Abstract
The effect of ozone in a chemical sludge disintegration process was evaluated. Sludge solution chemical oxygen demand (COD), total suspended solids (TSS) and settling were investigated in single and sequential processes. A significant influence of ozone dose on sludge disintegration was observed: ozone was utilised to degrade the soluble organic matter and to destroy cell surfaces and release the cell liquids. For a single ozonation step, we found an optimum ozone dose in the range of 0.008–0.013 g O3/g TSS to give the best COD and TSS removal efficiency. Disintegrated sludge was treated in a sequential process consisting of consecutive ozonation and bio-aeration (i.e. O3 + biological treatment). The tendency was dependent on accumulated ozone, treatment time and operational conditions. An accumulated ozone dose of 0.055 g O3/g TSS in two separate ozonation processes followed by biological treatments led to COD and TSS removal efficiency of 53 and 46.6%, respectively. The removal efficiency was improved by increasing aerobic treatment time and/or by mixing ozonated sludge with non-ozonated sludge. The settling ability of sludge was found to be fast at very low specific ozone doses. An observed tendency was the effect of ozone on cell disintegration and protein liberation. The use of sequential processes improved the settling tendency of sludge.
1. Introduction
Increasing concern about the environment has generated growing demands for the development of new environmental technologies, new materials and new ways to reduce and minimise wastes (Cecil et al. Citation1996, Jeyaseelan and Qing Citation1996, Hejazi et al. Citation2003, Mrayyan and Battikhi Citation2005, Chu et al. Citation2008). One of the hot topics of concern is waste activated sludge (WAS), which is produced in abundance during the biological treatment of wastewater (Lundin et al. Citation2004, Manterola et al. Citation2008). Part of this activated sludge should be removed and disposed in order to maintain the biomass concentration in the reactor. The cost for the treatment of the excess sludge can be as much as 50–60% of the total treatment cost (Low and Chase Citation1999, Liu and Tay Citation2001, Wei et al. Citation2003, Nowak Citation2006, Muruganandham et al. Citation2007).
Conventional disposal methods such as landfill or ocean dumping are listed as successful solutions. However, this may cause secondary pollution problems and so these disposal methods are strictly regulated in many countries. Other sludge treatment processes such as ultrasound (Dichtl et al. Citation1997, Yin et al. Citation2004, Feng et al. Citation2009), ball mill (Weemaes and Verstraete Citation1998, Yamashita et al. Citation2010), homogeniser treatments, electric beam, thermal treatment in the temperature ranges of 40–180°C (Tanaka et al. Citation1997), freezing, thawing and enzyme treatment have also been tested (Sakai et al. Citation1997, Tanaka et al. Citation1997, Muller et al. Citation1998, Chu et al. Citation1999, Kepp et al. Citation1999, Barjenbruch and Kopplow Citation2003, Yoon et al. Citation2004, Wawrzynczyk et al. Citation2008). However, the application of these methods turned out to be rather complicated and expensive (e.g. Ball mill), and needed high energy (ultrasound), aggressive reaction conditions (thermal treatment) or special material (freezing, thawing and enzyme).
Recently, sludge disintegration has been proposed as a promising treatment method for sludge reduction (Ray et al. Citation1990, Yasui and Shibata Citation1994, Yasui et al. Citation1996, Muller et al. Citation1998, Weemaes et al. Citation2000, Yeom et al. Citation2002, Goel et al. Citation2003). Among the various approaches, chemical treatments using ozone were most widely studied and adopted in many commercialised processes (Muruganandham et al. Citation2007, Chu et al. Citation2008, Yan et al. Citation2009, Zhang et al. Citation2009). Ozone is a strong oxidising gas and it reacts with inorganic and organic compounds directly or indirectly via the formation of hydroxyl radicals. It preferentially oxidises electron-rich molecules containing carbon–carbon double bonds and aromatics. Ozone treatment splits the long-chain compounds making them biodegradable. The combination of ozonation and aerobic bio-treatment was demonstrated to be an effective method for destroying lipophilic extractives and increasing the biodegradability [BOD/chemical oxygen demand (COD) ratio] of treated organic matter (OM) before returning them to the bio-treatment unit (Kamenev Citation2003, Bierbaum and Oller Citation2005, Carballa et al. Citation2007, Chu et al. Citation2008).
