Abstract
A chemical pathway combining reverse water gas shift, Fischer‐Tropsch synthesis and hydro‐cracking was considered to re‐synthesise jet fuel from CO2 captured at high purity by oxy‐fuelling of a typical coal‐fired power station (Drax, UK). The oxygen for oxy‐fuelling and hydrogen for the fuel re‐synthesis process are sourced by electrolysis of water. According to material and energy balances , 3.1 MT/year of jet fuel and 1.6 MT/year each of gas oil and naphtha can be produced from the Drax annual emissions of 20 MT of CO2, sufficient to supply 23% of the UK jet fuel requirements. The overall re‐synthesis requires 16.9 GW, to be sourced renewably from (offshore) wind power, and releases 4.4 GW of exothermic energy giving scope for improvements via process integration. The energy re‐synthesis penalty was 82% ideally and 95% on a practical basis. With the cost of offshore wind power predicted to reduce to 2.0 p/kWh by 2020, this ‘re‐syn’ jet fuel would be competitive with conventional jet fuel, especially if carbon taxes apply. The re‐use of CO2 sequestrated from coal power stations to form jet‐fuel would halve the combined CO2 emissions from the coal power and aviation sectors.
1. Introduction
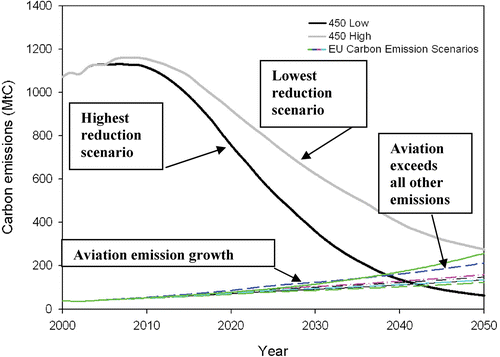
Emissions of CO2 have been linked to the greenhouse effect and global warming. Many countries worldwide are therefore examining ways to reduce their CO2 emissions. In the UK, the proposed Climate Change Bill is intended to legislate for a system to establish a credible emissions reduction pathway to 2050, with the UK's existing 60% target for 2050 placed in statute (Draft Climate Change Bill Citation2007). The EU has set a maximum allowable global atmospheric temperature rise by 2050 of 2°C. This is equivalent to an atmospheric CO2 concentration of 450 ppmv. Current estimations of the growth of the EU aviation industry predict that, by 2050, the CO2 emissions from aviation will by themselves exceed the necessarily reduced emission rates from all other EU sources that would require to be achieved to deliver such a 450 ppm target (Bows and Anderson Citation2007). This outcome is shown schematically in Figure for several aviation growth rates.
As a result of this ‘business as usual’ scenario, in the face of no apparent solution, the aviation industry is subjected to hostility and appeals for flying to be restricted. Any technical solution that was put forward would, in order to be acceptable, probably need to satisfy two key aviation industry criteria, at least in the medium term. The first is the established existence of a mature jet‐fuel supply chain infra‐structure that would be very expensive to replace. Second, present next‐generation aircraft (like the Airbus A380 super‐jumbo and the Boeing 787 Dreamliner) represents a long term 50‐year investment in current technology, albeit that these new designs will deliver some improvement in CO2 emissions per passenger km.
Re‐synthesising jet fuel from captured CO2 to give a net carbon‐neutral fuel offers a way to radically reduce the environmental impact of CO2 emissions from the aircraft industry whilst meeting both of these two key criteria. This in turn would provide a breathing space in which to innovate new aircraft zero‐emission propulsion technologies. Currently SASOL in South Africa produces synthetic jet fuel via the gasification of coal followed by Fischer‐Tropsch synthesis (FTS) (Dry Citation2001), where the fuel produced is of high quality with negligible contaminants. This fuel is moreover fully compatible with current aircraft designs. Therefore, there is a clear practical possibility of producing jet‐fuel from captured CO2 to give ‘net’ carbon‐neutral flying.
