was diluted with 0.5 mL dimethyl sulfoxide, aliquoted, and preserved at −20 °C. For each sample, 1 µmol of JC-1 was added to 1 mL of sperm suspension containing 1 × 106–2 × 106 cells and incubated for 15 min at 37 °C. After washing with phosphate buffer saline (PBS) and centrifugation, the spermatozoa were suspended again with 300 µL of PBS and evaluated using a flow cytometer (BD FACSCalibur, BD biosciences, San Jose, CA, USA) (Uribe et al. Citation2017). Forward and side scatter was used to separate the gated population of spermatozoa from other cells.
Sperm malondialdehyde
Lipid peroxidation in seminal plasma was analyzed using the thiobarbituric acid test. After preparation, the sperm concentration reached 10 × 106/mL. 50 µL samples were added to a 50 µL reagent. Then, 1 mL of chromogenic solution was added to the mixture and heated at 95 °C in a warm bath for 1 h. After cooling the mix, the samples were centrifuged at 3000–4000 g for 15 min. At a wavelength of 530–540 nm, the MDA level of supernatant was measured by a spectrophotometer and reported as nmol/mL (Suleiman et al. Citation1996; Agarwal et al. Citation2009).
Statistical analysis
The data are expressed as mean ± SD. The Shapiro‐Wilk test evaluated the normality of data. If the distribution of data was normal, one way‐ANOVA followed by the Tukey test was used to compare the parameters between different groups. Kruskal-Wallis performed the non-parametric analysis with the Dunn as a post-test. The level of significance was considered as p < 0.05.
Ethical approval
This study was approved by the ethics committee of Iran University of Medical Sciences (IR.IUMS.FMD.REC.1399.613). Informed consent was obtained from individuals participating in this research.
Authors’ contributions
Developed the project: IH, YA, FSA; collected the data: HGN; analysed the data: IH, YA; prepared the manuscript: HGN; wrote the manuscript: YA, FSA, IH; proofread the manuscript: YA, IH.
| Abbreviations | ||
| PRGF | = | plasma-rich in growth factors |
| MDA | = | malondialdehyde |
| ROS | = | reactive oxygen species |
| EGF | = | epidermal growth factor |
| PDGF | = | platelet-derived growth factor |
| VEGF | = | vascular endothelial growth factor |
| TGFβ | = | transforming growth factor-beta |
| HGF | = | hepatocyte growth factor |
| FGF | = | fibroblast growth factor |
| IGF-1 | = | and insulin-like growth factor |
| PRP | = | platelet-rich plasma |
| MMP | = | mitochondrial membrane potential |
| DGC | = | density gradient centrifugation |
| FGFR | = | FGF receptor |
| ERK | = | extracellular signal-regulated kinase |
| PKB | = | protein kinase B |
| IVF | = | in vitro fertilization |
| SCD | = | sperm chromatin dispersion |
| SD | = | standard deviation |
| SEM | = | standard error of mean |
| PBS | = | phosphate buffer saline |
| VCL | = | curvilinear velocity |
| VSL | = | straight line velocity |
| VAP | = | average path velocity |
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data of this study are available from the corresponding authors, upon reasonable request.
Additional information
Funding
References
- Agarwal A, Tvrda E, Sharma R. 2014. Relationship amongst teratozoospermia, seminal oxidative stress and male infertility. Reprod Biol Endocrinol. 12(1):45.
- Agarwal A, Varghese AC, Sharma RK. 2009. Markers of oxidative stress and sperm chromatin integrity. In: Park-Sarge OK, Curry TE, editors. Molecular endocrinology: methods and protocols. Totowa, NJ: Humana Press. p. 377–402.
- Agarwal A, Virk G, Ong C, Du Plessis SS. 2014. Effect of oxidative stress on male reproduction. World J Mens Health. 32(1):1–17.
- Amidi F, Pazhohan A, Shabani Nashtaei M, Khodarahmian M, Nekoonam S. 2016. The role of antioxidants in sperm freezing: a review. Cell Tissue Bank. 17(4):745–756.
- Anitua E, Muruzabal F, Tayebba A, Riestra A, Perez VL, Merayo-Lloves J, Orive G. 2015. Autologous serum and plasma rich in growth factors in ophthalmology: preclinical and clinical studies. Acta Ophthalmol. 93(8):e605–e614.
- Anitua E, Orive G. 2012. Endogenous regenerative technology using plasma- and platelet-derived growth factors. J Control Release. 157(3):317–320.
- Anitua E, Pascual C, Pérez-Gonzalez R, Antequera D, Padilla S, Orive G, Carro E. 2013. Intranasal delivery of plasma and platelet growth factors using PRGF-Endoret system enhances neurogenesis in a mouse model of Alzheimer’s disease. PLoS One. 8(9):e73118.
- Anitua E, Sánchez M, Orive G, Andia I. 2008. Delivering growth factors for therapeutics. Trends Pharmacol Sci. 29(1):37–41.
- Asimakopoulos B, Tiptiri-Kourpeti A, Metallinou C. 2021. IGF-I and NGFβ enhance in vitro progressive motility and vitality of human spermatozoa. Reprod Med Biol. 20(3):361–367.