Most disintegration methods propose the combination of ozone treatment with biological treatment as a typical process for accelerated sludge degradation (Chiu et al. Citation1997, Weemaes et al. Citation2000, Yeom et al. Citation2002, Lee et al. Citation2005, Campos et al. Citation2009). The objective of the ozonation step is to hydrolyse solids, to reduce excess biomass production and to increase biogas production (Yasui and Shibata Citation1994, Sakai et al. Citation1997). Several studies suggest treating part of the recycled sludge with ozone, where both solubilisation to biodegradable organics (due to disintegration of suspended solids) and to a lesser extent mineralisation to CO2 and H2O (due to oxidation of soluble OM) occur (Kamiya and Hirotsuji Citation1998). Then, the sludge should be returned to the wastewater stream for further biodegradation (Yasui and Shibata Citation1994, Boehler and Siegrist Citation2004). Overall, the basis for the sludge reduction processes is an effective combination of these methods for sludge disintegration and biodegradation of the treated sludge. The disintegration of the sludge biomass was reflected by a decrease in total suspended solids (TSS), volatile suspended solids (VSS), the ratio of VSS/TSS and inorganic solids in the ozonated sludge (Deleris et al. Citation2002). Yasui and Shibata (Citation1994) and Yasui et al. (Citation1996) combined the activated sludge process with ozone pre-treatment: the combined processes resulted in zero sludge production. Disintegration technology may also improve the performance of other wastewater treatment processes. For example, the membrane bioreactor process has been known as a process with relatively high decay rate and less sludge production due to much longer biomass retention in the reactor. However, the use of disintegration technology reduced the sludge production while maintaining high retention time (Shimizu et al. Citation1996, Yoon et al. Citation2004).
Although previous studies showed that the use of ozone for sludge reduction is successful, the amount of ozone dose required per gram of TSS was high; few references in the literature specify the economical required ozone dose for a specific mass of sludge (Chu et al. Citation2008, Manterola et al. Citation2008, Zhang et al. Citation2009). The present work was conducted first to confirm previous findings and second to relate the amount of ozone required for specific mass of sludge in an economical way (i.e. optimum conditions). The study also aimed to follow up and evaluate the behaviour of OM during the ozonation process and during sequential ozone-aerobic processes. Moreover, the effect of ozone on sludge characteristics such as settling ability and protein content during single and sequential process was assessed.
2. Material and methods
2.1 Ozone experiments
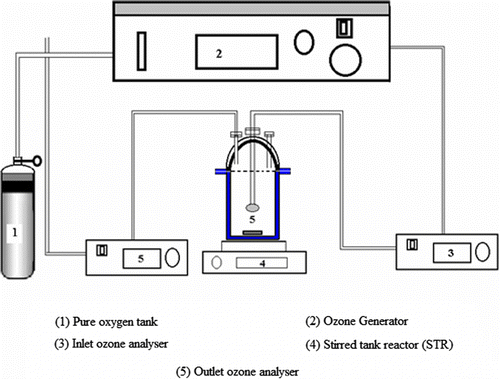
The experimental equipment arrangement used for this study is presented in Figure . The equipment consists of (1) pure oxygen tank, (2) ozone generator (Ozone generator COM-AD-01; Anseros, Tübingen, Germany); ozone was produced onsite from ultra pure O2, gas connections were all of PFA tubes, (3) Inlet ozone analyser (Ozomat GM-60000-OEM), (4) 2.5 l stirred tank reactor; the sludge mixing, inside the reactor, was performed using a magnetic bar and base agitator; ozone was distributed in the reactor by means of a ceramic diffuser and (5) outlet ozone analyser (Ozomat GM-60000-OEM).
2.2 Aerobic reactor
Aerobic digestion was carried out in 2.5 l aerobic reactors. The reactors were supplied with agitator and air supply. The air flow rate was controlled to be in the range of 8–10 l/h. The digestion was carried out at room temperature and in the pH range of 6.8–7.2.