The Drax coal‐fired power station in Yorkshire is the largest single industrial emitter of CO2 in the UK. The annual emissions from Drax are 20 million tonnes of CO2 (Drax Power Ltd Citation2005); this is considered here as a suitable and convenient source of CO2 for a jet fuel re‐synthesis plant. The re‐synthesis process proposed uses H2 and O2 sourced from abundant water. The energy required for electrolysis can be provided renewably from wind power. This CO2 to jet fuel process involves the reverse water gas shift reaction to produce syngas, and subsequent Fischer‐Tropsch synthesis to form liquid hydrocarbon fuels.
Material and energy balances for the Drax emissions will be evaluated over the proposed re‐synthesis process with the subsequent calculation of an energy re‐synthesis penalty, η. This quantifies the excess energy that must be supplied to re‐constitute the fuel starting from sequestrated CO2. These process evaluations then permit an economic appraisal whereby the cost of re‐synthesising jet‐fuel can be compared with that conventionally sourced from crude oil.
2. Re‐synthesis chemical pathway
Recent research has considered carbon‐neutral automobiles (Mann Citation2006) based on re‐synthesis of gasoline. However, the possibility of synthesising jet‐fuel from CO2 for aviation has not so far been considered.
2.1 Oxy‐fuelling for capture of high purity CO2
The CO2 produced from conventional coal‐fired power stations can be captured at high purity via oxy‐coal combustion (Riemer Citation1993, Veranth and Krishnamoorthy Citation2001), where the pulverised coal is burnt in an appropriately managed combustion environment being fed with pure oxygen instead of air. This can result in a flue gas containing over 90% CO2. However, the flame temperature in such an oxy‐coal furnace is potentially much higher than a standard air‐coal furnace. To adjust the flame temperature to that conventionally encountered in normal combustion with air, some of the flue gas can be externally recycled and/or internally re‐draughted back within the furnace to dilute the make‐up oxygen whilst still producing a flue gas with a very high CO2 content. This eliminates the complication of N2 dilution which is unavoidable and highly detrimental if air is used for combustion. Moreover, the separation problems associated with highly diluted CO2 are completely avoided, so that the resulting high purity CO2 stream is now conveniently available as a raw material for further chemical reaction(s) along a re‐synthesis path. A further benefit of oxy‐fuelling is that by avoiding the co‐flow of a large amount of nitrogen it creates zero‐emission operation of the power plant, thereby eliminating local nuisances from smoke, grit and fumes. Finally, it should be noted that the pure oxygen, co‐produced in the electrolysis to provide hydrogen, facilitates the oxy‐fuelling operation at no significant extra cost.
2.2 Reverse‐water‐gas shift
This CO2 is then converted into syngas, a mixture of CO and H2, by reducing the CO2 with an excess of H2 via the reverse water gas shift (rWGS) reaction:
2.3 Fischer‐Tropsch synthesis (FTS)
The syngas produced by the rWGS reactor is then fed forward into FTS where it is catalytically converted into liquid hydrocarbons with an approximate stoichiometry:
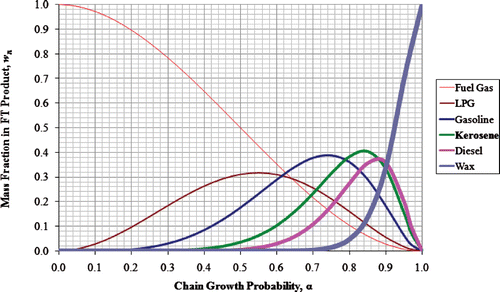
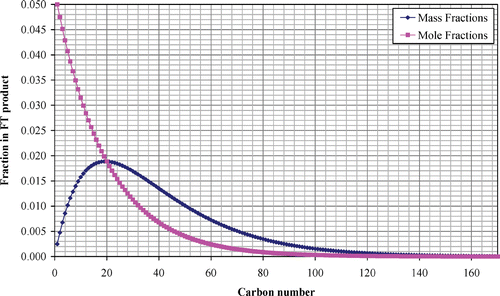
Using a chain growth probability of 0.95 [the highest severity value that has been used in an industrial FTS process (Eilers et al. Citation1990)], produces the product distribution as a function of carbon number shown in Figure . The majority of the products by weight are waxes with carbon numbers in the range 20 to 30. Conversley, on a molar basis, the majority of the products are light ends with around 5% methane. This FTS product can be broken down into three classes with carbon numbers in the range less than 10, between 10 and 20, and greater than 20. These ranges/fractions by mole and mass are shown in Table . These ranges are comparable with SMDS results, with a majority of waxes with carbon number greater than 20 which require to be hydro‐cracked back into the middle distillate range.