- Aziz N, Saleh RA, Sharma RK, Lewis-Jones I, Esfandiari N, Thomas AJ, Jr., Agarwal A. 2004. Novel association between sperm reactive oxygen species production, sperm morphological defects, and the sperm deformity index. Fertil Steril. 81(2):349–354.
- Azúa D, Saucedo L, Giordana s, Magri M, Buffone M, Neuspiller F, Vazquez-Levin M, Marín-Briggiler C. 2017. Fibroblast growth factor 2 (FGF2) is present in human spermatozoa and is related with sperm motility. The use of recombinant FGF2 to improve motile sperm recovery. Andrology. 5(5):990–998.
- Bader R, Ibrahim JN, Moussa M, Mourad A, Azoury J, Azoury J, Alaaeddine N. 2020. In vitro effect of autologous platelet-rich plasma on H(2) O(2) -induced oxidative stress in human spermatozoa. Andrology. 8(1):191–200.
- Bui AD, Sharma R, Henkel R, Agarwal A. 2018. Reactive oxygen species impact on sperm DNA and its role in male infertility. Andrologia. 50(8):e13012.
- Claassens OE, Kaskar K, Coetzee K, Lombard CJ, Franken DR, Kruger TF. 1996. Comparison of motility characteristics and normal sperm morphology of human semen samples separated by percoll density gradient centrifugation. Arch Androl. 36(2):127–132.
- Cocuzza M, Sikka SC, Athayde KS, Agarwal A. 2007. Clinical relevance of oxidative stress and sperm chromatin damage in male infertility: an evidence based analysis. Int Braz J Urol. 33(5):603–621.
- Collodel G, Moretti E, Micheli L, Menchiari A, Moltoni L, Cerretani D. 2015. Semen characteristics and malondialdehyde levels in men with different reproductive problems. Andrology. 3(2):280–286.
- Coughlan C, Clarke H, Cutting R, Saxton J, Waite S, Ledger W, Li T, Pacey AA. 2015. Sperm DNA fragmentation, recurrent implantation failure and recurrent miscarriage. Asian J Androl. 17(4):681–685.
- de Lamirande E, Gagnon C. 1992. Reactive oxygen species and human spermatozoa. I. Effects on the motility of intact spermatozoa and on sperm axonemes. J Androl. 13(5):368–378.
- Dorostghoal M, Kazeminejad SR, Shahbazian N, Pourmehdi M, Jabbari A. 2017. Oxidative stress status and sperm DNA fragmentation in fertile and infertile men. Andrologia. 49(10):e12762.
- Espinoza JA, Schulz MA, Sánchez R, Villegas JV. 2009. Integrity of mitochondrial membrane potential reflects human sperm quality. Andrologia. 41(1):51–54.
- Fanaei H, Khayat S, Halvaei I, Ramezani V, Azizi Y, Kasaeian A, Mardaneh J, Parvizi MR, Akrami M. 2014. Effects of ascorbic acid on sperm motility, viability, acrosome reaction and DNA integrity in teratozoospermic samples. Iran J Reprod Med. 12(2):103–110.
- Hall JA, Fishel SB, Timson JA, Dowell K, Klentzeris LD. 1995. Human sperm morphology evaluation pre- and post-Percoll gradient centrifugation. Hum Reprod. 10(2):342–346.
- Iyibozkurt AC, Balcik P, Bulgurcuoglu S, Arslan BK, Attar R, Attar E. 2009. Effect of vascular endothelial growth factor on sperm motility and survival. Reprod Biomed Online. 19(6):784–788.
- Keshtgar S, Fanaei H, Bahmanpour S, Azad F, Ghannadi A, Kazeroni M. 2012. In vitro effects of α-tocopherol on teratozoospermic semen samples. Andrologia. 44 (Suppl 1):721–727.
- Li Y, Higashi Y, Itabe H, Song YH, Du J, Delafontaine P. 2003. Insulin-like growth factor-1 receptor activation inhibits oxidized LDL-induced cytochrome C release and apoptosis via the phosphatidylinositol 3 kinase/Akt signaling pathway. Arterioscler Thromb Vasc Biol. 23(12):2178–2184.
- Martinez-Pastor F, Johannisson A, Gil J, Kaabi M, Anel L, Paz P, Rodriguez-Martinez H. 2004. Use of chromatin stability assay, mitochondrial stain JC-1, and fluorometric assessment of plasma membrane to evaluate frozen-thawed ram semen. Anim Reprod Sci. 84(1-2):121–133.
- Mirzaei J, Movahedin M, Halvaei I. 2022. Plasma-rich in growth factors ameliorates detrimental effects of cryopreservation on human sperm. Cell J. 24(6):330–336.
- Najafi L, Halvaei I, Movahedin M. 2019. Canthaxanthin protects human sperm parameters during cryopreservation. Andrologia. 51(10):e13389.