2.3 Experimental procedure
All the trials were conducted as batch experiments. The WAS used in this work was obtained from the wastewater treatment plant (WWTP) near Goslar in Germany. The WWTP has a capacity of approx. 150,000 PE and connected to a small industry (non-chemical industry). The WAS used in these experiments was the return sludge collected from the recycled sludge in the biological reactor. The experimental procedure was as follows: the obtained sludge from WWTP was stabilised for 24 h in an aerobic biological reactor. Next, a volume of 2.0 l of sludge was oxidised with ozone at a specific inlet ozone concentration (from 2.0 to 20.0 mg/l) and a specific gas flow rate (either 40 or 50 l/h). This was considered as a first ozonation step. Following this, the oxidised sludge was diverted to an aerobic reactor and aerated for a certain time (4, 24, 48 or 96 h). A similar procedure was performed for subsequent ozonation and biological aeration. The inlet and outlet ozone concentration levels were measured online by means of an ozone analyser.
2.4 Analytical methods
COD ( ± 3 mg O2/l) was followed as indicated in the Standard Methods (1985) section 5220D. Macherey–Nagel (Düren, Germany) analyses test-kits were used in the test.
The analyses of total solids (TS) and TSS were accomplished following German regulations (DIN).
The sludge settlement was followed by measuring the volume in milli litres occupied by 1 g of a suspension after a 30-min settling period.
3. Results and discussion
3.1 Sludge ozonation
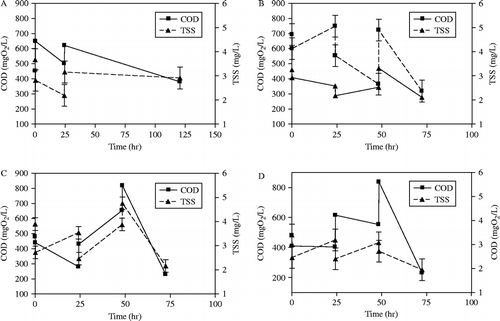
Figure presents the evolution of COD and TSS obtained during batch ozonation of stabilised sludge for different ozone doses. Each experiment was repeated at least 3 times (error bar on Figure was calculated based on 95% CI). Although the starting sludge had the same characteristics, it was observed that COD and TSS evolution depended on the ozone dose: at low ozone dose, in the range of 0.00025–0.0010 g O3/g TSS (see Figure (A),(B)), the contained COD increased progressively during the ozonation process reaching a maximum value (in Figure (A); from 452 to 620 mg O2/l), after that the COD started to decrease. The observed tendency can be explained for two reasons: (1) liberation of some of the OM from the ozonated sludge as a result of sludge disintegration by means of ozone and (2) disintegration of sludge as a result of shear between liquid and gas (air+O3). As the supplied ozone was increased, the sludge tendency was somehow different; for ozone dose in the range from 0.0015 to 0.022 g O3/g TSS (see Figure (C)), it was observed that the COD at first decreased reaching a minimum value, after that the COD started to increase. The decrease in COD value can be attributed to the fast direct reaction of ozone with soluble COD in the solution. After mineralisation of all soluble COD, ozone attacks the surface of the cells leading to release of cell liquids. These liquids are known to have high OM mostly measured as COD. Accordingly, the solution COD increases. The released cell liquids are considered as simple OM which can be oxidised easily. The oxidation of these compounds was noticed at higher ozone doses, 0.022–0.044 g O3/g TSS (see Figure (D)); it was observed that the COD at first decreased, as a result of consuming the soluble COD, then the COD increased (cell liquids release). Nevertheless, due to the presence of sufficient amounts of ozone, which attacks the released OM, the COD decreased again.
Figure 2 Evolution of COD and TSS during batch ozonation at different ozone dose: pH = 7, (A) [O3] i = 0.00025 O3/g TSS, (B) [O3] i = 0.0010 g O3/g TSS, (C) [O3] i = 0.0015 g O3/g TSS and (D) [O3] i = 0.022 g O3/g TSS.