Table 1. Fischer‐Tropsch synthesis product distribution with AFS kinetics for a severity factor of 0.95.
The water produced by the rWGS and FTS reactions can be separated from the hydrocarbons by simply cooling the FTS product and decanting. This also allows the separation of the small amount of gaseous hydrocarbons which contain less than four carbon atoms per molecule; these light ends can be used on‐site as a fuel source or sold as carbon‐neutral fuel gases.
2.4 Hydrocracking back to middle distillates
During hydro‐cracking the long‐chained n‐paraffin waxes are cracked in the presence of H2 into shorter chained iso‐paraffins which lie in the desired boiling range. At the same time, the middle distillate n‐paraffins are hydro‐isomerised into iso‐paraffins, which improves their cold‐flow properties making them better transportation fuels.
The results for the estimated product distribution after hydro‐cracking are shown in Table in accordance with experimental measurements (Sie et al. Citation1991). It can be seen from this product spectrum that the desired product jet fuel (kerosene) makes up the majority of the hydro‐cracked product on both a weight and molar basis but with significant amounts of naphtha and gas oil also co‐produced. The naphtha can be used as a chemical feedstock or further processed into gasoline to fuel automobiles. The gas oil can be used industrially as a fuel or processed into diesel again as an automotive fuel. This means that the sellable portion of the hydro‐cracked product consists of 98.4% of the product by mass and 94.4% by mol. Thus in kerosene mode, a hydro‐cracker can produce 50% jet fuel and 25% of both naphtha and gas oil by weight. In practice the severity can be adjusted to give flexibility to the re‐syn fuel product spectrum, which would allow the re‐synthesis plant to be tuned to match the demands of individual fuel markets. After being hydro‐cracked, the re‐syn fuels are then recovered in a process involving flash drums and fractionation, similar to a conventional crude distillation.
Table 2. Distribution of re‐synthesis plant products following hydrocracking and recovery.
2.5 Electrolysis to produce hydrogen and oxygen
Electrolysis is used to produce the H2 required for this re‐syn process and the O2 for the oxy‐fuel combustion. In this process an electrical current is used to split water molecules into H2 and O2, the two products being conveniently produced already separated at opposite electrodes:
In this scheme, the oxygen is no longer a waste by‐product, but directly facilitates the oxy‐fuelling of the coal combustion without the usual heavy cost penalty normally associated with prior separation of oxygen. Essentially, apart from relatively minor modifications to furnace draughting, the convenient recovery of high purity CO2 could be achieved almost without cost.
It might also be assumed that the use of water raises sustainability and cost issues. However, in the UK, water is plentiful and hence about two hundred times cheaper than the value of liquid hydrocarbons derived from fossil sources. Furthermore, the proposed re‐synthesis scheme represents perfectly sustainable water re‐use, since the hydrogen obtained by electrolysis returns to the atmosphere as water on combustion of the re‐synthesised fuel.