- Obermair A, Obruca A, Pöhl M, Kaider A, Vales A, Leodolter S, Wojta J, Feichtinger W. 1999. Vascular endothelial growth factor and its receptors in male fertility. Fertil Steril. 72(2):269–275.
- Oborna I, Fingerova H, Novotny J, Brezinova J, Svobodova M, Aziz N. 2009. Reactive oxygen species in human semen in relation to leukocyte contamination. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 153(1):53–57.
- Ozer OF, Akbulut H, Guler EM, Caglar HG, Gevher F, Koktasoglu F, Selek S. 2019. Oxidative stress and phenotype frequencies of paraoxonase-1 in teratozoospermia. Andrologia. 51(8):e13299.
- Padilha RT, Magalhães-Padilha DM, Cavalcante MM, Almeida AP, Haag KT, Gastal MO, Nunes JF, Rodrigues APR, Figueiredo JR, Oliveira MAL. 2012. Effect of insulin-like growth factor-I on some quality traits and fertility of cryopreserved ovine semen. Theriogenology. 78(4):907–913.
- Palani A, Alahmar A. 2020. Impact of oxidative stress on semen parameters in normozoospermic infertile men: a case–control study. African J Urol. 26(1):50.
- Said TM, Agarwal A, Sharma RK, Thomas AJ, Jr., Sikka SC. 2005a. Impact of sperm morphology on DNA damage caused by oxidative stress induced by beta-nicotinamide adenine dinucleotide phosphate. Fertil Steril. 83(1):95–103.
- Said TM, Aziz N, Sharma R, Lewis-Jones I, Thomas AJ, Jr, Agarwal A. 2005b. Novel association between sperm deformity index and oxidative stress-induced DNA damage in infertile male patients. Asian J Androl. 7(2):121–126.
- Saleh RA, Agarwal A. 2002. Oxidative stress and male infertility: from research bench to clinical practice. J Androl. 23(6):737–752.
- Saucedo L, Buffa GN, Rosso M, Guillardoy T, Góngora A, Munuce MJ, Vazquez-Levin MH, Marín-Briggiler C. 2015. Fibroblast growth factor receptors (FGFRs) in human sperm: expression, functionality and involvement in motility regulation. PloS One. 10(5):e0127297. eCollection 2015.
- Selley M, Lacey M, Bartlett M, Copeland C, Ardlie N. 1991. Content of significant amounts of a cytotoxic end-product of lipid peroxidation in human semen. J Reprod Fertil. 92(2):291–298.
- Sharma RK, Pasqualotto FF, Nelson DR, Thomas AJ, Agarwal A. 1999. The reactive oxygen species-total antioxidant capacity score is a new measure of oxidative stress to predict male infertility. Hum Reprod. 14(11):2801–2807.
- Suleiman SA, Ali ME, Zaki ZM, el-Malik EM, Nasr MA. 1996. Lipid peroxidation and human sperm motility: protective role of vitamin E. J Androl. 17(5):530–537.
- Susilowati S, Triana IN, Malik A. 2015. The effects of insulin-like growth factor I (IGF-I) complex from seminal plasma on capacitation, membrane integrity and DNA fragmentation in goat spermatozoa. Asian Pac J Reprod. 4(3):208–211.
- Symeonidis EN, Evgeni E, Palapelas V, Koumasi D, Pyrgidis N, Sokolakis I, Hatzichristodoulou G, Tsiampali C, Mykoniatis I, Zachariou A, et al. 2021. Redox Balance in Male Infertility: excellence through Moderation-"Μέτρον. ἄριστον". Antioxidants. 10(10):1534.
- Tohidnezhad M, Wruck CJ, Slowik A, Kweider N, Beckmann R, Bayer A, Houben A, Brandenburg LO, Varoga D, Sönmez TT, et al. 2014. Role of platelet-released growth factors in detoxification of reactive oxygen species in osteoblasts. Bone. 65:9–17.
- Uribe P, Villegas JV, Boguen R, Treulen F, Sánchez R, Mallmann P, Isachenko V, Rahimi G, Isachenko E. 2017. Use of the fluorescent dye tetramethylrhodamine methyl ester perchlorate for mitochondrial membrane potential assessment in human spermatozoa. Andrologia. 49(9):e12753.
- Venkatesh S, Singh G, Gupta NP, Kumar R, Deecaraman M, Dada R. 2009. Correlation of sperm morphology and oxidative stress in infertile men. Int J Reprod Biomed. 7(1):29–34.
- Walczak-Jedrzejowska R, Wolski JK, Slowikowska-Hilczer J. 2013. The role of oxidative stress and antioxidants in male fertility. Cent European J Urol. 66(1):60–67.
- Williams AC, Ford WCL. 2005. Relationship between reactive oxygen species production and lipid peroxidation in human sperm suspensions and their association with sperm function. Fertil Steril. 83(4):929–936.
- World Health Organization 2010. WHO laboratory manual for the examination and processing of human semen. Geneva: World Health Organization.
- Yan B, Zhang Y, Tian S, Hu R, Wu B. 2021. Effect of autologous platelet-rich plasma on human sperm quality during cryopreservation. Cryobiology. 98:12–16.