![Figure 2 Evolution of COD and TSS during batch ozonation at different ozone dose: pH = 7, (A) [O3] i = 0.00025 O3/g TSS, (B) [O3] i = 0.0010 g O3/g TSS, (C) [O3] i = 0.0015 g O3/g TSS and (D) [O3] i = 0.022 g O3/g TSS.](/cms/asset/08676c4f-46ae-422b-ae56-7d7b1eba53f2/tsue_a_534643_o_f0002g.gif)
The evolution of TSS for all ozone doses confirmed the disintegration of sludge; the supplied ozone dose was found to be related to the decrease in sludge TSS; for low ozone dose the decrease in TSS did not exceed 25%, while for high ozone dose the decrease in TSS was more than 33%.
Although the removal of more OM (COD) from the sludge is favourable, the cost of the ozonation step is expensive. However, the use of ozone to disintegrate the cell structure increases the sludge biodegradability. On the basis of the experimental results presented in Figure , sludge cells were destroyed in the ozonation process at the point where the COD content of the solution increased. Thus, the ozonation process can be cut-off at this point and the sludge can bio-oxidise in the subsequent biological treatment. On the basis of the experimental results, an optimum ozone dose of 0.008–0.013 g O3/g TSS is proposed. The results are interesting for two reasons: (1) the efficient interaction between the sludge and ozone and (2) the low amount of ozone required per g of TSS in the sludge. A similar ozone dose value (0.02 g O3/g TSS) was reported by Chu et al. (Citation2008). According to our literature review, the present ozone dose is lower by 15% when compared with published data. Zhang et al. (Citation2009) reported that an optimum ozone dose of 50 mg O3/g of dissolved solids was required to achieve sludge disintegration of 46.7% after 105 min. A lower ozone dose (between 25 and 35 mg O3/g TSS) was reported by Manterola et al. (Citation2008).
3.2 Ozonation followed by bio-treatment
After the first ozonation (with different ozone doses), the sludge was put in an aerobic biological reactor and incubated for a specified time. The sludge was then removed from the reactor and ozonated again for a specified time and this procedure was repeated for a specified number of times. Table summarises the experimental results obtained in each set of experiments (i.e. for sequential processes). The sludge tendency for each set of experiments (results are the average of three experiments under the same conditions) was found to be related to the cumulative amount of ozone, biological incubation time and aerobic treatment operational conditions. The following are the conditions for sludge treated with ozone and biological treatment: the process sequence was O3 + bio1 + O3 + bio2 + O3 + bio3, the ozonation time was 5 min, the biological incubation time was 24 h, and accumulated ozone dose was 0.0014 g O3/g TSS; the COD and TSS reductions were by 11.9 and 15%, respectively. Increasing the accumulated ozone dose to 0.039 g O3/g TSS under similar conditions led to COD and TSS reductions of approximately 48 and 42%, respectively. Increasing the ozone still further to 0.055 g O3/g TSS (see Runs 3 and 4) improved the removal efficiency to 52 and 46.6% for COD and TSS, respectively. These values are considered significant when compared with published data.
Table 1 Experimental results obtained for different sequential processes.
Figure illustrates the evolution of COD and TSS for sequential processes related to the sludge used in the single ozonation step shown in Figure . The obtained data are in agreement with the observations of Table ; for an accumulated ozone dose of 0.014 g O3/g TSS, the COD and TSS were reduced by 15.6 and 20%, respectively. Higher accumulated ozone of 0.099 g O3/g TSS led to COD and TSS reduction by 51 and 43%, respectively.
Figure 3 Evolution of COD and TSS during the sequential process (A) ΔO3 = 0.014 g O3/g TSS, (B) ΔO3 = 0.015 g O3/g TSS, (C) ΔO3 = 0.099 g O3/g TSS and (D) ΔO3 = 0.141 g O3/g TSS.
In Section 3.2 (single ozonation), it was concluded that the optimum ozone dose for a single ozonation was in the range of 0.008–0.013 g O3/g TSS. The results obtained in the sequential process confirm the previous results (see Table ); accumulated ozone dose was in the range 0.034 g O3/g TSS, and the COD and TSS were reduced by 48 and 42%, respectively. Moreover, it was observed that higher ozone doses did not enhance the removal efficiency, but simply oxidised the biodegradable material. Consequently, the process feasibility decreased.