3. Material and energy balances for a coal‐fired power plant
3.1 Material balances
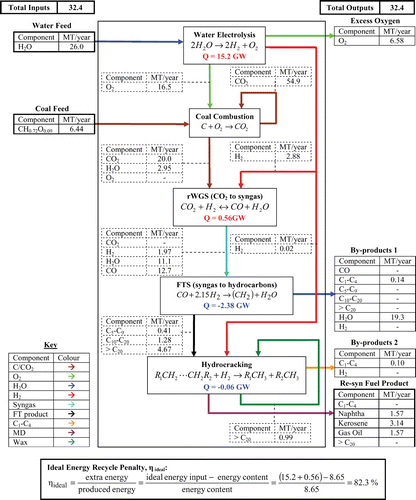
To determine the feasibility of re‐synthesising jet fuel from CO2, material and energy balances were performed over the re‐syn plant. The basis chosen was the annual CO2 emissions from Drax power station, which are around 20 MT/year. From this value the predicted annual production of jet fuel, naphtha and gas oil were calculated. The results of this material balance are shown in Figure , where the re‐syn fuel products are 3.14 MT/year of jet fuel (kerosene) and 1.57 MT/year of both gas oil and naphtha. The calculated yield on carbon from the CO2 for jet fuel is 48.9%. However, there is a high yield of 97.8% for the set of useful hydrocarbon fuels naphtha, kerosene and gas oil. The associated naphtha and gas oil fractions could be processed further into gasoline and diesel fuel respectively. This gives an overall mass yield to transport hydrocarbons of 97.8% based on the original carbon in the captured CO2.
The jet fuel produced would supply the UK aviation industry with sufficient fuel to fly 22.6% of the total distance travelled by air in the UK (UK Department for Transport Citation2006). To provide all the aircraft flying from the UK with carbon‐neutral jet fuel some 13.9 MT of fuel would be required, which would be equivalent to 88.6 MT of feedstock CO2.
3.2 Energy balances
The re‐synthesis chemical pathway was then analysed by an energy balance for two cases. The first ‘ideal’ case only considers the enthalpy changes of the chemical reactions along the re‐synthesis pathway according to Hess's Law. For the second case, a ‘practical’ energy balance was performed. The energy changes involved in the practical heating, cooling and distillation were considered as well as the chemical reaction energy changes. Therefore, the practical case will have a higher energy requirement than the ideal case, because of the irreversibility and non‐recovery losses. The ideal case is shown embedded within Figure . The total energy input requirement is equal to 15.8 GW with 2.44 GW of energy being released. For the practical case the total energy requirement was calculated to equal 16.9 GW with the release of 4.40 GW of energy, thereby providing scope for efficiency gains by energy integration.
4. Energy re‐synthesis penalty
The energy re‐synthesis penalty η is a measure of the extra energy that is required to re‐synthesise the hydrocarbon fuels from CO2 as a percentage of the energy content of the fuels themselves. Thus:
The energy content of all the re‐syn fuels is 8.65 GW. Thus, for the ideal case the re‐synthesis penalty, ηideal, is equal to 82.3%. This increases to 94.9% for the practical case, ηpractical. The practical value could be improved by process heat integration to reduce the required energy input into the re‐syn process. More exothermic heat energy is actually released by the FTS process than is required by the process pre‐heaters, therefore it should be possible by good process design to recover and integrate these heating and cooling duties. This would potentially result in the energy for the water electrolysis being the only required energy input to the process. In this case ηpractical would reduce down to 76%.
5. Economic evaluation
The predicted annual operating costs of the re‐synthesis plant were calculated which included the maintenance, labour and electricity costs of the plant. This allowed the prediction of the prices of the hydrocarbon fuels produced, to determine the feasibility of producing jet fuel, and other transport hydrocarbon fuels, from CO2.