3.3 Operational mode
To test the effect of aerobic incubation time on sludge treatment, we performed two sets of experiments (see Runs 5 and 6). The first set had the following conditions: sequential process O3 + bio1 + O3 + bio2, ozonation time of 5 min; accumulated ozone dose of 0.011 g O3/g TSS and biological incubation time of 24 h. The second set had similar operational conditions but the incubation time of the second biological step was increased to 96 h. The increase in the time of the second biological treatment led to an increase in removal efficiency of both COD and TSS. As the second biological treatment time was increased from 24 to 96 h, the COD and TSS reductions were improved from 30.6 and 36.8% up to 42.6 and 44.7%, respectively. A similar tendency was observed for different ozone doses (see Runs 7 and 8).
The sequence of processes was changed in order to increase the removal efficiencies (see Runs 9 and 10). The accumulated ozone doses for these runs were 0.075 and 0.054 g O3/g TSS, respectively. There was a significant difference in terms of COD and TSS removal between the two runs. Although in Run 9, the TSS was reduced by 45.9%, there was a noticeable decrease in COD reduction of the solution. This tendency can be attributed to high-cell disintegration from the ozone dose and/or the short biological incubation time, which are not enough to bio-oxidise the free cell ingredients. In Run 10, repeating the biological incubation stage twice improved the OM removal efficiency and increased both COD and TSS removal efficiency to 50.0 and 42.0%, respectively.
3.4 Sludge mixing effect
Another set of experiments was performed by mixing the ozonated sludge with stabilised non-ozonated sludge. The procedure was as follows: a specific volume (2000 ml) of sludge was ozonated for a specified time (5, 10 or 20 min), next the ozonated sludge was mixed with stabilised non-ozonated sludge with a volume ratio of 3:1 (i.e. 1500 ml of ozonated sludge with 500 ml of non-ozonated sludge). The sludge mixture was aerated for 24 or 48 h, after that a specific volume (2000 ml) of this sludge mixture was taken and ozonated (second ozonation stage) with the same ozone dose and time. After the second ozonation stage, the sludge was again mixed with stabilised sludge and aerated for a certain time. Table presents the results and operational conditions of these experiments. It can be seen that for all the operational conditions, the accumulated ozone dose was low (less than 0.03 g O3/g TSS) and there was enhancement of both COD and TSS removal; better than 37 and 44% for COD and TSS, respectively. A maximum COD and TSS removal efficiency of 57 and 44.3%, respectively, was achieved at accumulated ozone dose of 0.024 mg/l. The enhanced removal efficiencies can be attributed to the fact that ozonation degraded the non-biodegradable materials in the sludge and transformed them to small biodegradable materials. Thus, using non-ozonated stabilised sludge helps in biodegradation by removing the OM from cell disintegration. Consequently, the COD removal increases. Moreover, it was mentioned previously that the ozone breaks up the cell structure, thus releasing the cell ingredients for degradation. As a result the active stabilised sludge also bio-oxidises these ingredients, and thus the removal efficiency increases.
Table 2 Operational conditions and removal efficiencies for sludge mixing experiments.
3.5 Lessons to learn
On the basis of these experimental results, it can be seen that sludge disintegration and biodegradability improvement is a complex task, because so many different influences exist. Each WWTP has its own sludge due to the differences in wastewater characteristics, treatment size, process design, operational parameters and sludge treatment type. Thus, optimisation of disintegration technology requires an understanding of the influence of the chemical process of sludge behaviour. Some of important factors to take into account are
-
The amount and dispersion of the TS. A special oxidation reagent concentration related to the TS is commonly used for plant operation. It is often correlated with the release of cell content in terms of COD. The dispersion involves the floc size distribution and influences the micro-kinetic regime (reaction on particle surfaces, diffusion into flocs).
-
The soluble compounds and their concentration in the wastewater, e.g. organics, nitrogen, nitrate or metals. Many soluble organic compounds react with ozone as well as with the volatile solids resulting in lower efficiency for sludge reduction applications. This is also valid for the oxidation of the mostly biodegradable cell content released during ozonation and, therefore, the released compounds should be reduced by the cheaper biological oxidation if possible.
-
The hydrodynamics of the ozone reaction system. The mean residence time distribution of continuous ozone reaction systems involves different ozone contact times for each sludge volume entering the system. Accordingly, inside the same reactor, different sludge oxidation states can be found: part of the sludge is attacked by ozone leading to high oxidation state, whereas the other part of the sludge faced lower oxidation and has no contribution to sludge integration and/or COD reduction.
-
The size and operational type of biological system. The sludge age (solid retention time, SRT) in the biological system can compensate the ‘degree’ of partial oxidation. A lower degree of partial oxidation resulting in less biodegradable COD may result in higher total volatile solids reduction using adapted sludge of higher SRT.
3.6 Settling effect
The effect of the ozonation process and sequential processes on sludge settlement was investigated. Table presents the fraction of sludge after 30 min settling time for different processes. It was observed that the settling efficiency increased at first at very low specific ozone dose rates below approx. 0.005 g O3/g TSS. Higher ozone dose rates led to a decrease in the settling ability of sludge. This turning point depends also on the sludge characteristics. For example, single ozonation of sludge with ozone dose of 0.005 g O3/g TSS gave a sludge settling of 40% in 30 min. At a higher ozone dose (0.024 g O3/g TSS), the sludge settling was reduced to 18%. These results can be explained as follows. A high available ozone level disintegrates the cell structures and liberates their content to the solution. The released COD contains proteins as a part of the cell liquid; these proteins have negative effects on sludge settling due to their surface charge. Moreover, the fraction of smaller solid particles increases with the ozone dose. Smaller particles need more time for settling. Similar results were reported by Ried et al. (Citation2002).
Table 3 Effect of the ozonation process and sequential processes on the sludge settlement.
There was a significant effect of the biological process on the settling tendency (see Table ). A reasonable subsequent biodegradation process for the ozonated sludge improved the settling tendency of the sludge. This can be attributed to bio-oxidation of the released portions and to eliminate their negative effect of sludge settling.
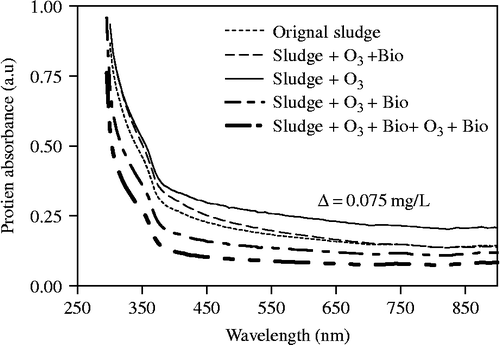
The previous finding was confirmed by protein tests on the treated solutions. Figure presents the protein test for one set of experiments. It was observed that the solution settling tendency was highly dependent on the amount of protein in the water solution: as the protein content in the solution decreased, the water solution settling tendency increased.
4. Conclusions
Ozonation can be used for sludge disintegration and to significantly reduce the production of excess sludge in bio-reactors. The reduction of COD and suspended solids in the bioreactor after ozone treatment was proportional to the ozone dose. Ozonation of sludge increased the soluble biodegradable COD in the solution due to the release of cell liquid. The total removal efficiency in sequential ozone-biological treatment can be controlled by relating the ozone dosage to the TSS content. Significant COD and TSS removal percentage was observed at moderate ozone dose for a single-step process in the range of 0.008–0.013 g O3/g TSS. Maximum removal efficiency can be achieved by increasing the bio-oxidation time or by mixing fresh sludge with bio-oxidised disintegrated sludge.
The disintegrated sludge was found to have low settling ability due to the presence of high surface charge proteins in the solution. The settling efficiency can be enhanced by the removal of these proteins either by chemical or by biological oxidation.
Acknowledgements
The authors acknowledge the financial support provided by Deutsche Forschungsgemeinschaft. The authors also acknowledge their appreciation of the technical support from the technical staff at the CUTEC-Institut GmbH.
References
- Barjenbruch , M. and Kopplow , O. 2003 . Enzymatic, mechanical and thermal pre-treatment of surplus sludge . Advances in Environmental Research , 7 ( 3 ) : 715 – 720 .