5.1 Use of wind energy
The main operating cost of the re‐synthesis plant is the cost of the electricity to run the water electrolyser. This electricity is to be sourced renewably from wind power. The cost of generating electricity from wind power in the UK is currently quoted as 2.8 p/kWh onshore and 5.1 p/kWh offshore (British Wind Energy Association Citation2007). This cost is, however, expected to drop in the future due to technical improvements in turbine productivity, the development of much larger turbines and a reduction in the capital cost of the turbines themselves, due to economies of scale and mass production. The predicted 2020 prices of offshore and onshore wind energy (British Wind Energy Association Citation2007) are shown in Table , along with those for electricity produced via natural gas and coal for comparison. It is widely accepted that electricity sourced from modern wind power technology is, relative to conventional fossil fuels, able to provide energy services in a sustainable way and with low or virtually zero GHG emissions (Sims et al. Citation2003).
Table 3. Current and predicted electricity prices.
It can be seen that currently it is marginally cheaper to use energy sourced from fossil fuels. However, this would not produce carbon neutral jet fuel unless CO2 capture and storage were to be used, such as the (recently deferred) BP Peterhead project where natural gas was to be converted into CO2. This CO2 was subsequently to be captured and sequestrated underground, and the H2 then used to generate electricity. The cost of the electricity from the Peterhead project was predicted to be between 2.75 and 3.25 p/kWh, more expensive than electricity generated from fossil fuels (EAC Citation2006); this makes the cost equivalent to offshore wind power which, however, has the advantage of being renewable. On the other hand, by 2020 it will be more economical to use wind energy than fossil fuels, with onshore prices as low as 1.5 p/kWh and 2.0 p/kWh offshore (taking the most favourable case in Table with the ‘minus’ sign operating). The offshore price is also predicted to drop even further by 2050, with a smaller relative decrease in the price of onshore wind energy.
5.2 Price of re‐synthesised jet fuel
Using the prices of wind power, the cost of the re‐synthesised fuels was predicted using the required energy input of the re‐synthesis process. There are two cases for the re‐synthesis plant, the first case is when no heat integration is used in the plant giving an energy requirement of 16.9 GW. The second case is where heat integration is used in the plant, and due to the fact that more heat energy is produced than is required, the only energy input that is required is for the electrolysis of water. For this case where the energy efficiency of the plant is increased, the energy requirement is lower at 15.2 GW.
The predicted wholesale prices of the re‐synthesised naphtha, kerosene and gas oil were calculated for both energy cases in 2007 and 2020 for onshore and offshore wind energy. For the predicted 2020 prices the cost of electricity was taken to equal the average of the predicted ranges; i.e. 2.0 p/kWh for onshore generation and 2.5 p/kWh for offshore. The results of these predictions are shown in Tables and .
Table 4. Predicted fuel prices without heat integration.
Table 5. Predicted fuel prices implementing heat integration.
The predicted fuel prices show the importance of including heat integration in the design of the re‐synthesis plant. This heat integration uses the energy removed from the FTS reactor and after‐cooler to heat the rWGS reactor and process stream pre‐heaters in the plant, thus increasing energy efficiency. This optimisation of the re‐syn plant design therefore reduces the energy requirement of the re‐synthesis process, which in turn reduces the predicted price of the fuels in all energy price scenarios.
The predicted prices of the re‐synthesised fuels can be compared with the current price of these fuels when they are sourced conventionally from crude oil. The commodity prices, in April 2008, for naphtha, gas oil and jet fuel sourced from crude oil are shown in Table (The Financial Times 10 April 2008).
Table 6. Commodity prices for oil products (2008).
By comparison with the wholesale prices of traditional naphtha, jet fuel and gas oil, the predicted prices of the re‐syn fuels are not yet cost competitive in 2008. However, by 2020 the situation changes with the re‐syn fuels becoming more than competitive with the conventional fuels, especially for electricity at onshore prices. However, it is more likely that wind farms will be built (out of sight) offshore due to the fact that they will raise fewer planning objections and the turbines will beneficially have a higher productivity. Even so, with offshore wind power in the most favourable case (with a ‘minus’ in Table ) potentially as low as 2.0 p/kWh by 2020, we calculate this would give a re‐syn jet fuel price of 36 pence per litre (p/l) without energy integration and potentially down to 32 p/l with an optimised integrated design.