- Bierbaum , S. and Oller , H.-J. 2005 . “ Online-controlled ozone dosage for the treatment of paper mill effluents ” . In Proceedings of the IOA 17th World Ozone Congress VII.1-21 – VII.1.2-10 . Strasbourg, France
- Boehler , M. and Siegrist , H. 2004 . Partial ozonation of activated sludge to reduce excess sludge, improve denitrification and control scumming and bulking . Water Science and Technology , 49 ( 10 ) : 41 – 49 .
- Campos , J.L. 2009 . Ozonation strategies to reduce sludge production of a seafood industry WWTP . Bioresource Technology , 100 ( 3 ) : 1069 – 1073 .
- Carballa , M. 2007 . Influence of ozone pre-treatment on sludge anaerobic digestion: removal of pharmaceutical and personal care products . Chemosphere , 67 ( 7 ) : 1444 – 1452 .
- Cecil , L.-H. 1996 . Sludge management in highly urbanized areas . Water Science and Technology , 34 ( 3–4 ) : 517 – 524 .
- Chiu , Y.C. 1997 . Alkaline and ultrasonic pretreatment of sludge before anaerobic digestion . Water Science and Technology , 36 ( 11 ) : 155 – 162 .
- Chu , C.P. 1999 . Reduction in microbial density level through freezing and thawing . Water Research , 33 : 3532 – 3535 .
- Chu , L.-B. 2008 . Enhanced sludge solubilization by microbubble ozonation . Chemosphere , 72 ( 2 ) : 205 – 212 .
- Deleris , S. 2002 . Minimization of sludge production in bio-logical processes: an alternative solution for the problems of sludge disposal . Water Science and Technology , 46 ( 10 ) : 63 – 70 .
- Dichtl , N. 1997 . Waste sludge disintegration – a recent overview . Korrespondenz Abwasser , 44 : 1726 – 1739 .
- Feng , X. 2009 . Dewaterability of waste activated sludge with ultrasound conditioning . Bioresource Technology , 100 ( 3 ) : 1074 – 1081 .
- Goel , R. 2003 . Optimal process configuration for anaerobic digestion with ozonation . Water Science Technology , 48 ( 10 ) : 86 – 96 .
- Hejazi , R.F. , Tahir , H. and Khan , F.I. 2003 . Landfarming operation of oily sludge in arid region – human health risk assessment . Journal of Hazardous Materials , 99 ( 3 ) : 287 – 302 .
- Jeyaseelan , S. and Qing , L.G. 1996 . Development of adsorbent/catalyst from municipal wastewater sludge . Water Science and Technology , 34 ( 3–4 ) : 499 – 505 .
- Kamenev , I. 2003 . Aerobic bio-oxidation combined with ozonation in recalcitrant water treatment Doctoral thesis. Tallinn Technical University
- Kamiya , T. and Hirotsuji , J. 1998 . New combined system of biological process and intermittent ozonation for advanced wastewater treatment . Water Science and Technology , 38 ( 8–9 ) : 145 – 153 .
- Kepp , U. 1999 . Enhanced stabilisation of sewage sludge through thermal hydrolysis – three years of experience with a full scale plant . Water Science and Technology , 42 ( 9 ) : 89 – 96 .
- Lee , J.W. 2005 . Operational strategies for an activated sludge process in conjunction with ozone oxidation for zero excess sludge production during winter season . Water Research , 39 ( 7 ) : 1199 – 1204 .
- Liu , Y. and Tay , J.-H. 2001 . Strategy for minimization of excess sludge production from the activated sludge process . Biotechnology Advances , 19 ( 2 ) : 97 – 107 .
- Low , E.W. and Chase , H.A. 1999 . Reducing production of excess biomass during wastewater treatment . Water Research , 33 ( 5 ) : 1119 – 1132 .
- Lundin , M. 2004 . Environmental and economic assessment of sewage sludge handling options . Resources, Conservation and Recycling , 41 ( 4 ) : 255 – 278 .
- Manterola , G. , Uriarte , I. and Sancho , L. 2008 . The effect of operational parameters of the process of sludge ozonation on the solubilisation of organic and nitrogenous compounds . Water Research , 42 ( 12 ) : 3191 – 3197 .