5.3 Carbon tax effect
Since conventional jet fuel results in a net increase in CO2 emissions upon combustion, it is a candidate for a carbon tax under the proposed EU carbon trading scheme (although aviation is currently exempted). The UK Government has priced the social cost of carbon as £35 to £140 per tonne of carbon or around £10 to £40 per tonne of CO2 (Sustainable Development Commission Citation2005). In comparison, the market price of CO2 in the EU Emissions Trading Scheme has varied between $25 and $35 per tonne of CO2, approximately £12 to £18, throughout the majority of 2005 and 2006 (Energy Information Administration Citation2006). The prices of naphtha, jet fuel and gas oil were estimated with a carbon tax ranging from £10 to £40 per tonne of CO2 for comparison with the re‐synthesised fuels, these are also shown in Table .
The cost of the re‐syn jet fuel in 2008 is still higher than the current price of conventional jet fuel at 37 p/l. However, it is likely that this price will increase by 2020 due to the fact that the worldwide crude oil supplies will become scarcer. Also, if carbon taxation of the conventional oil‐based fuels is implemented, the price of conventional jet fuel could be as high as 45 p/l, using the UK Government's carbon tax predictions (Sustainable Development Commission Citation2005). This would in fact be potentially more expensive than the re‐syn jet fuel, produced from a plant with fully optimised heat integration, at 32 p/l, as well as being non‐sustainable.
5.4 Impact on the aviation business
The impact that an increase in the price of jet fuel will have on the aviation industry is relatively minor compared to other operating costs. For a large aviation company, the cost of jet fuel is around 20% of the operating costs, meaning that an increase of 50% in the price of jet fuel will only increase the total operating costs by around 10% (Cox Citation2005). Therefore, the use of the re‐syn jet fuel in 2008 although potentially more expensive, would have only a marginal impact on the operating costs of the current aviation industry, with the added advantage of being net carbon‐neutral via fully renewable energy. In effect, planes would be flying using wind power!
6. Conclusions
Jet fuel can be re‐synthesised from CO2, conveniently captured from a coal‐fired power station, and H2, from the electrolysis of water, using a combination of the rWGS reaction, FTS and hydrocracking in a re‐synthesis plant. This would make the CO2 emissions from the aircraft industry carbon‐neutral. Otherwise aviation emissions alone are predicted to exceed EU CO2 targets by 2020. This re‐syn fuel is of a higher quality than conventional jet fuel and is fully compatible with new aircraft designs which have expected lifetimes of 50 years. The capture of CO2 by oxy‐fuelling has the additional benefit of creating zero‐emission operation of a coal‐fired power station.
The use of offshore wind energy, although currently more expensive than the use of fossil fuels, provides a renewable way of supplying the re‐syn plant with the energy required for the fuel re‐synthesis process. At current prices, the re‐syn jet fuel at 90 p/l is not competitive with conventional fuels at around 37 p/l. However, by 2020 the price of re‐syn fuel for the projected median price of onshore wind electricity should fall to 32 p/l. For the most favourable cost of offshore wind‐power at the low end of the range (the ‘minus’ case in Table ) and with efficient energy integration, the price would now be the same for the politically more acceptable offshore wind. In comparison, the price of conventional fuel is likely to rise due to scarcity and carbon taxation.
Re‐syn jet fuel, produced by renewable energy from CO2, will become an economically viable alternative to jet fuels sourced from crude oil in the future. The side products, naphtha and gas oil, can also be converted into carbon‐neutral transportation fuels. These re‐syn fuels will help to mitigate the CO2 emissions from the transportation sector, whilst avoiding future problems of crude oil supply. The re‐use of power plant CO2 halves the combined greenhouse gas impact of the coal power and aviation sectors, providing a means of meeting post‐Kyoto reduction targets of 50%.
Acknowledgement
An earlier version of this paper was presented at the joint CHEMRAWN‐XVII and ICCDU‐IX Conference ‘Greenhouse gases: mitigation and utilisation’ held in Kingston, ON, Canada, 9–12 July 2007.
References
- Bows , A. and Anderson , K. 2007 . Policy clash: can projected aviation growth be reconciled with the UK Government's 60% carbon reduction target? . Transport Policy , 14 (2) : 103 – 110 .
- British Wind Energy Association (BWEA), 2007. The economics of wind energy. Available from: http://www.bwea.com/ref/econ.html [Last accessed 4 April 2007]
- Cox, J. 2005. The high cost of aviation fuel. Available from: http://www.globalair.com/discussions/cox/article.asp?msgID = 68 (http://www.globalair.com/discussions/cox/article.asp?msgID = 68) (Accessed 26 August 2005)
- Draft Climate Change Bill . 2007 . : 21 Department for Environment, Food and Rural Affairs (DEFRA), HM Government
- Drax Power Ltd . 2005 . Environmental Performance Review
- Dry , M. E. 1990 . The Fischer‐Tropsch process – commercial aspects. . Catalysis Today , 6 : 183 – 205 .
- Dry , M. E. 2001 . High quality diesel via the Fischer‐Tropsch process – a review. . Journal of Chemical Technology and Biotechnology , 77 : 43 – 50 .
- EAC . 2006 . Keeping the lights on: Nuclear, renewables and climate change, UK House of Commons, Environmental Audit Committee
- Eilers , J. , Posthuma , S. A. and Sie , S. T. 1990 . The Shell middle distillate synthesis process (SMDS). . Catalysis Letters , 7 : 253 – 270 .
- Energy Information Administration . 2006 . International Energy Outlook . : 77 US Department of Energy
- FT Online . 2008 . FT Commodities and Agriculture, retrieved 10 April 2008
- Mann , R. 2006 . Carbon recycling for zero‐emission carbon‐neutral transportation. . Energy Materials , 1 (2) : 1 – 3 .
- Mignard , D. 2003 . Methanol synthesis from flue‐gas CO2 and renewable electricity: a feasibility study. . International Journal of Hydrogen Energy , 28 : 455 – 464 .
- Park , S.‐W. 2001 . Development of ZnO/Al2O3 catalyst for reverse‐water‐gas‐shift reaction of CAMERE (carbon dioxide hydrogenation to form methanol via a reverse‐water‐gas‐shift) process. . Applied Catalysis A , 211 : 81 – 90 .
- Riemer , P. 1993 . The capture of carbon dioxide from fossil fuel fired power stations, IEA Greenhouse Gas R&D Programme, Cheltenham, UK
- Sie , S. T. , Senden , M. M. G. and van Wechem , H. M. H. 1991 . Conversion of natural gas to transportation fuels via the Shell middle distillate synthesis process (SMDS). . Catalysis Today , 8 : 371 – 394 .
- Sims , R. E. H. , Rogner , H. ‐H. and Gregory , K. 2003 . Carbon emission and mitigation cost comparisons between fossil fuel, nuclear and renewable energy resources for electricity generation. . Energy Policy , 31 : 1315 – 1326 .
- Sustainable Development Commission (SDC) . 2005 . Wind power in the UK . 35 : 40 – 42 .
- UK Department for Transport . 2006 . Transport statistics for Great Britain
- Veranth , J. M. and Krishnamoorthy , G. 2001 . “ Oxygen enriched coal combustion with carbon dioxide recovery and recycle: simulation and experimental study ” . 1 – 2 . Department of Chemical and Fuels Engineering, University of Utah, UT, USA