- Mrayyan , B. and Battikhi , M.N. 2005 . Biodegradation of total organic carbons (TOC) in Jordanian petroleum sludge . Journal of Hazardous Materials , 120 ( 1–3 ) : 127 – 134 .
- Muller , J.A. 1998 . Disintegration of sewage sludges and influence on anaerobic digestion . Water Science and Technology , 38 ( 8–9 ) : 425 – 433 .
- Muruganandham , M. , Chen , S.H. and Wu , J.J. 2007 . Evaluation of water treatment sludge as a catalyst for aqueous ozone decomposition . Catalysis Communications , 8 ( 11 ) : 1609 – 1614 .
- Nowak , O. 2006 . Optimizing the use of sludge treatment facilities at municipal WWTPs . Journal of Environmental Science and Health , A41 ( 9 ) : 1807 – 1817 .
- Ray , B.T. , Lin , J.G. and Rajan , R.V. 1990 . Low-level alkaline solubilization for enhanced anaerobic digestion . Journal of the Water Pollution Control Federation , 62 ( 1 ) : 81 – 87 .
- Ried , A. 2002 . Optimierungsmöglichkeiten beim Betrieb von biologischen Kläranlagen durch den Einsatz von Ozon . Korrespondenz Abwasser , 49 ( 5 ) : 648 – 661 .
- Sakai , Y. 1997 . An activated sludge process without excess sludge production . Water Science and Technology , 36 ( 11 ) : 163 – 170 .
- Shimizu , Y. 1996 . Filtration characteristics of hollow fiber microfiltration membranes used in membrane bioreactor for domestic wastewater treatment . Water Research , 30 ( 10 ) : 2385 – 2392 .
- Tanaka , S. 1997 . Effects of thermochemical pre-treatment on the anaerobic digestion of waste activated sludge . Water Science and Technology , 35 ( 8 ) : 209 – 215 .
- Wawrzynczyk , J. 2008 . The function of cation-binding agents in the enzymatic treatment of municipal sludge . Water Research , 42 ( 6–7 ) : 1555 – 1562 .
- Weemaes , M. and Verstraete , W. 1998 . Evaluation of current wet sludge disintegration techniques . Journal of Chemical Technology and Biotechnology , 73 : 83 – 92 .
- Weemaes , M. 2000 . Anaerobic digestion of ozonized biosolids . Water Research , 34 ( 8 ) : 2330 – 2336 .
- Wei , Y. 2003 . Minimization of excess sludge production for biological wastewater treatment . Water Research , 37 ( 18 ) : 4453 – 4467 .
- Yamashita , Y. , Sasaki , C. and Nakamura , Y. 2010 . Development of efficient system for ethanol production from paper sludge pretreated by ball milling and phosphoric acid . Carbohydrate Polymers , 79 ( 2 ) : 250 – 254 .
- Yan , S.-T. 2009 . Systematic analysis of biochemical performance and the microbial community of an activated sludge process using ozone-treated sludge for sludge reduction . Bioresource Technology , 100 ( 21 ) : 5002 – 5009 .
- Yasui , H. and Shibata , M. 1994 . An innovative approach to reduce excess sludge production in the activated sludge processes . Water Science and Technology , 30 ( 9 ) : 11 – 20 .
- Yasui , H. 1996 . A full-scale operation of a novel activated sludge process without excess sludge production . Water Science and Technology , 34 ( 3–4 ) : 395 – 404 .
- Yeom , I.T. 2002 . Effects of ozone treatment on the biodegradability of sludge from municipal wastewater treatment plants . Water Science and Technology , 46 ( 4–5 ) : 421 – 425 .
- Yin , X. 2004 . A review on the dewaterability of bio-sludge and ultrasound pretreatment . Ultrasonics Sonochemistry , 11 ( 6 ) : 337 – 348 .
- Yoon , S.H. , Kim , H.S. and Lee , S.H. 2004 . Incorporation of ultrasonic cell disintegration into a membrane bioreactor for zero sludge production . Process Biochemistry , 39 ( 12 ) : 1923 – 1929 .
- Zhang , G. 2009 . Sludge ozonation: disintegration, supernatant changes and mechanisms . Bioresource Technology , 100 ( 3 ) : 1505 – 1509 .